What’s the diagnosis?
A man with bilateral pink plaques in the axillae
Case presentation
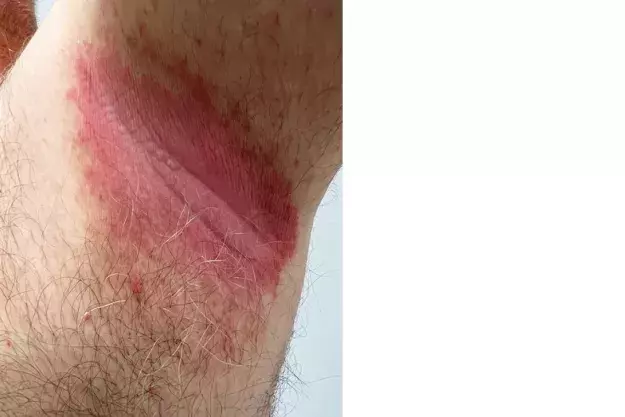
A 56-year-old man presents with a two-year history of pink plaques in his axillae. The plaques are slightly uncomfortable but not painful or pruritic. He has stopped using deodorants because they cause stinging, but the eruption has not improved. He has a history of chronic plaque psoriasis, type 2 diabetes and obesity.
On examination, well-demarcated pink to erythematous plaques with lichenification are observed in the axillae (Figure). Skin scrapings are taken for Candida and bacterial cultures, and negative results are returned.
Differential diagnoses
Conditions to consider among the differential diagnoses for a patient presenting with bilateral pink to erythematous plaques in the axillae include the following.
Contact dermatitis
Contact dermatitis is a common inflammatory skin condition. There are two main forms: allergic and irritant.
Allergic contact dermatitis is a delayed-type hypersensitivity reaction that occurs when skin comes in contact with a chemical to which it has previously been sensitised.1 In the acute setting, these reactions are often intensely pruritic and eczematous, with or without blistering, weeping and oedema.1 Allergic contact dermatitis can affect anyone and can occur at any age. Fragrances are one of the most common causes.2 About 1 to 4% of the general population and 6 to 14% of patients with contact dermatitis suffer from fragrance contact allergy.3 The gold standard diagnostic tool for allergic contact dermatitis is patch testing.
Irritant contact dermatitis involves a nonimmune process and is caused by direct skin injury or inflammation as a result of contact with an irritating substance.4 It can be precipitated by antiperspirants such as aluminium chloride hexahydrate, especially when used in high concentrations for the treatment of hyperhidrosis.5
Axillary skin is particularly prone to contact dermatitis because it is thin, moist and under occlusion. Some manifestations of contact dermatitis can be both allergic and irritant.4
For the case patient, the axillary lesions were not pruritic, which makes a diagnosis of allergic contact dermatitis less likely, and they were not painful, which makes a diagnosis of irritant contact dermatitis less likely. There was also no improvement when he stopped using deodorant, which makes either form of contact dermatitis less likely. Furthermore, the lesions had remained intensely pink to erythematous for two years – if contact dermatitis was the cause then the lesions would be expected to become increasingly scaly and poorly demarcated over a protracted period with repeated exposures.1
Seborrhoeic dermatitis
Seborrhoeic dermatitis is an erythematous scaly skin condition that is typically confined to regions with sebaceous glands and body folds.6 The scalp is most commonly affected, followed by the face, chest and intertriginous sites. The pathophysiology of seborrhoeic dermatitis remains poorly understood, but it is thought to be caused by an inflammatory response to the commensal yeast Malassezia (particularly Malassezia furfur [Pityrosporum ovale]) and linked to the overproduction of sebum.6
The diagnosis of seborrhoeic dermatitis is made clinically. Patients present with erythematous, pink-yellow, dull red or red-brown plaques with a bran-like or flaky ‘greasy’ scale. It is considered one of the most common skin disorders, with a prevalence estimated to be 5%, but the lifetime incidence is likely much higher.7 Men are more commonly affected than women.6
Seborrhoeic dermatitis has a chronic and relapsing course. Patients feel well and are generally systemically well. However, extensive and therapy-resistant seborrhoeic dermatitis should raise the suspicion of underlying immunosuppression, such as HIV infection.6
For the case patient, this diagnosis is less likely because the plaques are confluent and thick, without bran-like scale. It would be unusual for seborrhoeic dermatitis to be isolated to the axillae with no involvement of the scalp, face or ears. In addition, the lesions are often pruritic, which was not present for this case.
Candida intertrigo
Candidiasis is a fungal infection caused by the yeast Candida. It is a normal inhabitant of the human digestive tract from early infancy, where it does not cause disease in the majority of cases.
Candida infections have a wide spectrum of clinical presentations on the mucosa and skin and, rarely, can be invasive.8 Almost 90% of all skin Candida infections are caused by Candida albicans.9 Predisposing factors include diabetes mellitus, obesity, the use of corticosteroids or broadspectrum antibiotics and immunosuppression (such as HIV infection).8,10 Inflammatory skin conditions such as psoriasis also increase the risk of candidiasis.10
Cutaneous Candida infections typically present as deeply erythematous and sometimes erosive patches, often with satellite papules and pustules.8 Patients frequently complain of a burning sensation, pruritus, odour and pain. Sites most commonly affected are submammary, inguinal creases, intergluteal folds, axillae and scrotum, as well as the nappy area of babies and young children. The moisture and warm temperature on the surface of opposing skin folds are favourable for the growth of Candida.9
The clinical appearance of candida intertrigo is usually sufficient for a diagnosis.9 However, in chronic, resistant and recurrent cases, a skin scraping may be required for potassium hydroxide examination (looking for budding yeast and pseudohyphae) and/or culture.
For the case patient, the dry (rather than macerated) appearance of the plaque and absence of satellite lesions makes candidiasis a less likely diagnosis. Candida infections are characterised by rapid development, whereas this patient’s plaques had expanded over years. Furthermore, results of skin scrapings for culture were negative.
Erythrasma
Erythrasma is a superficial bacterial skin infection caused by Corynebacterium minutissimum. The worldwide incidence is around 4%, but it is more common in tropical and subtropical areas because of the warm and humid climate.11
Erythrasma commonly affects the axillae, groin, intergluteal fold, umbilicus and submammary region, as well as the webbed spaces of the toes.12 The condition typically presents as well-defined pink to brown patches with fine scale and is either asymptomatic or only mildly pruritic.12 Recurrence is common. Although erythrasma is typically a clinical diagnosis, the finding of coral-pink fluorescence on Wood’s lamp examination (caused by illumination of porphyrin produced by the bacteria) supports the diagnosis.8
For the case patient, erythrasma is an important diagnosis to consider because he has two risk factors (type 2 diabetes and obesity) for the condition.12 Other predisposing factors include poor hygiene, hyperhidrosis and immunosuppression.12 However, it is not the most likely diagnosis because it typically presents as brown plaques with fine scale; in addition, the plaques did not show fluorescence with Wood’s lamp examination.
Inverse psoriasis
This is the correct diagnosis. Inverse (intertriginous) psoriasis is a relatively rare form of psoriasis in which the flexural areas are the only sites of involvement.13 The inguinal fold is most commonly affected, followed by the axillae and the external genitalia.14 Up to 7% of patients with psoriasis present with the inverse form.15,16 Its appearance and distribution in the flexural skin folds make it difficult to diagnose and differentiate from more common causes of intertrigo, such as candidiasis.17
The lesions of inverse psoriasis are characterised by shiny, pink to red, well-demarcated plaques that are commonly symmetrical.10 Central fissures are often seen.13 There is much less scale than for typical untreated chronic plaque psoriasis.13
For the case patient, the symmetry, colour and clear demarcation of the plaques support the diagnosis of inverse psoriasis. These features are not typical of the aforementioned conditions. The history of psoriasis is also significant, as between 21 and 30% of patients with plaque psoriasis will also develop the inverse form.18
Management
Inverse psoriasis often warrants a treatment strategy that is distinct from chronic plaque psoriasis because of the intrinsic sensitivity, thinness and occlusion of involved areas.19 A systematic review of treatments published in 2020 found that first-line treatment for inverse psoriasis should commence with low-to-mid potency topical corticosteroids (such as daily 0.1% betamethasone valerate or 0.005% fluticasone propionate).19-21 Although not as efficacious as topical corticosteroids, topical immunomodulators (such as pimecrolimus 1% once or twice daily and tacrolimus 0.1% twice daily) as well as vitamin D analogues (such as calcipotriol 0.005% ointment once daily) were also ranked as first-line treatments.20,22,23 These do not confer the same risk of skin atrophy as topical corticosteroids and are therefore preferred for long-term (more than four weeks) therapy.19 Coal tar is also used in the treatment of inverse psoriasis. The evidence for use of systemic treatments for inverse psoriasis is very low, although ixekizumab (a monoclonal antibody) has shown efficacy in patients with moderate-to-severe genital psoriasis.19,24
For all patients with a new or existing diagnoses of psoriasis, it is imperative to screen for psoriatic arthritis and other comorbidities such as depression, metabolic syndrome and cardiovascular disease.14 Obesity is both a risk factor and an aggravating factor for inverse psoriasis; weight reduction has been shown to reduce the severity of psoriasis for patients with obesity.25,26
Outcome
For the case patient, it was important to take skin scrapings for fungal and bacterial culture for several reasons. Candida, bacterial (e.g. streptococcal species) and dermatophyte infections can be a trigger for inverse psoriasis and, if present, would require additional treatment.13 Skin affected by psoriasis and other disorders may also be secondarily infected by Candida or bacteria – and this patient’s comorbidities of diabetes mellitus and obesity put him at an increased risk for infections in general.10,27 He was treated with topical hydrocortisone 1% ointment, followed by 3% liquor picis carbonatum (LPC). Weight reduction may improve his psoriasis and would also improve control of his diabetes.28MT
COMPETING INTERESTS: None.
References
1. Nixon RL, Mowad CM, Marks JG. Allergic contact dermatitis. In: Bolognia JL, Schaffer JV, Cerroni L (eds). Dermatology, 4th ed. Philadelphia, PA: Elsevier; 2018. pp. 242-261.
2. Firooz A, Nassiri-Kashani M, Khatami A, et al. Fragrance contact allergy in Iran. J Eur Acad Dermatol Venereol 2010; 24: 1437-1441.
3. Marks JG, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatology 1998; 38(6 Pt 1): 911-918.
4. Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician 2010; 82: 249-255.
5. Woolery-Lloyd H, Valins W. Aluminum chloride hexahydrate in a salicylic acid gel: a novel topical agent for hyperhidrosis with decreased irritation. J Clin Aesthet Dermatol 2009; 2: 28-31.
6. Reider N, Fritsch PO. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Cerroni L (eds). Dermatology, 4th ed. Philadelphia, PA: Elsevier; 2018. pp. 228-241.
7. Wikramanayake TC, Borda LJ, Miteva M, Paus R. Seborrheic dermatitis – looking beyond Malassezia. Exp Dermatol 2019; 28: 991-1001.
8. Elewski BE, Hughey LC, Hunt KM, Hay RJ. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L (eds). Dermatology, 4th ed. Philadelphia, PA: Elsevier; 2018. pp. 1329-1363.
9. Metin A, Dilek N, Bilgili SG. Recurrent candidal intertrigo: challenges and solutions. Clin Cosmet Investig Dermatol 2018; 11: 175-185.
10. Metin A, Dilek N, Demirseven DD. Fungal infections of the folds (intertriginous areas). Clin Dermatol 2015; 33: 437-447.
11. Ramírez L, Moreno G, Arenas R, Gorzelewski A, Fernández R. Treatment of interdigital foot erythrasma with ozonated olive oil. J Am Acad Dermatol 2016; 74 (Suppl 1): AB155.
12. Sommer LL, Reboli AC, Heymann WR. Bacterial Diseases. In: Bolognia JL, Schaffer JV, Cerroni L (eds). In: Dermatology, 4th ed. Philadelphia; PA: Elsevier; 2018. pp. 1259-1295.
13. van de Kerkhof PCM, Nestlé FO. Psoriasis. In: Bolognia JL, Schaffer JV, Cerroni L (eds). Dermatology, 4th ed. Philadelphia, PA: Elsevier; 2018. pp. 138-160.
14. van de Kerkhof PCM, Murphy GM, Austad J, Ljungberg A, Cambazard F, Duvold LB. Psoriasis of the face and flexures. J Dermatolog Treat 2007; 18: 351-360.
15. Wang G, Li C, Gao T, Liu Y. Clinical analysis of 48 cases of inverse psoriasis: a hospital-based study. Eur J Dermatol 2005; 15: 176-178.
16. Kundakci N, Türsen Ü, Babiker MOA, Gürgey E. The evaluation of the sociodemographic and clinical features of Turkish psoriasis patients. Int J Dermatol 2002; 41: 220-224.
17. Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol 2011; 12: 143-146.
18. Merola JF, Li T, Li W-Q, Cho E, Qureshi AA. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol 2016; 41: 486-489.
19. Reynolds KA, Pithadia DJ, Lee EB, Wu JJ. Treatments for inverse psoriasis: a systematic review. J Dermatolog Treat 2020; 31: 786-793.
20. Kreuter A, Sommer A, Hyun J, et al. 1% pimecrolimus, 0.005% calcipotriol, and 0.1% betamethasone in the treatment of intertriginous psoriasis: a double-blind, randomized controlled study. Arch Dermatol 2006; 142: 1138-1143.
21. Lebwohl MG, Tan M-H, Meador SL, Singer G. Limited application of fluticasone propionate ointment, 0.005% in patients with psoriasis of the face and intertriginous areas. J Am Acad Dermatol 2001; 44: 77-82.
22. Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol 2004; 51: 731-738.
23. Freeman AK, Linowski GJ, Brady C, et al. Tacrolimus ointment for the treatment of psoriasis on the face and intertriginous areas. J Am Acad Dermatol 2003; 48: 564-568.
24. Ryan C, Menter A, Guenther L, et al. Efficacy and safety of ixekizumab in a randomized, double‐blinded, placebo‐controlled phase IIIb study of patients with moderate‐to‐severe genital psoriasis. Br J Dermatol 2018; 179: 844-852.
25. Jensen P, Skov L. Psoriasis and obesity. Dermatology 2016; 232: 633-639.
26. Mahil SK, McSweeney SM, Kloczko E, McGowan B, Barker JN, Smith CH. Does weight loss reduce the severity and incidence of psoriasis or psoriatic arthritis? A critically appraised topic. Br J Dermatol 2019; 181: 946-953.
27. Brook I. Secondary bacterial infections complicating skin lesions. J Med Microbiol 2002; 51: 808-812.
28. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018; 391: 541-551.


