New symmetrical hyperpigmented patches of skin
Test your diagnostic skills in our regular dermatology quiz. What is the cause of this skin hyperpigmentation?
Case presentation
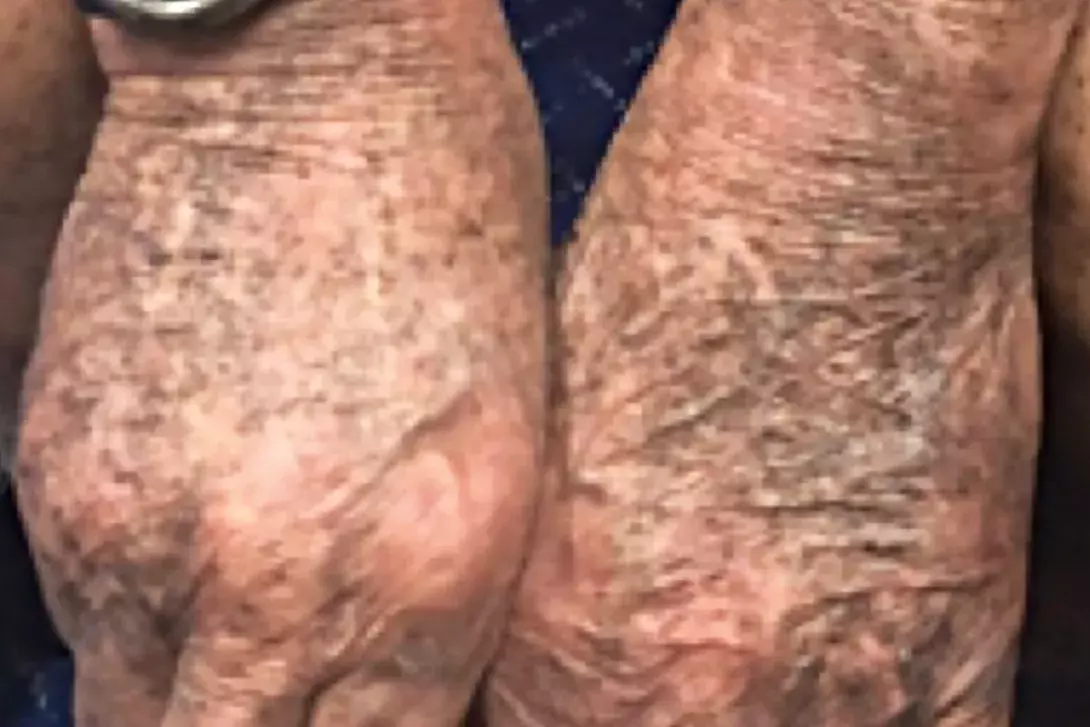
A 52-year-old man presents with hyperpigmented patches of skin on his upper and lower limbs that have been developing over the previous six months (Figures 1a to c). The hyperpigmentation is symmetrical and diffuse, with black-grey macules involving sun-exposed areas.
The patient’s other medical issues include hypertension and osteoarthritis, for which he has been taking amlodipine and slow-release paracetamol as required. He has been treated previously for rosacea. He does not have any allergies and works full-time in an office. The patient does not have a personal history or family history of melanoma, but he does report a family history of diabetes mellitus.
Differential diagnoses
Conditions to consider in a patient with symmetrical, diffuse hyperpigmented patches of skin who is otherwise well include the following.
- Drug-induced hyperpigmentation. The medications that cause hyperpigmentation are myriad, and include rifampicin, isoniazid, amiodarone, tetracycline (see below) and, rarely, amlodipine.1 Drug-induced hyperpigmentation is thought to account for up to 20% of cases of acquired idiopathic hyperpigmentation, and the latency between initiation of drug treatment and adverse hyperpigmentation can vary from hours to years.1 These reactions are generally associated with an unusual variegated appearance and colour – amiodarone-induced hyperpigmentation, for example, has a blue-grey hue.1
- Ochronosis. This rare disorder is characterised by blue-grey hyperpigmentation involving connective tissue.2,3 The endogenous form, a metabolic disorder known as alkaptonuria, is inherited in an autosomal recessive pattern and causes accumulation of homogentisic acid, which results from a lack of homogentisic acid oxidase.3 Accumulated homogentisic acid binds collagen, resulting in hyperpigmentation of the skin, conjunctivae and nails as well as calcification of the prostate and heart valves.3 Exogenous ochronosis, which is usually caused by drugs or heavy metals and generally asymptomatic, is staged according to severity: stage I (mild hyperpigmentation of the face and neck with associated erythema), stage II (more severe hyperpigmentation with papules) and stage III (papules and nodules with associated inflammatory change).2 The common culprits include hydroquinone, phenol, mercury and antimalarials, and it usually occurs after at least six months of drug exposure.2 Histological features correlate with clinical severity: early stage I lesions show yellow-brown and green ochronotic fibres, while advanced lesions show a florid mixed inflammatory infiltrate containing giant cells, plasma cells and histiocytes.2
- Endocrinopathy. Hyperpigmentation is a feature of many endocrinopathies, including Addison’s disease, thyrotoxicosis, Cushing’s syndrome, acromegaly and diabetes mellitus.4 Addison’s disease classically presents with diffuse hyperpigmentation, which occurs because of hypermelanosis secondary to ACTH-induced stimulation of melanocytes, and often precedes other features of systemic disease. Hyperpigmentation is generalised but accentuated in photoexposed areas (face, head, dorsum of hands), extensor surfaces prone to chronic pressure (elbows, knees), acral surfaces and the anogenital region.4 The palmar creases are often hyperpigmented. Causes of thyrotoxicosis include Grave’s disease, toxic multinodular goitre and thyroiditis, and can present with diffuse hyperpigmentation similar to that seen in Addison’s disease in up to 40% of cases.4
- Mercury toxicity. Toxicity from mercury is highly variable and correlates with the extent of exposure. It varies from an initial flu-like prodrome of myalgia, headache and chills, to respiratory, gastrointestinal and genitourinary dysfunction – neuropsychiatric features, with associated Parkinsonian-like symptoms, are associated with prolonged toxicity and can be permanent.5 There are many clinical variants of mercury exposure, such as acrodynia, tattoo reactions, mercury exanthema and oral lichenoid papules caused by dental amalgam. Mercury was historically used for skin bleaching yet known to worsen hyperpigmentation of affected areas, with associated slate-grey to light brown discolouration that is reversible on withdrawal of mercury-containing treatment.5 There may be hyperpigmentation of neck folds, nose and eyelids. Histopathology is diagnostic and shows coarse black-brown granular aggregates in the papillary dermis.5
- Minocycline-induced hyperpigmentation (MIH). This is the correct diagnosis. MIH is an adverse effect of minocycline therapy and is classified into three clinical types. Type I is characterised by localised, blue-grey macules that commonly involve scars.6 Type II presents as diffuse, well-demarcated blue-black hyperpigmentation involving normal skin of the distal lower and upper limbs. Type III appears as dark brown, diffuse symmetrical hyperpigmented macules predominantly affecting photoexposed areas.6,7 Type I MIH does not have a dose-dependent effect, whereas types II and III develop after a cumulative dose greater than 70 g.7 The pathogenesis underlying MIH is unknown but postulated to occur as a result of its immunogenic, lipophilic structure, which results in reactive metabolite and autoantibody formation in the dermis and subcutis.6 Biopsy of a type I lesion shows macrophages laden with black pigmented granules with sparse involvement of the dermis, whereas a type II lesion shows black pigment granules involving the dermis and macrophages in a perivascular and periadnexal pattern.6 Type III differs from types I and II because of increased melanosis, which is reflected in histological features showing hypermelanosis of the stratum basalis with an absence of dermal iron.6 Adjunctive immunohistochemical staining with Perls’ and Fontana-Masson stains, which are positive in the presence of iron and melanin, respectively, confirm the diagnosis. Perls’ and Fontana-Masson staining is positive in types I and II MIH, whereas type III MIH is positive for Fontana-Masson stain only.6
Management
MIH is a relatively common adverse effect of minocycline therapy, usually seen in patients who require extended periods of therapy for chronic conditions such as rosacea and acne. This patient had been treated previously for rosacea, for which he was started on minocycline 100 mg twice daily 18 months prior. He was then lost to follow up and continued taking the medication, with prescriptions provided by his general practitioner.
Cutaneous hyperpigmentation may be the most apparent tissue involvement, but other sites that are adversely affected by minocycline therapy require examination – these include the nails, thyroid, heart valves and the oral and ophthalmic mucosae.7 Types I and II MIH are the most common types and resolve on cessation of minocycline, although patients should be warned this may take months. Type III MIH is rare and may persist despite withdrawal of minocycline.
Outcome
In this patient, biopsy was performed and confirmed the diagnosis of type II MIH. Results of blood tests showed normal thyroid function, HbA1c, growth hormone and early morning cortisol levels. A serum test for mercury did not show a toxic level. He was agreeable to ceasing oral minocycline and to transitioning to topical metronidazole for his rosacea instead. His hyperpigmented lesions resolved within 24 months of stopping oral minocycline and his rosacea was controlled with topical therapy and intermittent short courses of doxycycline for acute flares. MT
