A bilateral scaly eruption on the palms and soles
Test your diagnostic skills in our regular dermatology quiz. What are these painful plaques on a woman’s hands and feet?
Case presentation
A 63-year-old woman presents with a one-year history of pruritic scaly plaques on her hands and feet that are causing her pain and difficulty walking. She has tried treatment with topical terbinafine 10 mg/g, without response.
The patient’s past medical history is significant for coeliac disease, which is managed with a gluten-free diet, and Hashimoto’s thyroiditis, which is treated with oral levothyroxine sodium 50 mcg daily. She is a current smoker (five cigarettes daily) and she drinks two standard drinks daily. She swims at the local pool three times weekly. Her family history is significant for chronic plaque psoriasis (father).
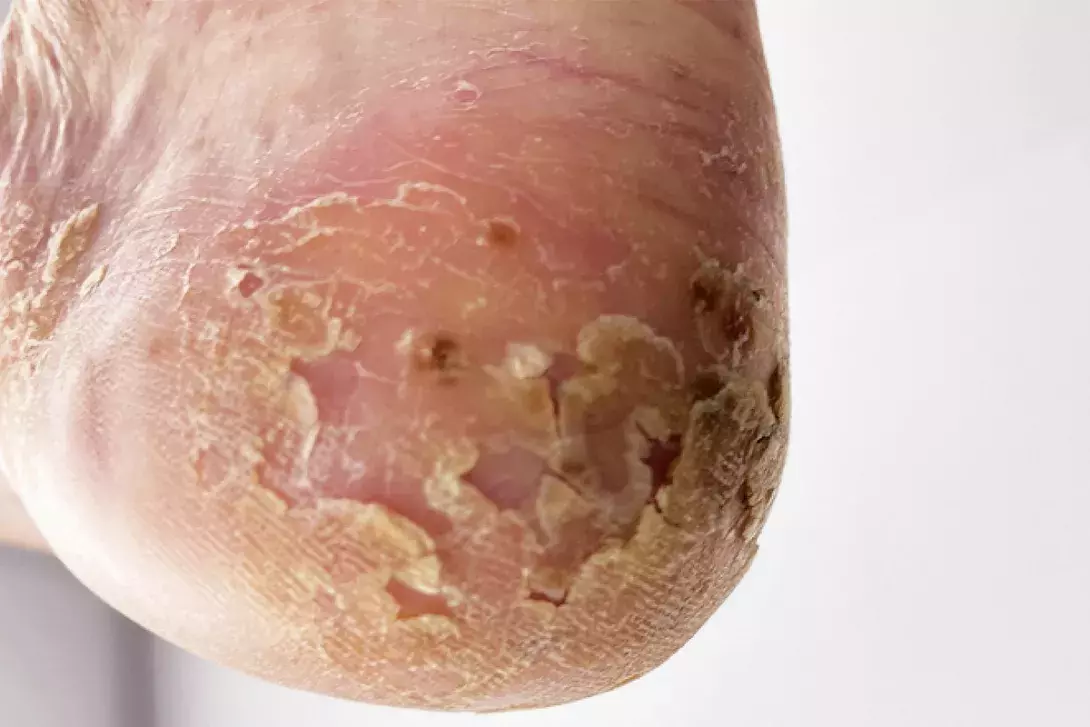
On examination, hyperkeratotic plaques studded with red-brown macules are observed on the patient’s palmar and plantar surfaces (Figures 1a and b).
Differential diagnoses
Conditions to consider in a patient presenting with a bilateral scaly eruption involving palmar and plantar surfaces include the following.
Atopic dermatitis
This inflammatory skin disease usually coincides with a history of atopy, which includes asthma, allergic rhinitis and keratoconjunctivitis. The cardinal symptom of atopic dermatitis (AD) is pruritus, which is accompanied by a primary eczematous eruption (e.g. erythema, papules, scaling) or secondary changes caused by itching (e.g. excoriations, lichenification).1 The typical distribution involves the flexural surfaces, such as the antecubital and popliteal fossae, and the flexural surface of the neck and ankles.2 AD is one of the strongest predictors for the development of chronic hand dermatitis.3
AD is one of the most common inflammatory diseases in the world, affecting up to 20% of children and up to 10% of adults.4 Disease onset is usually in infancy, between 2 and 6 months of age, but can occur at any age.1,4 Risk factors for AD include a family history of atopy and filaggrin mutations that lead to defects in the skin barrier.4
For the case patient described above, the scaly hyperkeratotic morphology of the lesions, presence of red-brown macules and absence of atopy, as well as the family history of psoriasis, do not support a diagnosis of AD.
Dermatophytosis
Dermatophytes cause superficial infections of the hair, skin and nails, which are most commonly due to Trichophyton, Epidermophyton or Microsporum species.5,6 Tinea pedis is a dermatophytic infection of the feet; tinea manuum is a dermatophytosis of the hands.
Tinea pedis presents with scaly plaques, or desquamation, often with associated pruritus. It can affect the interdigital spaces and/or lead to hyperkeratosis of the plantar foot, and may also affect the nails (onychomycosis). Occasionally, tinea pedis can be inflammatory and resemble pompholyx/dishidrotic eczema with vesiculobullae appearing on inflamed, erythematous plantar skin.6 Tinea manuum is usually unilateral, with scaly plaques on the palmar surface of the hand, and may have erythema, vesicles and/or pustules.6 A combination of tinea pedis and manuum where there is involvement of both feet and one hand (‘one hand-two feet’ syndrome) is more likely to be of the chronic moccasin-type tinea characterised by dry hyperkeratosis.5 The diagnosis of tinea is typically clinical, supported by skin scrapings taken from the active, scaly edge of the eruption sent for microscopy with potassium hydroxide stain and culture to isolate the causative dermatophyte.
Dermatophytoses are very common. At least 70% of the population worldwide will have a superficial infection due to a dermatophyte during their lifetime, resulting in a cost of at least $US500 million annually to treat.5 Tinea pedis is far more common than tinea manuum; both are most frequently caused by Trichophyton rubrum (>80%).7
For this case patient, the lack of response to topical terbinafine and the presence of red-brown macules make a diagnosis of tinea pedis and manuum less likely.
Allergic contact dermatitis
This delayed-type hypersensitivity reaction occurs when a chemical, to which an individual has previously been sensitised, comes in contact with the skin.8 The lesions of allergic contact dermatitis (ACD) are typically well-demarcated and localised to the allergen site of contact; however, ACD may present in a diffuse or even disseminated manner in the case of autosensitisation dermatitis.8 Reactions are typically very pruritic, and eczematous, and may have blistering, weeping and/or oedema in the acute setting or be lichenified and scaly in the chronic setting.8 In severe ACD reactions, the skin may feel tight or painful. The most common allergens are nickel, fragrances, p-phenylenediamine (in permanent hair dye) and preservatives.9 Furthermore, rubber accelerators (chemicals used to speed up the manufacturing process for rubber) are important causes of hand dermatitis from gloves and foot dermatitis from footwear made of rubber.8 The average individual is in contact with hundreds of chemicals daily and identifying the causative allergen(s) is crucial to resolution of this process.10 The gold standard diagnostic tool for ACD is epicutaneous patch testing.
ACD is common, but the true incidence is difficult to capture because individuals often self-diagnose or seek medical care through emergency or general practice clinics.10 In Europe, about 20% of the general population suffers from an allergy to at least one contact allergen.9
For this case patient, the presence of red-brown macules and lack of erythema, together with her smoking history, autoimmune comorbidities and family history of psoriasis, make other conditions more likely.
Palmoplantar pustulosis
This is the correct diagnosis. Palmoplantar pustulosis (PPP) is a chronic inflammatory disorder that results in recurrent sterile pustules involving the palmar and plantar surfaces.11 Known associations with PPP include chronic plaque psoriasis (25% of cases), coeliac disease, autoimmune thyroid disease, type 1 diabetes mellitus, recurrent bacterial upper respiratory tract infection (e.g. tonsillitis) and drugs (TNF-α inhibitors). In addition, PPP is associated with SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome.12,13 The pathogenesis of PPP is unknown but is proposed to arise from a pro-inflammatory milieu established by antecedent triggers that promote neutrophil chemotaxis, which include interleukin-8, interleukin-17, C5a and platelet-activating factor, setting the stage for development of the characteristic intraepidermal neutrophil aggregates (pustules).14
Clinical features of PPP include scaly plaques with associated erythema, pustules and brown-red macules that involve the palmar skin first, with plantar involvement usually following several months later.11 Severity can range from localised aggregates on the palms and soles to extensive coalescent plaques with fissuring that cause significant pain, limiting mobility and function.14 Occasionally, there may be extra-palmoplantar cutaneous involvement, characterised by thin scaly erythematous plaques and patches involving extensor surfaces of the limbs and the buttocks, and peripheral and axial arthritis that is radiologically and clinically distinct from psoriatic arthritis.11,14 PPP is a clinical diagnosis and skin biopsy findings vary, correlating to the stage of cutaneous disease; these range from intraepidermal vesicles to sterile intraepidermal pustules.14
PPP is a rare condition, with prevalence ranging between 0.01% and 0.05% in different countries, although it is more common in Japan where the prevalence is 0.12%.13,15 The peak onset of PPP occurs between 45 and 62 years of age, and it has a predominance in females of about 4.5:1.13
The morphological features seen in Figures 1a and b are characteristic of PPP. Our case patient exhibits multiple risk factors for PPP, which include cigarette smoking, and associated diseases (coeliac disease, Hashimoto’s thyroiditis), and she has a family history of psoriasis.
Management
PPP is a difficult condition to treat and can be recalcitrant to therapy. Topical and systemic agents used for chronic plaque psoriasis and other inflammatory skin disorders, including ciclosporin, methotrexate and TNF-α inhibitors, have been used with varying success in PPP, although this data stems from case reports and retrospective audits.14 A 2006 Cochrane review identified topical corticosteroids (under occlusion), PUVA (a combination treatment of psoralens and UVA radiation) and acitretin as the only treatments to show efficacy in the context of placebo- controlled trials.16 Despite the success of biologics in treating other conditions, such as chronic plaque psoriasis, the response in PPP is variable. An open-label case series showed unsuccessful treatment of PPP with ustekinumab in half of the patient cohort.17 Conversely, secukinumab and guselkumab (anti-interleukin-23 antibodies) have shown benefit in treatment of PPP in placebo-controlled studies, with the latter recently approved for PPP in Japan.18
Surgical modalities may also have a role in recalcitrant PPP, as tonsillectomy was associated with resolution of refractory PPP from a retrospective cohort study of 138 Japanese adults.19 The patients in this study were referred for tonsillectomy by dermatologists, irrespective of whether they had a history of recurrent bacterial upper respiratory tract infection (e.g. tonsillitis associated with PPP) or not.19 Given the chronic, recurrent nature of PPP, management of modifiable disease factors such as smoking is critical.12
Outcome
The case patient was treated with topical corticosteroids (under occlusion) and oral acitretin 20 mg daily. She was also advised about the importance of smoking cessation as part of the management of her condition, which continues to improve. MT
