What’s the diagnosis?
A rapidly growing nodule on the scalp
Case presentation
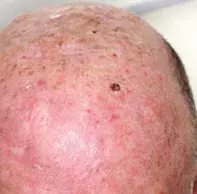
An 80-year-old man presents with a four-week history of a rapidly growing nodule on his scalp (Figures 1a and b). He has noticed occasional bleeding from the lesion. The lesion is not itchy or painful. He denies unintentional weight loss, night sweats or anorexia.
On examination, a violaceous, keratotic, indurated nodule with some ulceration is noted on the left mid-scalp. There is adjacent actinic damage on the surrounding skin, indicating significant sun exposure.
The patient has a previous history of skin cancer, including multiple squamous and basal cell carcinomas of the head and neck region. There is no palpable cervical lymphadenopathy.
Differential diagnoses
Conditions to consider among the differential diagnoses for a rapidly growing nodule in a patient with risk factors for cutaneous malignancy include the following.
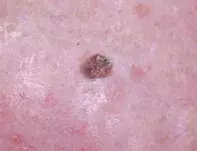
- Squamous cell carcinoma (SCC). SCC is the second most common nonmelanoma skin cancer (NMSC) and a worrisome diagnosis because of metastatic potential, particularly in older patients with head and neck lesions. A pink-red, indurated nodule on the dorsum of the hand, consistent with cutaneous SCC, is shown in Figure 2. Risk factors for SCC are shared with other cutaneous malignancies and include sun exposure, advanced age and immunosuppression. SCC commonly occurs in the seventh decade of life although may occur earlier – for instance, in the context of solid organ transplant recipients, patients with chronic lymphocytic leukaemia and patients taking immunosuppressant medication.1 Exposure to certain chemicals (e.g. arsenic, tar, alkylating agents) and ionising radiation can predispose to the development of cutaneous SCC. Human papilloma virus exposure is associated with periungual, oral and anogenital SCC.1 Dermoscopic assessment of SCC reveals atypical vascular patterns, which include dotted, glomerular, hairpin or serpentine vessels. Skin biopsy shows eosinophilic aggregates of glassy keratinocytes with cellular pleomorphism, intercellular bridging and keratin pearls.1
- Keratoacanthoma (KA). These skin neoplasms are characterised by a clinical pattern of rapid proliferation, plateau and then regression.2 Risk factors for KA are shared with SCC; however, there are additional risk factors that are unique for KA, including presence of foreign bodies and drugs that modulate the cell cycle (e.g. BRAF and Hedgehog pathway inhibitors).2 KA usually presents as a solitary, rapidly growing, hyperkeratotic plaque that can reach up to 2 cm in diameter before regressing; however, there is a variant, KA centrifugum, of which lesions can reach up to 20 cm in diameter.2 KAs usually occur on cutaneous, sun-exposed sites but they can involve mucosal surfaces.2 Dermoscopic features of KA overlap with SCC, typically showing circumferential white halos encircling a central crater with adjacent dot vessels. Many biopsy features are shared between KA and SCC, but a specimen showing ground-glass keratinocytes with a clearly demarcated border between tumour and stroma and absence of ulceration or cellular pleomorphism would favour a diagnosis of KA.2
- Pleomorphic spindle cell tumour (PSCT). Previously known as ‘atypical fibroxanthoma’, PSCT is an uncommon tumour originating from fibroblast- and/or myofibroblast-like cells. This tumour has metastatic potential.3 Risk factors include age (peak onset is in the seventh and eighth decades of life), male sex, immunosuppression, and exposure to ultraviolet and/or x-ray radiation.3 PSCT usually presents on photoexposed sites of the head and neck in older individuals but may involve the trunk or extremities in younger individuals. The lesions appear as pale-pink to red, solitary, indurated papules and nodules that are not painful or itchy but can ulcerate and bleed.3 Due to the nonspecific macroscopic features, biopsy is essential to confirm the diagnosis. Histopathological examination shows dermal aggregates of spindle, epithelioid and/or giant cells with cellular pleomorphism and overlying epidermal atrophy.3
- Amelanotic/hypomelanotic melanoma (AHM). This subtype of malignant melanoma, which is characterised by little to no pigment and therefore presents as an erythematous nodule, comprises less than 10% of all melanomas.4 The clinical appearance of AHM is variable and includes pale to pink, erythematous papules, macules or patches with asymmetrical, irregular borders arising on sun-exposed skin. AHM can present as a fungating, exophytic lesion that may resemble haemangioma. Dermoscopic features that are indicative of, but not exclusive to, AHM include milky-red areas, as well as dotted and linear vessels.4 Biopsy is the gold standard for diagnosis of melanoma, and AHM are characterised by pagetoid spread of atypical melanocytes in aggregated nests within a hyperplastic dermis.4
- Merkel cell carcinoma (MCC). This is the correct diagnosis. MCC is a rare, aggressive tumour of neuroectodermal origin.5 Risk factors for MCC include age (usually occurring after seventh decade of life), ultraviolet light exposure, a history of other skin cancers and immunosuppression. The Merkel cell polyomavirus (MCPyV) has been postulated to play a role in supporting oncogenesis, although this differs between geographical locations. MCPyV-dependent MCC comprises 80% of cases in the US, while only 20% of cases of MCC in Australia are due to MCPyV.6,7 MCC usually presents as a pink to violaceous papule or nodule, commonly affecting the head, neck and extremities.6 The ulceration and scale seen in the case patient described above are not typical features. MCC may be rapidly growing, as in this case, or have an initial slow-growth phase followed by a rapid-growth phase. Early spread is common and 30% of patients will have metastatic disease on diagnosis.6 Biopsy is diagnostic and shows dermal and/or subcuticular aggregates of blue dysplastic cells with granular ‘salt and pepper’ chromatin and a high nuclear-to-cytoplasm ratio.5
Management
Initial management of a patient with suspected skin cancer should include: (i) evaluation for evidence of locoregional or distal metastatic spread, and (ii) management of the primary lesion. A full skin examination with comprehensive review of lymph nodes should be performed to determine evidence of lymphadenopathy, as up to one-third of patients with MCC have lymph node involvement or disease on sentinel lymph node biopsy.6 Treatment of MCC is complex and requires a multidisciplinary approach, with medical oncology, radiation oncology and surgical oncology involvement to determine the optimal treatment. MCC is exquisitely sensitive to radiation and occurs in elderly individuals in whom surgical fitness may be an issue, and recent reports have shown the promise of urgent wide-field radiation therapy as initial management of these lesions.8 Chemotherapy agents such as cyclophosphamide and doxorubicin have been used for metastatic MCC but do not confer any additional clinical survival benefit.6 Recently identified immunotherapies have shown promise for metastatic MCC, such as pembrolizumab (anti-programmed death [PD-1] monoclonal antibody) and avelumab (anti-programmed death ligand 1 [PD-L1] monoclonal antibody).6 Up to 90% of MCC recurrence occurs in the first two years, so patients with this diagnosis require intense monitoring with three-monthly skin checks for up to three years after treatment.6
Outcome
In this patient, a biopsy returned the unexpected diagnosis of MCC, and he was urgently referred to a multidisciplinary unit specialising in MCC management. He underwent urgent wide-field radiation therapy and decided to forego surgical excision due to perioperative risk. He continues to attend his dermatologist’s clinic for regular three-monthly skin checks, with no clinically apparent disease 18 months since treatment.
References
1. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 2018; 78: 237-247.
2. Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol 2016; 74: 1220-1233.
3. Iorizzo LJ 3rd, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg 2011; 37: 146-157.
4. Pizzichetta MA, Talamini R, Stanganelli I, et al. Amelanotic/hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol 2004; 150: 1117-1124.
5. Pulitzer MP, Amin BD, Busam KJ. Merkel cell carcinoma: review. Adv Anat Pathol 2009; 16: 135-144.
6. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol 2018; 78: 445-454.
7. Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol 2009; 129: 246-248.
8. Nghiem P, Park SY. Less toxic, more effective treatment – a win-win for patients with Merkel cell carcinoma. JAMA Dermatol 2019; 155: 1223-1224.
Skin cancer

