What’s the diagnosis?
Painful vesicles following vaccination
Case presentation
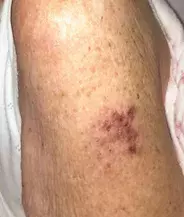
A 90-year-old woman is admitted to hospital with decompensated congestive cardiac failure and community acquired pneumonia requiring intravenous antibiotics and diuresis. During her admission, she develops a painful vesicular eruption on her arm where, 18 days previously, she was administered the live-attenuated varicella zoster virus (VZV) vaccine (Figure 1a). Aside from her age and a recent 10-day course of prednisone for podagra, she has no other cause for immunosuppression.
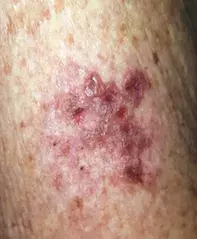
On examination, grouped vesicles surrounding the vaccination site are observed on the left deltoid (Figure 1b). She has no oral or genital lesions, and there are no vesicles or bullae elsewhere on her body.
Differential diagnoses
Conditions to consider among the differential diagnoses in a patient with an acute onset of painful vesicles following vaccination include the following.
- Injection site reaction. Injection site reactions are the second most commonly observed adverse event following immunisation (AEFI), and were responsible for 22% of AEFIs reported to the Vaccine Adverse Event Reporting System in the USA between 1991 and 2001.1 Despite this, no standard definition exists for an injection site reaction. The term is used to describe physiological or morphological changes at the site of immunisation, and include both symptoms (pain, pruritus) and signs (erythema, warmth, swelling, ecchymosis) that develop immediately after or in the days following the administration of a vaccine. Injection site reactions are a diagnosis of exclusion – any morphological changes specific to other diagnoses, such as urticarial plaques indicative of a IgE-mediated hypersensitivity reaction or inflammatory bullae indicative in bullous impetigo, are not considered part of an injection site reaction. In this patient’s case, the cutaneous changes did not develop immediately after her VZV vaccination but more than two weeks later, making an injection site reaction less likely.
- Herpes simplex infection. The herpes simplex virus (HSV) is one of the most common viruses responsible for mucocutaneous disease. Worldwide prevalence of seropositivity to HSV is estimated to be between 45 and 98%; risk factors for HSV infection include increasing age, female gender, African-American ethnicity, chronic skin diseases (atopic dermatitis, psoriasis, benign familial pemphigus), and increasing number of sexual partners.2 Transmission of HSV is primarily by direct inoculation from an individual with active or subclinical HSV infection, but respiratory droplets and mucosal secretions can also transmit the virus. Primary HSV infections are usually more severe than recurrent infections, causing primary gingivostomatitis, herpes simplex labialis or genital HSV infection. Any HSV type can cause infection; however, the majority of orofacial HSV infections are caused by HSV1 whereas HSV2 is more likely to cause genital disease.2 Clinical features of HSV infection include prodromal pain and burning sensation, which are followed by painful grouped vesicles on an erythematous base and pustules that erode (cutaneous surfaces) and ulcerate (mucosal surfaces). Patients with HSV infections involving periorbital and ocular surfaces need ophthalmic assessment because of the risk of keratoconjunctivitis, which can lead to permanent visual impairment.
- Localised bullous pemphigoid (BP). Localised BP describes variants of the supepidermal autoimmune blistering disease that are characterised by pruritic, tense bullae arising in various anatomical sites, including the vulva, umbilicus and pretibial skin.3 Localised BP can also arise at the distal end of an amputated limb (‘stump pemphigoid’), sites of joint arthroplasty and radiotherapy.3,4 Biopsy is diagnostic, with histopathological examination demonstrating a subepidermal blister, and direct immunofluorescence revealing IgG and C3 linearly deposited along the dermoepidermal junction.3
- Herpes zoster. This is the correct diagnosis. Herpes zoster (shingles) is a cutaneous viral infection caused by reactivation of VZV. It is primarily a disease of adults, with incidence that increases with age (5.09 cases per 1000 persons per year for adults aged between 51 and 79 years; 10.1 cases per 1000 persons per year for those aged 80 years and over).5 Risk factors include immunosuppression, such as concomitant HIV infection, receipt of a transplant, treatment with immunosuppressive medications and malignancy requiring treatment with radio- and/or chemotherapy.5 Herpes zoster classically presents with a prodromal burning pain and sometimes systemic symptoms, which precede a dermatomal eruption by 24 to 72 hours. The cutaneous lesions evolve from an erythematous patch or plaque that develops into vesicles and/or pustules before crusting, sometimes leaving scars. Constitutional symptoms are common and include fever, malaise and regional lymphadenopathy. The most common complication of herpes zoster is postherpetic neuralgia, which occurs in 10 to 50% of patients and can persist for months after the infection has resolved.5
Diagnosis and management
Herpes zoster is a clinical diagnosis and is confirmed by taking a skin swab from the base of an eroded vesicle, or fluid from a ruptured vesicle, for polymerase chain reaction (PCR) testing and confirmation of VZV DNA. For patients with suspicion of or risk factors for disseminated zoster (defined as 20 or more vesicles outside the primary dermatomal site of infection), further assessment for visceral VZV infection is warranted – this may include full blood counts; electrolytes, urea and creatinine levels; liver function tests; chest x-ray and a lumbar puncture if clinically indicated.
Herpes zoster following live-attenuated VZV vaccination as seen in our patient is a rare event, constituting only six of 23,092 zoster reports to Vaccine Adverse Event Reporting System between 2006 and 2015.6 The timing of onset of herpes zoster after live-attenuated VZV vaccination is important because development of characteristic vesiculopustular lesions within 14 days of vaccination is likely to be due to wild-type VZV whereas the development of lesions between 15 and 42 days of vaccination is more likely to be due to vaccine-specific VZV (Oka strain).7 Prompt diagnosis and initiation of antiviral treatment are essential, particularly in patients who are immunosuppressed because disseminated herpes zoster infection can result in fatal VZV pneumonitis, hepatitis or encephalitis and the risk of mortality is high (about 40%).5,8
Outcome
For this patient, a skin swab was taken from an eroded vesicle and VZV DNA was demonstrated on PCR testing, which excluded a diagnosis of an injection site reaction to the vaccine. Genotyping confirmed the vaccine-specific Oka strain of the VZV virus as the causative agent. Chest x-rays were arranged, which did not show any signs of VZV pneumonitis. The patient was commenced on intravenous aciclovir at a dose of 10 mg/kg eight-hourly and then stepped down to oral valaciclovir 1000 mg three times daily until her herpetic lesions resolved.
References
1. Gidudu J, Kohl KS, Halperin S, et al. A local reaction at or near injection site: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2008; 26: 6800-6813.
2. Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol 2007; 57: 737-763.
3. Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol 2017; 18: 513-528.
4. Truss A, Papalexandris S, Gardner S, Harvey R. Localised bullous pemphigoid overlying knee arthroplasty: a diagnostic challenge. BMJ Case Rep 2019; 12(4): e227440.
5. McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol 1999; 41: 1-14.
6. Miller ER, Lewis P, Shimabukuro TT, et al. Post-licensure safety surveillance of zoster vaccine live (Zostavax®) in the United States, Vaccine Adverse Event Reporting System (VAERS), 2006-2015. Hum Vaccin Immunother 2018; 14: 1963-1969.
7. Harada K, Heaton H, Chen J, Vazquez M, Meyer J. Zoster vaccine-associated primary varicella infection in an immunocompetent host. BMJ Case Rep 2017; 2017: bcr2017221166.
8. Alexander KE, Tong PL, Macartney K, Beresford R, Sheppeard V, Gupta M. Live zoster vaccination in an immunocompromised patient leading to death secondary to disseminated varicella zoster virus infection. Vaccine 2018; 36: 3890-3893.
Immunisation

