
Could antivirals reduce your risk of long COVID? Where the research is up to on prevention and treatment
Evidence is continuing to accumulate on the burden and frequency of chronic effects after a COVID infection, which fall under the umbrella term “long COVID”.
At least 5%–10% of people who contract COVID experience long COVID. This can include symptoms (for example, fatigue, brain fog and breathlessness) or conditions (for example, heart conditions, neurological conditions and diabetes) after the initial infection that may be persisting, new or relapsing.
Studies show the symptoms and increased risk of chronic conditions can persist for up to two years after infection. The individual impact of long COVID can range from temporary to severely disabling, and the societal cost – for example due to reduced workforce and increased health-care costs – is enormous
The lower risk of developing long COVID with up-to-date COVID vaccinations is substantially offset by the high levels of infections and re-infections globally. As a result, the cumulative burden of long COVID has increased, including in lower and middle income countries. A conservative estimate suggests 65 million people may be currently affected globally.
So where are we at with reducing the risk of, and treating, long COVID?
Could antivirals reduce the risk of long COVID?
COVID antiviral drugs, taken orally, continue to play an important role in reducing acute severe disease after infection. In Australia, they’re available to those at highest risk from COVID.
Observational research has suggested taking antivirals during a COVID infection can reduce the risk of long COVID in people with at least one risk factor for acute severe COVID.
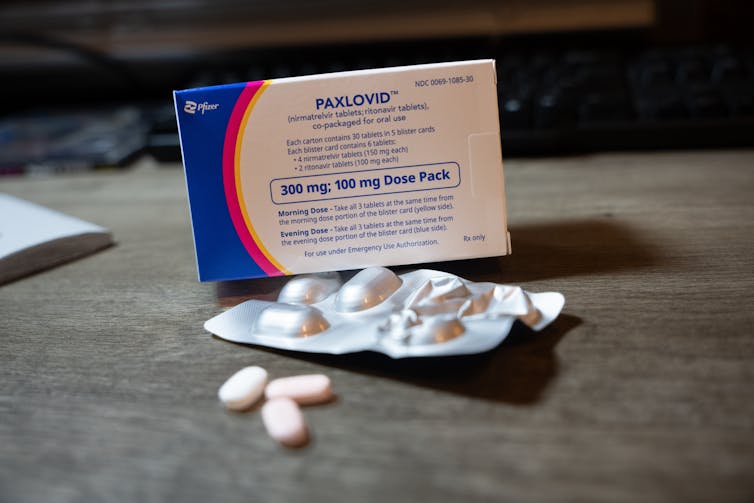
In one study, nirmatrelvir and ritonavir, known as Paxlovid, was associated with a 26% reduced risk of developing long COVID. It was also linked to a 47% reduced risk of death and a 24% reduced risk of hospitalisation after the acute infection phase.
A similar 14% reduction in long COVID risk has been reported for molnupiravir (Lagevrio).
Ensitrelvir – a COVID antiviral available in Japan – could also reduce the risk of long COVID, preliminary analyses suggest.
More research is needed, but this data indicates antiviral medications may be a key approach to lessening the risk of long COVID.
The population most at risk of long COVID (often working-age adults) differs from those most at risk of severe disease from a COVID infection (older adults or those with chronic medical conditions). Eligibility criteria to access antivirals do not currently include consideration of long COVID.
Meanwhile, one randomised trial found metformin, a commonly prescribed diabetes medication, could also reduce long COVID risk. The study offered people with symptomatic COVID who were overweight or obese metformin for two weeks (beginning within a week of symptoms starting). This group was 41% less likely to develop long COVID compared with a placebo group that didn’t take metformin.
The way this works might involve an effect on the powerhouses of our cells, mitochondria, or directly on the virus. Whatever the precise mechanism, further research should be priortised to fast-track this potential.
We’re understanding more about long COVID
There are no effective or approved treatments for long COVID at present. Currently about 12 clinical trials are testing potential drugs. A number of candidate treatments exist for certain components of long COVID that may be useful in subgroups of patients.
However, recently we’ve seen major advances in understanding what’s actually driving long COVID in the body. This knowledge opens up approaches for both diagnosis and treatments or interventions.
Research on treatments is lacking
An Australian parliamentary inquiry into long COVID stressed the best way to avoid the condition is to lower the risk of getting infected with COVID in the first place (through protective behaviours such as vaccination, mask wearing and cleaner indoor air).
While these are all important measures, we would benefit from having more tools at our disposal to prevent and treat long COVID. After all, COVID is still evolving rapidly and vast numbers of people are likely to be reinfected in the months and years ahead.
Overall, the quantity and speed of clinical trials into long COVID treatments has been insufficient. And most public health policy approaches are focused on preventing severe disease from a COVID infection, rather than the long-term effects.
That said, Australia recently announced an initial A$22 million of funding and a plan for research into long COVID through the Medical Research Future Fund.
In July 2023, the White House established the Office of Long COVID Research and Practice which will coordinate the US government’s response to long COVID, as well as two randomised trials of Paxlovid.
What now?
Given what we now know about long COVID, and the additional concern of what we don’t know (for example, could organ damage reveal itself many years down the track?), we desperately need diagnostic tools, clinical care pathways coupled with health worker training, and treatments to prevent and cure long COVID.
Unaddressed, long COVID may well lead to a new and substantial health and societal burden for many years to come. The response must involve prioritisation of research, such as that which led to the fast development of COVID vaccines and antivirals.
While there are some positive signs in the policy and research space, we need to see stronger recognition of long COVID and a greater sense of urgency around finding solutions.
Disclosure statement
Suman Majumdar, through the Burnet Institute receives grant funding from the Australian Governemnt via the National Health & Medical Research Council of Australia, the Medical Research Future Fund and DFAT's Centre for Health Security.
Brendan Crabb and the Institute he leads receives research grant funding from the National Health & Medical Research Council of Australia, the Medical Research Future Fund, DFAT's Centre for Health Security and other Australian federal and Victorian State Government bodies. He is the Chair of The Australian Global Health Alliance and the Pacific Friends of Global Health, both in an honorary capacity. And he serves on the Board of the Telethon Kids Institute, on advisory committees of mRNA Victoria, the Sanger Institute (UK), the Institute for Health Transformation (at Deakin University), The Brain Cancer Centre (Australia), the WHO Malaria Vaccine Advisory Committee; MALVAC, and is a member of OzSAGE and The John Snow Project, all honorary positions.
Michelle Scoullar received funding from the RACP Foundation in 2018 and 2020 as part of her PhD. She is the lead paediatrician with Clinic Nineteen, a clinic specialised in the care and treatment of people with long COVID.
Ziyad Al-Aly consulted for Pfizer (uncompensated) and Tonix pharmaceuticals. He receives funding from the US government.
Emma Pakula does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
This article is republished from The Conversation under a Creative Commons license. Read the original article, published on 5 December 2023.
Opinions expressed in Something Borrowed are those of the original authors and do not necessarily reflect those of the Editors, the Editorial Board or the Publisher of Medicine Today.
![]()


