Familial hypercholesterolaemia – enhancing the care of patients and families
Familial hypercholesterolaemia (FH) is a common genetic disorder of LDL-cholesterol, that, if untreated, leads to premature atherosclerotic cardiovascular disease (ASCVD). With early diagnosis and initiation of cholesterol-lowering treatment, the risk of ASCVD can be substantially reduced. Yet, many people with FH remain undiagnosed and undertreated. GPs can play an important role in the care of patients and families with FH.
- Familial hypercholesterolaemia (FH) is a common genetic disorder of the LDL receptor pathway, with the heterozygous form affecting one in 250 individuals.
- A diagnosis of FH should be suspected in people with an elevated LDL-cholesterol (LDL-C) level (>5 mmol/L), especially if there is a personal or family history of premature coronary artery disease.
- The Dutch Lipid Clinic Network Criteria can be used to make a phenotypic diagnosis of FH; however, it should not be used in children.
- Genetic testing plays a role in confirming the diagnosis (MBS item 73352), atherosclerotic cardiovascular disease risk stratification and cascade testing (MBS item 73353).
- Because FH is an autosomal dominant condition, cascade testing is important to identify undiagnosed relatives of index cases.
- High-intensity statins remain the cornerstone of therapy; however, the addition of ezetimibe and monoclonal antibodies targeting PCSK9 may be required to attain LDL-C goals.
- A multidisciplinary approach and integration of services are required to implement optimal care of patients and families with FH.
Familial hypercholesterolaemia (FH) is an autosomal dominant and highly penetrant genetic disorder affecting the LDL receptor pathway. FH is characterised by lifelong elevated plasma LDL-cholesterol (LDL-C) levels due to impaired hepatic clearance, and by an increased risk of atherosclerotic cardiovascular disease (ASCVD), particularly coronary artery disease.1 Heterozygous FH is one of the most common monogenic conditions, with an estimated prevalence of one in 250 people worldwide.2 Some ethnic groups may have a higher frequency of FH due to gene founder effects, including the Afrikaans, Christian Lebanese and French Canadian populations. In Australia, there are about 100,000 people living with FH.3 Compared with the general population, the risk of coronary artery disease may be over 10-fold higher in patients with heterozygous FH.4,5 Homozygous or compound heterozygous FH has an estimated prevalence of one in 160,000 to 300,000 people and manifests as very elevated levels of LDL-C (typically >10 mmol/L), with severe ASCVD and aortic valve sclerosis often present by the late teenage years, or earlier if untreated.6
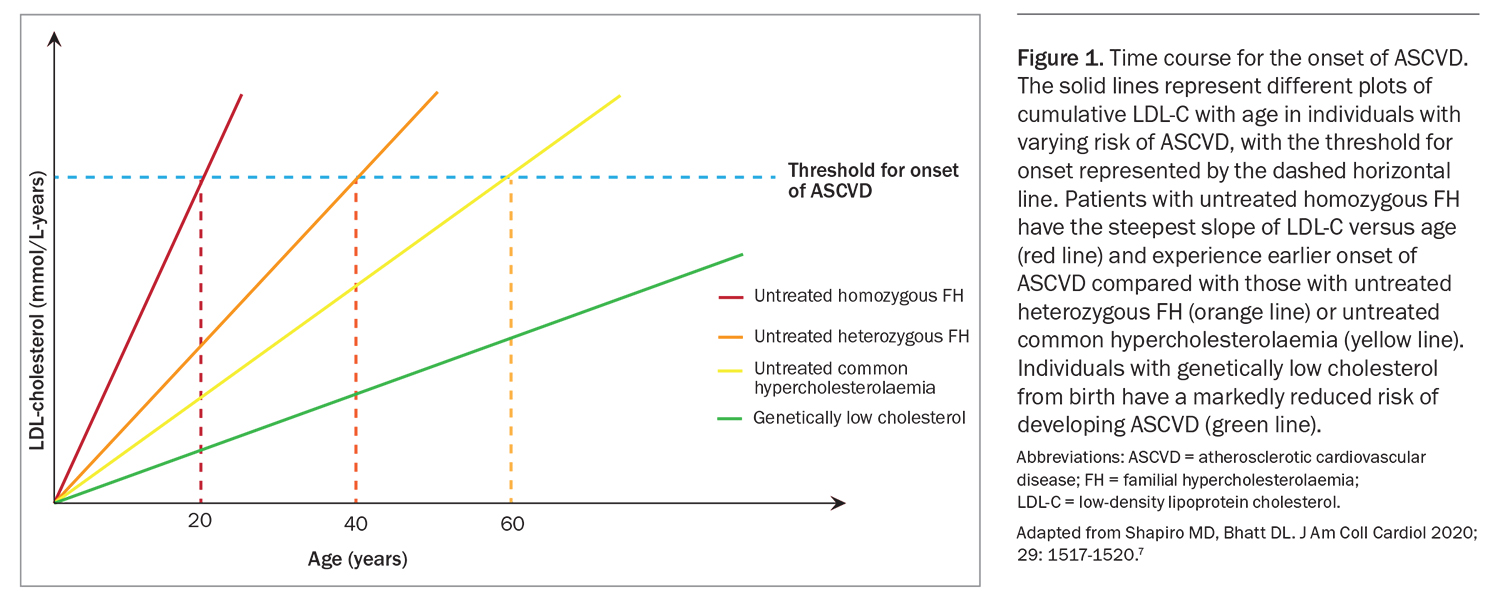
The major driver of ASCVD risk in people with FH is the cumulative exposure to elevated plasma LDL-C levels from birth (Figure 1).7 Early initiation of lifestyle measures and medications to reduce LDL-C levels in people with FH can effectively reduce the risk of ASCVD.8 However, most people with FH remain undiagnosed and undertreated, representing a missed opportunity for ASCVD prevention and a major public health problem.9 GPs request more than 90% of LDL-C measurements and 88% of Australians present to GPs annually.10,11 A survey of Australian GPs showed that most considered they were the most effective health practitioners for managing FH.12 GPs are ideally placed to enhance the care of patients and families with FH.11 The purpose of this article is to provide a contemporary overview of the diagnosis and management of FH.
Screening
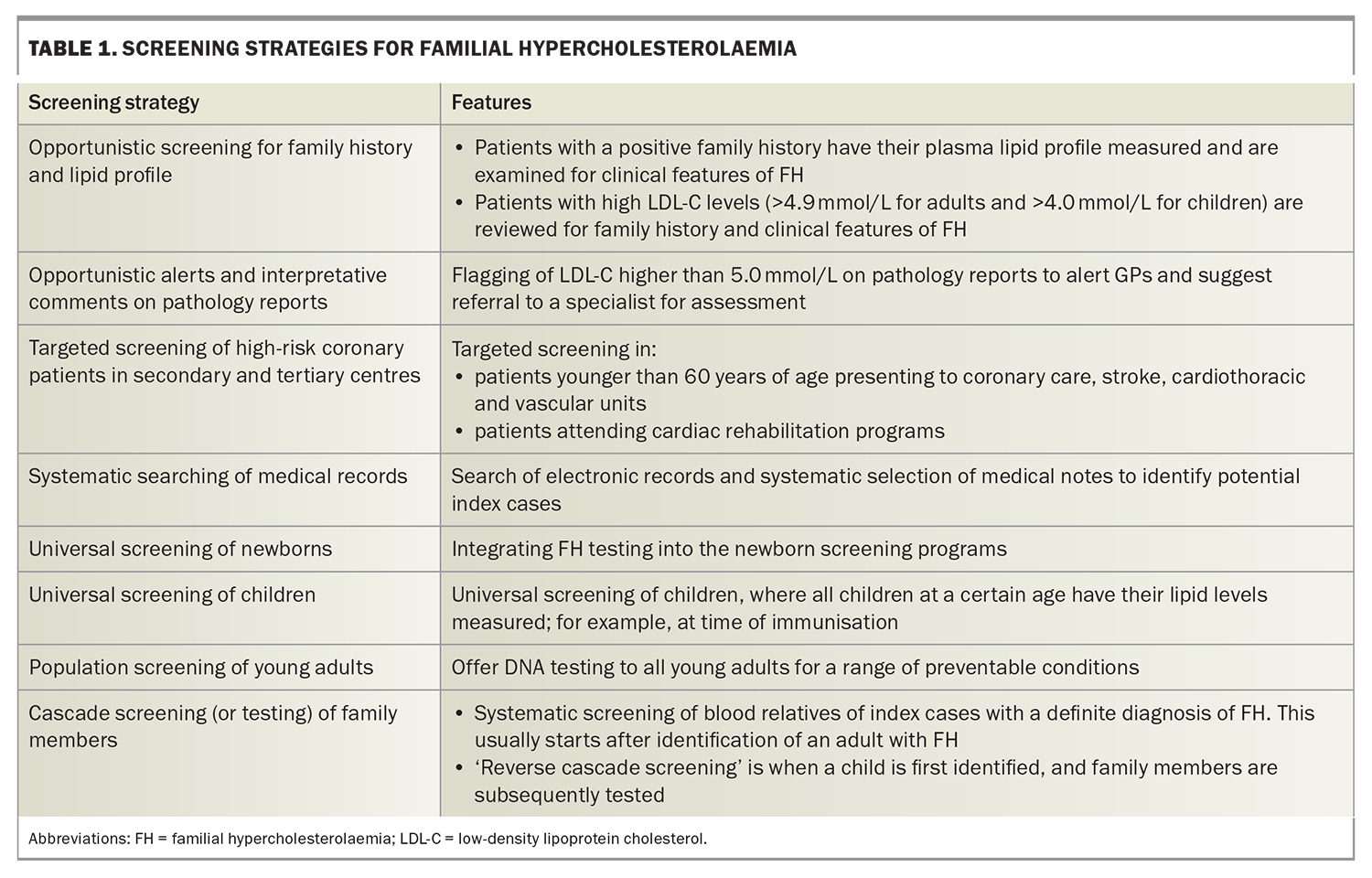
FH meets all criteria for screening for a condition. Several strategies for universal, targeted, systematic and opportunistic screening have been proposed to detect probands (i.e. the first person diagnosed with FH in a kindred).13 Examples of FH screening strategies (Table 1) include universal screening of children at the time of immunisations coupled with ‘reverse’ cascade testing of parents if the child is found to have the condition, targeted screening of patients with premature ASCVD in coronary care units or rehabilitation programs, application of electronic tools to systematically search GP patient databases, and interpretative comments and alerts on lipid profile results issued by pathology laboratories.13,14
Opportunistic screening of adults, such as during a health check, should be employed in primary care to detect FH, based on an LDL-C level above 5 mmol/L.3 Owing to practical advantages, nonfasting samples can be considered for FH screening but should be used with caution in patients with hypertriglyceridaemia (triglyceride level >4.5 mmol/L), who should have fasting samples tested.3 It has also been advocated that FH be considered part of the newborn bloodspot screening program in Australia; however, such a strategy needs further development.15 Recommended ages for screening children for FH are as early as possible (no later than 2 years) for suspected homozygotes and from the age of 5 years (no later than 10 years) for suspected heterozygotes.
Providing educational resources and national programs to raise awareness among the public and health professionals about the health impacts of high cholesterol, and FH specifically, are also important.16 The FH Australasia Network provides online resources on FH (https://www.athero.org.au/fh/).
Phenotypic diagnosis
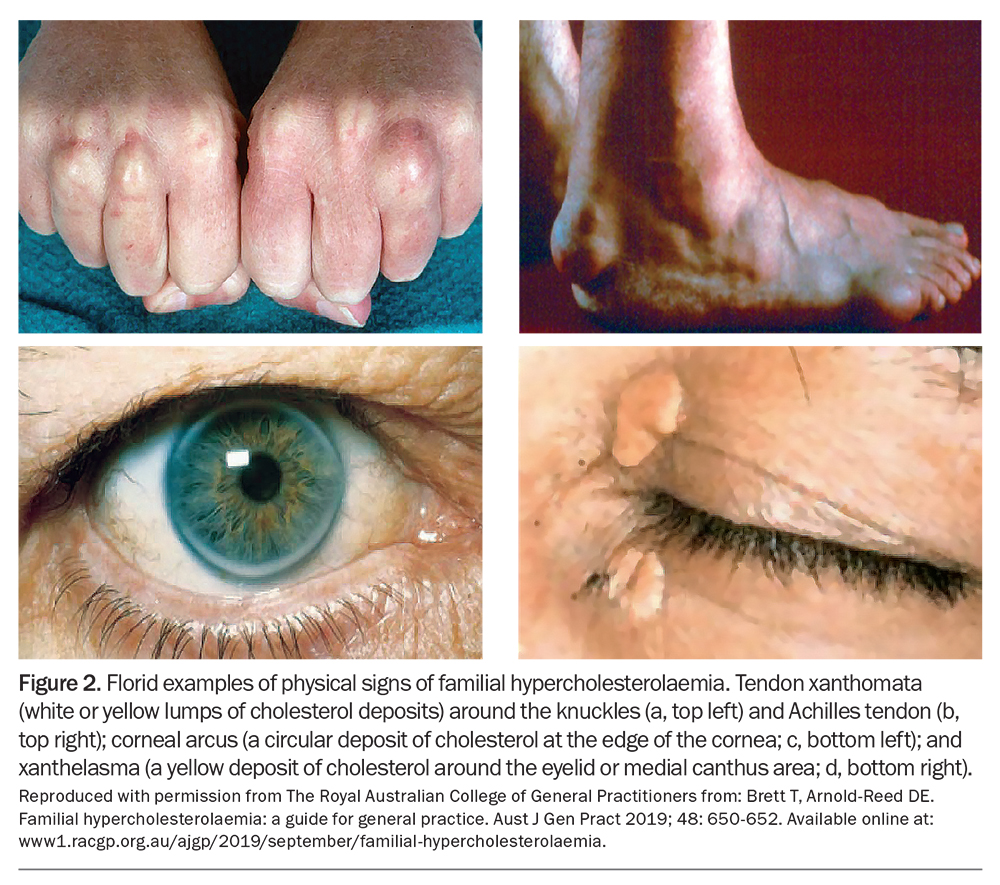
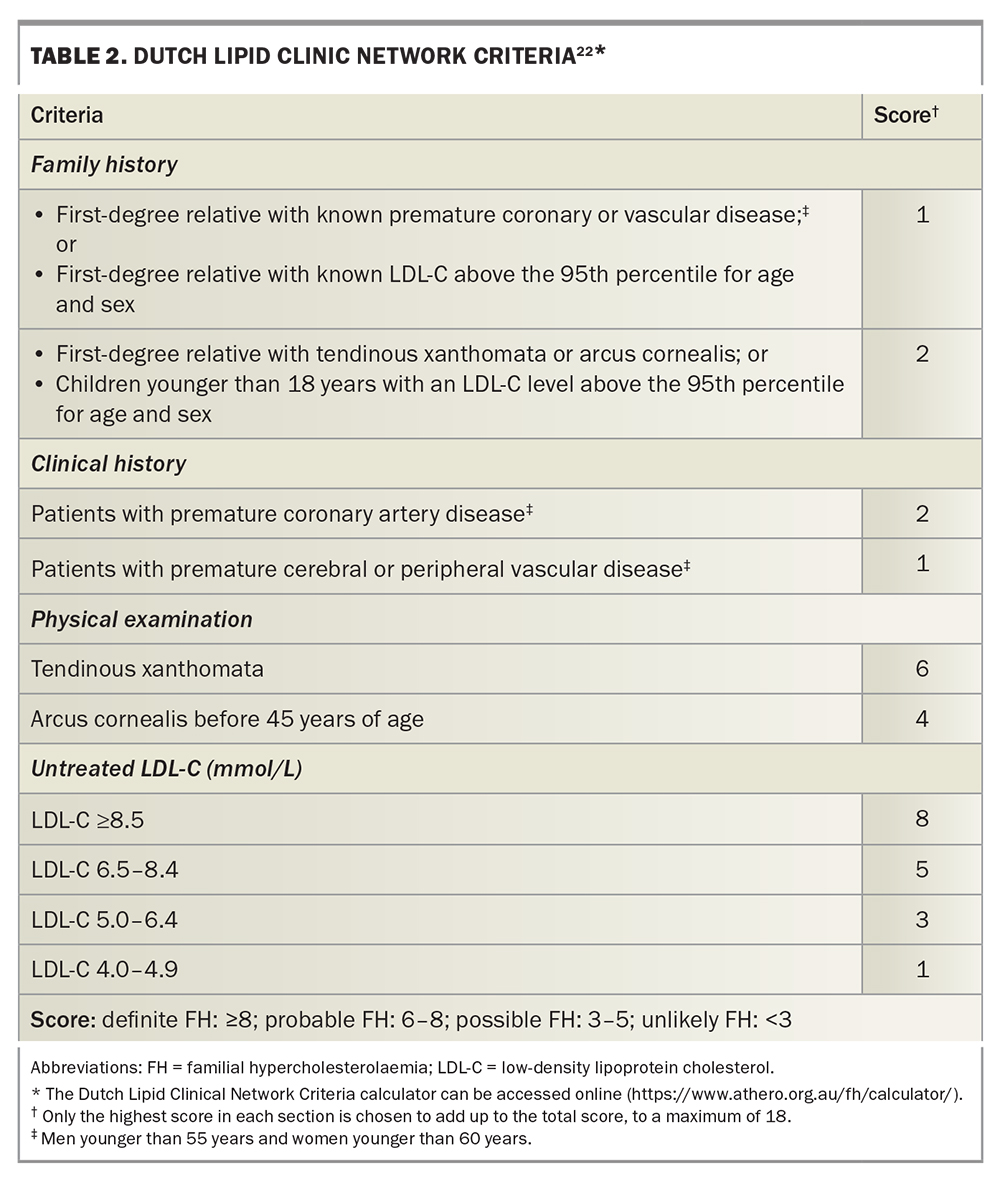
FH can be diagnosed using criteria combining personal and family history of hypercholesterolaemia and premature ASCVD, LDL-C levels and physical signs of cholesterol deposition, such as tendon xanthoma, arcus cornealis and xanthelasma (Figure 2).17-20 Physical stigmata can be subtle and are less prevalent due to statin therapy. Thus, their absence does not exclude the diagnosis of FH.21 Although several diagnostic tools are available, the Dutch Lipid Clinic Network Criteria score (DLCNC) (Table 2) is the preferred tool in Australia for adults.3,22 The DLCNC tool assigns a score to each diagnostic criterion and the total score is used to categorise the likelihood of FH as ‘definite’, ‘probable’, ‘possible’ or ‘unlikely’. In patients taking a statin or ezetimibe, the pretreatment LDL-C level can be estimated using a correction factor.23
In children, the clinical diagnosis of FH relies on LDL-C levels and family history. Testing for suspected heterozygous FH using a phenotypic and/or a genotypic strategy should be considered in children between the ages of 5 and 10 years.3 The DLCNC tool is not suitable for children.21 A probable diagnosis of FH should be considered in children with an LDL-C level higher than:21,24
- 5.0 mmol/L in the absence of parental history of hypercholesterolaemia or premature ASCVD
- 4.0 mmol/L with parental history of hypercholesterolaemia or premature ASCVD
- 3.5 mmol/L with a parent with a pathogenic or likely pathogenic gene variant.
If FH is suspected, a fasting plasma or serum lipid profile should be performed, ideally on two occasions, in both adults and children, and secondary causes of hypercholesterolaemia, such as hypothyroidism, nephrotic syndrome, cholestatic liver disease and medication use (e.g. steroids, isoretinoids), excluded.3 After a diagnosis is made, patients with FH should be referred to, or discussed with, a specialist with expertise in lipidology.3
Genetic testing
Genetic testing is the gold standard for diagnosing FH and should be offered when available.3 However, the absence of a causative variant does not exclude the diagnosis, especially when the phenotypic expression is strong.25 Pathogenic gene variants are detected in 60 to 80% of those with ‘definite’ FH and in 20 to 40% with ‘probable’ FH.25 Variants in the LDL receptor (LDLR), apolipoprotein B (APOB) and proprotein convertase subtilisin/kexin type 9 (PCSK9) genes contribute to more than 95% of genetically confirmed cases. There is also a very rare form of autosomal recessive FH due to variants in the LDL receptor adaptor protein 1 (LDLRAP1) gene.26,27 Patients with phenotypic FH, but without a detectable FH-causing variant, may have an unidentified genetic variant or polygenic hypercholesterolaemia.25 Genetic testing remains underutilised, despite its value in:25,28
- increasing the specificity of diagnosis
- enabling more efficient genetic counselling
- improving the accuracy of risk stratification
- improving adherence to treatment
- enabling access to special therapies.
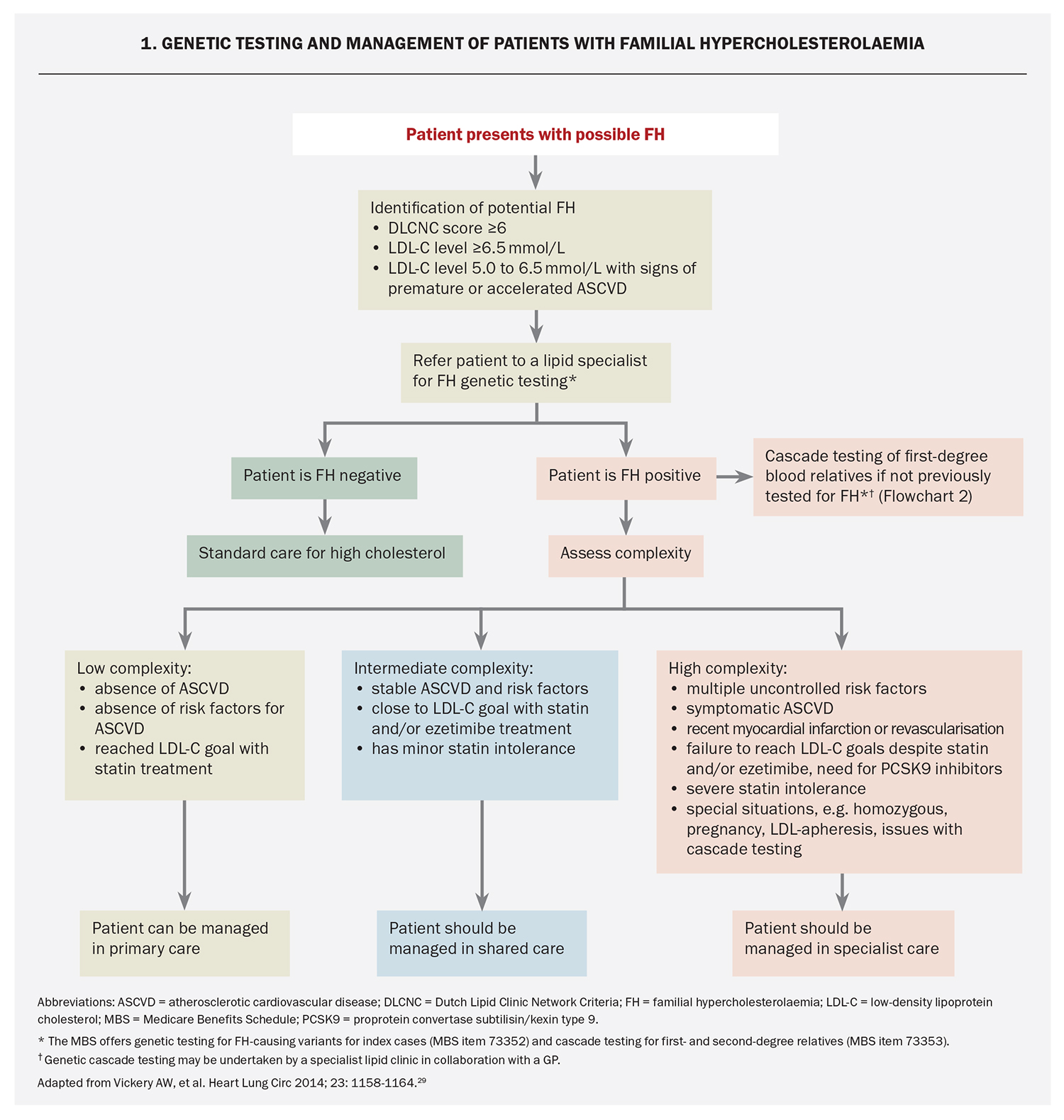
Genetic testing should ideally be offered to adult index cases with a definite or probable diagnosis of FH by the DLCNC and be performed using accredited methods in certified laboratories.3 The MBS has introduced new pathology services to facilitate genetic testing for FH-causing variants in index cases (MBS item 73352), which requires non-GP specialist authorisation.11,29
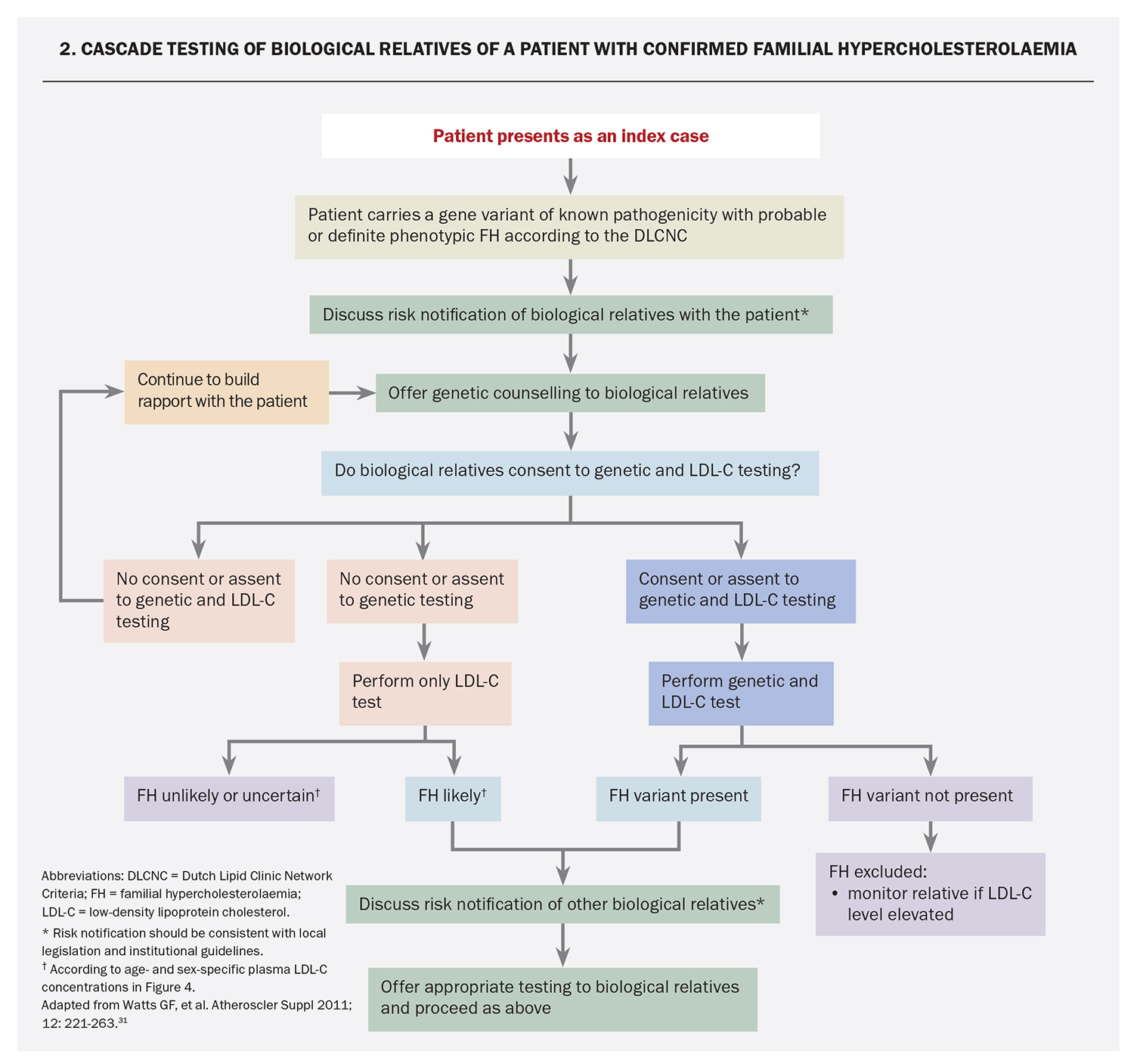
One of the major benefits of genetic testing for FH is to enable cascade testing of at-risk family members. If an index case has a pathogenic FH-causing variant, cascade genetic testing of first- or second-degree relatives should be offered, and GPs can request this (MBS item 73353).11 Pre- and post-test counselling is an integral part of the process for FH genetic testing and should be delivered by a healthcare professional with skills in genetic counselling.25 Information for GPs on genetic counselling is available through the WA HealthPathways (https://wa.communityhealthpathways.org/981755.htm). A shared-care approach between GP and non-GP specialists can facilitate genetic testing of index cases and cascade testing.29 An algorithm for genetic testing and management of patients with FH is presented in Flowchart 1.
Cascade testing
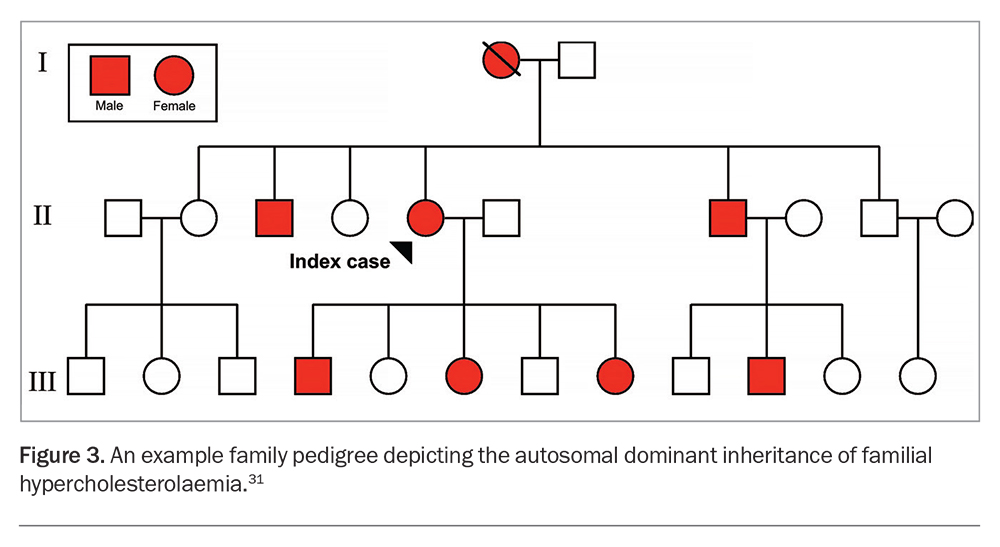
Cascade testing is the systematic testing of blood relatives of an index case with the condition.30 Since FH is a monogenic autosomal dominant genetic disorder with high penetrance, 50% of first-degree relatives of index cases will have the condition (Figure 3).14,25,31 After FH is diagnosed in an index case, cascade testing of first- and second-degree relatives should be offered using phenotypic (i.e. LDL-C levels) and genotypic (MBS item 73353) approaches where feasible. An approach to cascade testing of biological relatives of a patient with confirmed FH is summarised in Flowchart 2.3,31 Genetic testing should be offered to diagnose FH in children after a pathogenic or likely pathogenic gene variant has been identified in a parent or first-degree relative.3,21 Cascade genetic testing also identifies relatives who do not inherit the genetic variant, which gives reassurance that they do not have FH.25
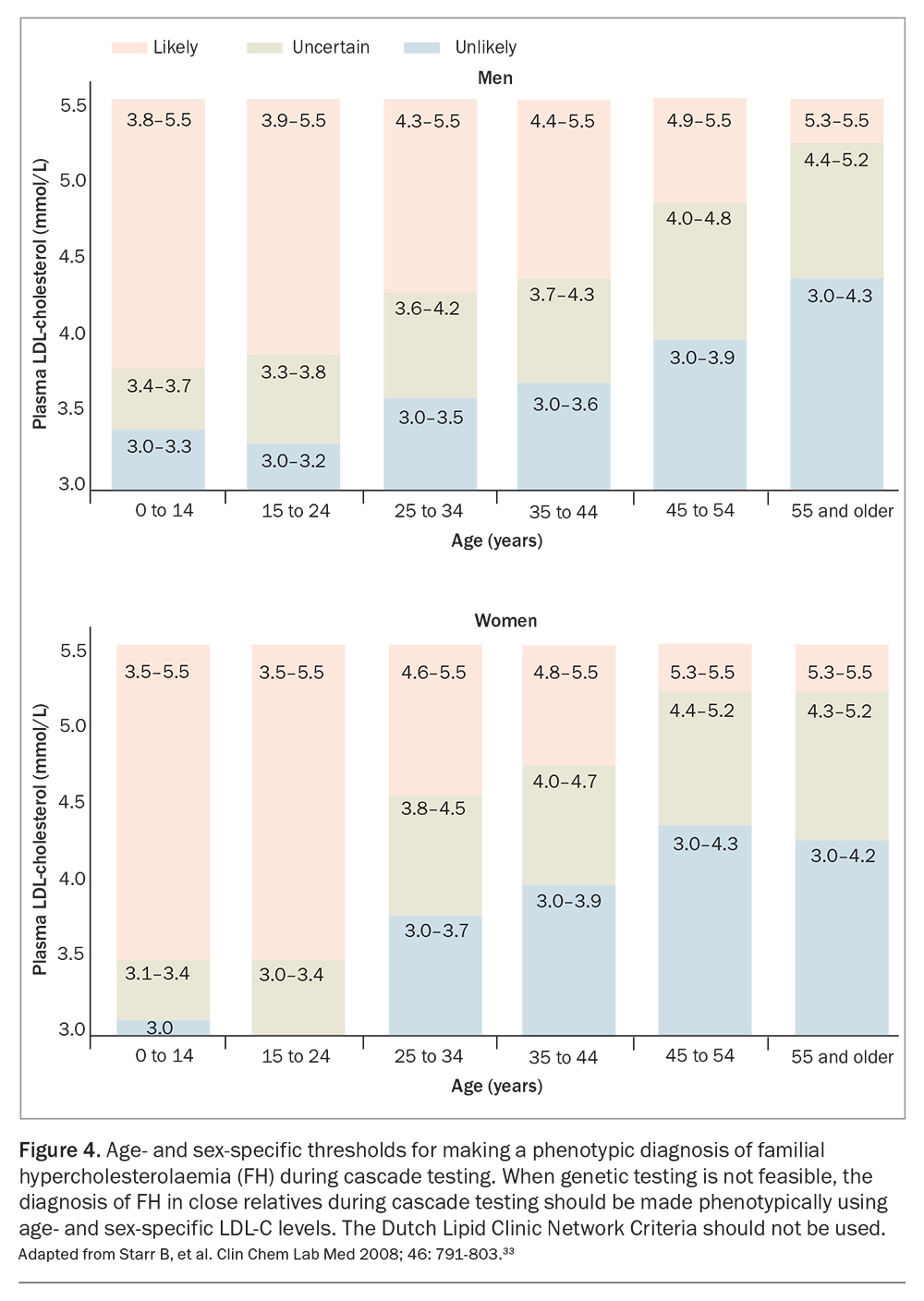
Cascade screening programs that use variant-specific genetic testing have been shown to be feasible and cost-effective.32 However, cascade testing should ideally be co-ordinated by a well-resourced centre that can also enable testing via GPs.3 Risk notification of family members should be performed according to local legislation and institutional guidelines.3 As with genetic testing of index cases, pre- and post-test counselling for genetic cascade screening should be delivered by a healthcare professional with skills in genetic counselling.25 In the absence of genetic testing, cascade testing of relatives of index cases may be carried out using measurement of fasting LDL-C, with age-and gender-specific levels (Figure 4); the DLCNC should not be used to make the diagnosis of FH in relatives.3,25,33
ASCVD risk stratification
After FH is diagnosed, a comprehensive ASCVD risk assessment should be conducted to develop a personalised treatment plan and guide management.34 Traditional ASCVD risk factors remain significant predictors of ASCVD in patients with FH, highlighting the importance of a multifactorial approach to risk reduction.35,36 In women, female-specific ASCVD risk factors, such as hypertension or toxaemia in pregnancy, gestational diabetes and premature menopause, should be considered. Tendon xanthomata reflect cumulative exposure to elevated plasma LDL-C levels and are associated with higher ASCVD risk.37 Furthermore, lipoprotein(a) [Lp(a)] is a genetically determined LDL-like particle bound to apolipoprotein(a) that is a causal risk factor for ASCVD and calcific aortic valve stenosis.38 In patients with FH, an elevated Lp(a) level is common and is a potent risk factor for coronary artery disease; it may also mimic the FH phenotype diagnostically, especially when markedly elevated or if coexisting with common hypercholesterolaemia.38,39 Measurement of Lp(a) should be considered as part of routine evaluation, noting that an MBS item is not yet available and that out of pocket costs may be incurred. However, Lp(a) measurement is a one-off test, as Lp(a) levels are considered stable throughout a person’s lifetime.40 Genetic testing provides information on ASCVD risk, as the presence of a pathogenic gene variant is associated with a higher cumulative exposure to LDL-C and is predictive of ASCVD.41
Primary prevention ASCVD risk calculators for the general population (such as the Australian CVD Check or the Framingham Risk Score) should not be used in patients with FH, as lifelong exposure to elevated LDL-C levels are not considered, thus risk will be underestimated.42 FH-specific equations for absolute ASCVD risk have been developed for people with genetically confirmed FH. For instance, the Spanish Familial Hypercholesterolemia Cohort Study risk equation (SAFEHEART-RE) takes into account age, sex, previous ASCVD, hypertension, obesity, smoking, LDL-C levels and Lp(a) levels; however, requires further validation in an Australian population.43
Noninvasive imaging for atherosclerosis, such as carotid ultrasonography, coronary artery calcium scoring and CT coronary angiography, can facilitate personalised risk assessment; it enables the identification of patients with atherosclerotic disease who may require more intensive treatment, including referral for further cardiac evaluation.42 Patients with symptoms of ASCVD should be referred to a cardiologist for further investigation and management.
Lifestyle modifications and management of non-cholesterol risk factors
Modifications targeting a heart-healthy diet, smoking cessation, regular exercise, weight loss, moderation in alcohol consumption and mitigation of psychological stress are emphasised by all guidelines for the prevention of ASCVD and are recommended for all patients with FH.3 Hypertension, diabetes and obesity should be managed according to relevant guidelines. The recommended cardioprotective diet for patients with FH is one that is low in saturated fat and cholesterol, coupled with the preference for unsaturated fat, particularly within the context of the Mediterranean-style or DASH-style (Dietary Approaches to Stop Hypertension) dietary patterns.44 Referral to a dietitian for specialised advice is recommended.3,45 However, a heart-healthy diet alone does not usually provide adequate reduction of LDL-C levels in patients with FH. Nutraceutical regimens, including plant sterols, red yeast rice, soluble fibre and berberine, may be useful adjunctive therapies in certain patients.3,46,47
Pharmacotherapy for lowering LDL-cholesterol
Based on extensive clinical trial, registry and genetic data, cholesterol lowering with statins remains the mainstay therapy for reducing the cumulative burden to elevated LDL-C levels and to prevent ASCVD.48
Adults with heterozygous FH
In adults with heterozygous FH, high-potency statins (such as high-dose atorvastatin or rosuvastatin) should be initiated as soon as possible after the diagnosis, with shared decision making to improve adherence. According to the FH Australasia Network guidelines, the LDL-C goal for adults with FH is a greater than 50% reduction in LDL-C levels and either an LDL-C level below:3
- 2.5 mmol/L in the absence of ASCVD or other major ASCVD risk factors
- 1.8 mmol/L if there is imaging evidence of ASCVD or if other major ASCVD risk factors are present
- 1.4 mmol/L if there is clinical ASCVD.
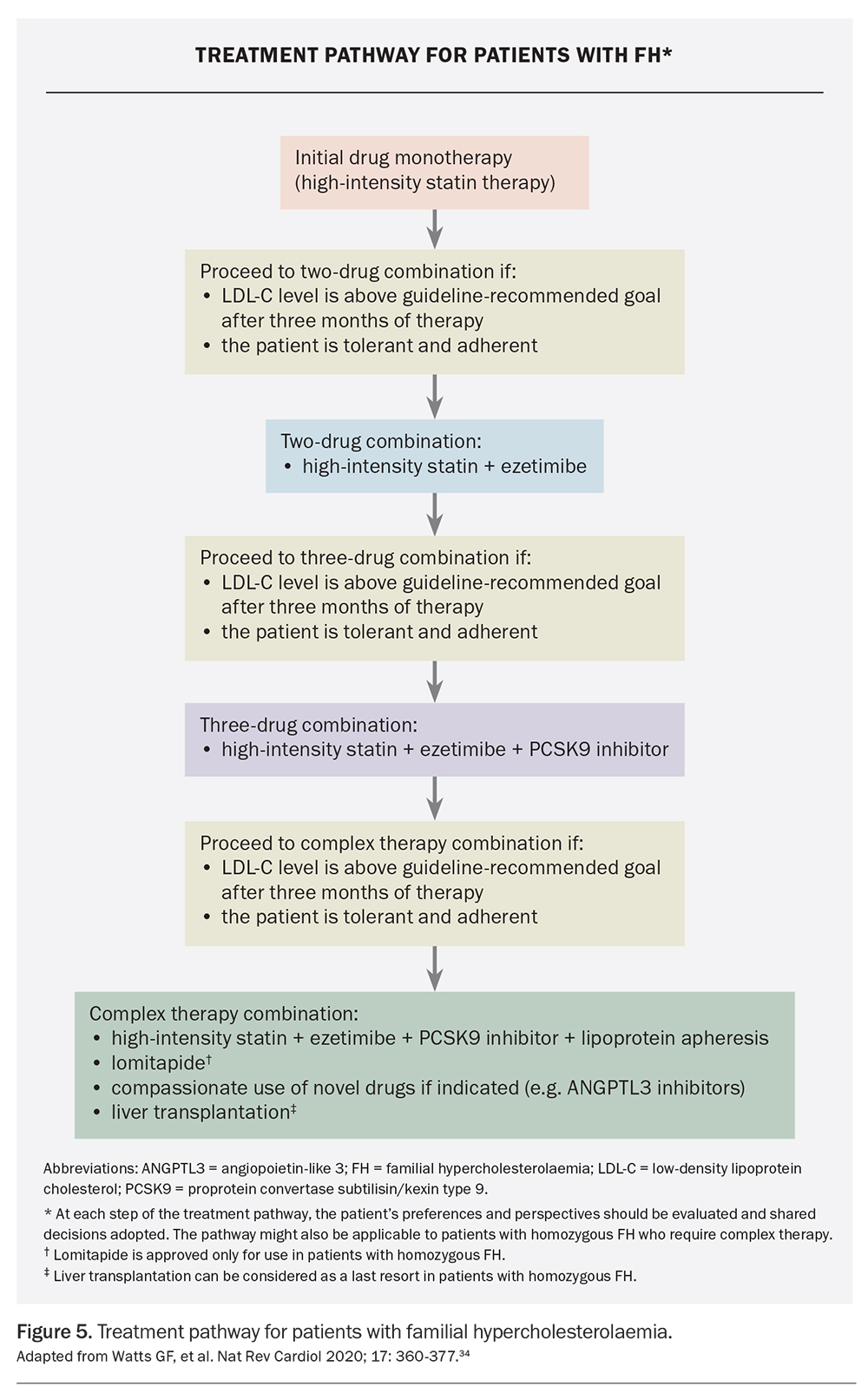
However, statins alone are often insufficient to attain LDL-C goals in most patients and sequential addition of other therapies is needed (Figure 5).34,49 The addition of ezetimibe (a cholesterol absorption inhibitor) can further reduce LDL-C levels by up to 20%. It is recommended as add-on therapy, or as monotherapy in those who are statin-intolerant, with good safety and efficacy and low cost.50,52 Bile acid sequestrants modestly reduce LDL-C levels but are seldom used, owing to gastrointestinal adverse effects.52 Bempedoic acid (an adenosine triphosphate citrate lyase inhibitor) is well tolerated and can be considered in patients who are statin-intolerant; however, it is not yet available in Australia.53 Statin intolerance is an important issue and should be managed according to established guidelines.54
Therapies targeting PCSK9 (a protein that binds to the LDL receptor resulting in degradation of the receptor) are also available.55 Inhibition of PCSK9 leads to upregulation of LDL receptor activity, thereby lowering plasma LDL-C levels. The PCSK9 inhibitors alirocumab and evolocumab are fully human monoclonal antibodies that are usually administered subcutaneously every two weeks. They lower LDL-C levels by 50 to 60% and reduce CVD events when added to statin therapy.56,57 These agents are safe and efficacious in patients with FH, with injection site reactions being the main adverse effect.58-62 Accordingly, these agents are recommended as third-line therapy after statins and ezetimibe.3 Monoclonal antibodies targeting PCSK9 are reimbursed on the PBS for patients with FH and can be initiated by GPs in consultation with a specialist.3
Pregnancy and FH
Women of child bearing age should be offered individualised prepregnancy counselling and contraception advice, as statins are associated with risk of teratogenesis.3 Safe and reliable forms of contraception should be recommended during statin treatment. Statins and other systemically absorbed cholesterol-lowering medications should be ceased three months prior to planned conception and during pregnancy and breastfeeding.3 However, prolonged periods off cholesterol-lowering treatment due to conception planning, pregnancy and breastfeeding may increase ASCVD risk.63 In pregnant women with FH, the severity of hypercholesterolaemia, presence of ASCVD and risks and benefits of cholesterol-lowering therapies need to be considered.64 Bile acid sequestrants are safe to use in pregnancy but can affect absorption of fat-soluble vitamins, such as vitamin K.64 Additionally, lipoprotein apheresis can be performed safely during pregnancy, and may be required for patients with homozygous FH or severe heterozygous FH, depending on ASCVD risk.65 A multidisciplinary approach involving experienced lipidologists, obstetricians and cardiologists should be followed.3
Children with heterozygous FH
In children with FH, modest and sustained reductions in LDL-C levels have a major impact in preventing ASCVD.21,24 Statins licensed for this age group (pravastatin, fluvastatin and simvastatin) should be initiated after age 8 to 10 years.3 An LDL-C goal below 3.5 mmol/L or a 50% reduction in LDL-C levels can be considered for children older than 10 years with FH.3,21 Studies have shown that statins are safe and efficacious in children with FH, with 20-year observational follow-up data showing a reduction in ASCVD events and death from ASCVD.66,67 Ezetimibe and monoclonal antibodies targeting PCSK9 are approved for patients older than 10 years with FH, with recent studies showing their safety and efficacy.68-71 Weight, growth, physical and sexual development, and wellbeing should be monitored in children receiving cholesterol-lowering therapies.3,21 Children with FH should preferably be reviewed by a paediatrician with expertise in lipidology.3,21 A multidisciplinary approach founded on shared decision making with the parents and an all-inclusive approach should be undertaken, addressing barriers and enablers to treatment. Transition clinics from childhood to adulthood are recommended, and should be planned well in advance.3,21
Children and adults with homozygous FH
Patients with homozygous FH should be referred to specialist centres as management is substantially more difficult.3 In children and adults with homozygous FH, statins should be initiated as soon as possible after the diagnosis.3 Ezetimibe and monoclonal antibodies targeting PCSK9 can be used; however, response to treatment may be reduced in patients carrying LDLR-negative variants who have minimal or no residual LDL-receptor function.62 Lipoprotein apheresis may therefore be required to attain adequate reduction in LDL-C levels and has been performed safely in children.65 Apheresis is performed weekly or every two weeks, with adequate vascular access and appropriately trained staff being essential.65 Barriers include lack of availability, high cost, expertise required, patient inconvenience and adverse effects. Other therapies for patients with homozygous FH include lomitapide (a microsomal triglyceride transfer protein inhibitor) and evinacumab (a monoclonal antibody targeting angiopoietin-like 3 protein); both work independently of LDL-C receptor function but require specialist consultation as they are obtained via special access or compassionate use schemes.72,73 Liver transplantation may be considered as a very last resort for patients with homozygous FH; however, it has rarely been performed in Australia.74
On the horizon
Inclisiran is a double-stranded small interfering ribonucleic acid therapy that inhibits hepatic synthesis of PCSK9. Inclisiran is administered subcutaneously every six months and can reduce LDL-C levels by 40 to 50% in patients with heterozygous FH, with the main adverse effects being injection site reactions and nasopharyngitis.75,76 Bempedoic acid may also play a role in the management of FH, as it has been shown to lower LDL-C levels by about 20% and reduce ASCVD events in patients with statin intolerance and ASCVD or ASCVD risk factors.53 Several novel cholesterol-lowering agents are also in clinical development.77
Conclusion
FH is a common genetic disorder that GPs will encounter in their practice. GPs can play a central role in the continuity of care of patients with FH, spanning screening, diagnosis, cascade testing, ASCVD risk stratification and lipid management. Earlier diagnosis and initiation of effective cholesterol-lowering treatments is required to reduce the burden of ASCVD in patients and their families with FH. Advances in cholesterol-lowering therapies have provided strategies that allow more patients with FH to approach recommended treatment goals. Effective implementation of FH care requires an integrated health system approach, with shared care between GPs and other specialities. MT
COMPETING INTERESTS: Dr Pang: None. Dr Lan has received research funding from Sanofi as part of a Clinical Fellowship in Endocrinology and Diabetes; education support from Amgen, Bayer, Boehringer Ingelheim, Eli Lilly and Novartis; speaker honoraria from Boehringer Ingelheim, Eli Lilly and Sanofi; and has participated in advisory boards for Eli Lilly. Professor Watts has received honoraria related to consulting, research and speaker activities from Amgen, Arrowhead, AstraZeneca, CRISPR Therapeutics, Esperion, Novartis and Sanofi.
References
1. Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29: 431-438.
2. Akioyamen LE, Genest J, Shan SD, et al. Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open 2017; 7: e016461.
3. Watts GF, Sullivan DR, Hare DL, et al. Integrated guidance for enhancing the care of familial hypercholesterolaemia in Australia. Heart Lung Circ 2021; 30: 324-349.
4. Wong B, Kruse G, Kutikova L, Ray KK, Mata P, Bruckert E. Cardiovascular disease risk associated with familial hypercholesterolemia: a systematic review of the literature. Clin Ther 2016; 38: 1696-1709.
5. Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab 2012; 97: 3956-3964.
6. Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014; 35: 2146-2157.
7. Shapiro MD, Bhatt DL. "Cholesterol-Years" for ASCVD risk prediction and treatment. J Am Coll Cardiol 2020; 76: 1517-1520.
8. Besseling J, Hovingh GK, Huijgen R, Kastelein JJP, Hutten BA. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all-cause mortality. J Am Coll Cardiol 2016; 68: 252-260.
9. Wilemon KA, Patel J, Aguilar-Salinas C, et al. Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol 2020; 5: 217-229.
10. Bell DA, Hooper AJ, Bender R, et al. Opportunistic screening for familial hypercholesterolaemia via a community laboratory. Ann Clin Biochem 2012; 49(Pt 6): 534-537.
11. Brett T, Radford J, Heal C, et al. Implications of new clinical practice guidance on familial hypercholesterolaemia for Australian general practitioners. Aust J Gen Pract 2021; 50: 616-621.
12. Bell DA, Garton-Smith J, Vickery A, et al. Familial hypercholesterolaemia in primary care: knowledge and practices among general practitioners in Western Australia. Heart Lung Circ 2014; 23: 309-313.
13. Lan NSR, Martin AC, Brett T, Watts GF, Bell DA. Improving the detection of familial hypercholesterolaemia. Pathology 2019; 51: 213-221.
14. Pang J, Vickery AW, Watts GF. Familial hypercholesterolemia: bridging and minding the gap in healthcare. Advances in dyslipidemia. Future Medicine Ltd; UK, 2013. p. 18-41.
15. Brett T. Universal screening for familial hypercholesterolaemia in newborns: time for general practice to contribute. Aust J Gen Pract 2023; 52: 246-248.
16. Bulsara C, Brett T, Radford J, et al. Awareness of familial hypercholesterolaemia in Australian primary care: a qualitative descriptive study. Aust J Gen Pract 2021; 50: 634-640.
17. Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ, Scheerder RL, Kastelein JJ. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 2001; 357: 165-168.
18. Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ 1991; 303(6807): 893-896.
19. Williams RR, Hunt SC, Schumacher MC, et al. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol 1993; 72: 171-176.
20. Harada-Shiba M, Arai H, Ishigaki Y, et al; Working Group by Japan Atherosclerosis Society for Making Guidance of Familial Hypercholesterolemia. Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb 2018; 25: 751-770.
21. Horton AE, Martin AC, Srinivasan S, et al. Integrated guidance to enhance the care of children and adolescents with familial hypercholesterolaemia: practical advice for the community clinician. J Paediatr Child Health 2022; 58: 1297-1312.
22. World Health Organization (WHO). Human Genetics Programme. Familial Hypercholesterolemia: report of a Second WHO Consultation. Document number: WHO/HGN/FH/Cons/99.2. WHO; Geneva, 1999. Available online at: https://apps.who.int/iris/handle/10665/66346 (accessed May 2023).
23. Haralambos K, Whatley SD, Edwards R, et al. Clinical experience of scoring criteria for familial hypercholesterolaemia (FH) genetic testing in Wales. Atherosclerosis 2015; 240: 190-196.
24. Wiegman A, Gidding SS, Watts GF, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J 2015; 36: 2425-2437.
25. Sturm AC, Knowles JW, Gidding SS, et al. Clinical genetic testing for familial hypercholesterolemia: JACC Scientific Expert Panel. J Am Coll Cardiol 2018; 72: 662-680.
26. Berberich AJ, Hegele RA. The complex molecular genetics of familial hypercholesterolaemia. Nat Rev Cardiol 2019; 16: 9-20.
27. Usifo E, Leigh SE, Whittall RA, et al. Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Ann Hum Genet 2012; 76: 387-401.
28. Watts GF, Gidding SS, Hegele RA, et al. International Atherosclerosis Society guidance for implementing best practice in the care of familial hypercholesterolaemia. Nat Rev Cardiol Accepted; In Press.
29. Vickery AW, Bell D, Garton-Smith J, Kirke AB, Pang J, Watts GF. Optimising the detection and management of familial hypercholesterolaemia: central role of primary care and its integration with specialist services. Heart Lung Circ 2014; 23: 1158-1164.
30. Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, Wierzbicki AS. Familial hypercholesterolaemia. Nat Rev Dis Primers 2017; 3: 17093.
31. Watts GF, Sullivan DR, Poplawski N, et al. Familial hypercholesterolaemia: a model of care for Australasia. Atheroscler Suppl 2011; 12: 221-263.
32. Ademi Z, Watts GF, Pang J, et al. Cascade screening based on genetic testing is cost-effective: evidence for the implementation of models of care for familial hypercholesterolemia. J Clin Lipidol 2014; 8: 390-400.
33. Starr B, Hadfield SG, Hutten BA, et al. Development of sensitive and specific age- and gender-specific low-density lipoprotein cholesterol cutoffs for diagnosis of first-degree relatives with familial hypercholesterolaemia in cascade testing. Clin Chem Lab Med 2008; 46: 791-803.
34. Watts GF, Gidding SS, Mata P, et al. Familial hypercholesterolaemia: evolving knowledge for designing adaptive models of care. Nat Rev Cardiol 2020; 17: 360-377.
35. Besseling J, Kindt I, Hof M, Kastelein JJ, Hutten BA, Hovingh GK. Severe heterozygous familial hypercholesterolemia and risk for cardiovascular disease: a study of a cohort of 14,000 mutation carriers. Atherosclerosis 2014; 233: 219-223.
36. Akioyamen LE, Genest J, Chu A, Inibhunu H, Ko DT, Tu JV. Risk factors for cardiovascular disease in heterozygous familial hypercholesterolemia: a systematic review and meta-analysis. J Clin Lipidol 2019; 13: 15-30.
37. Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J 2017; 38: 1573-1579.
38. Kronenberg F, Mora S, Stroes ESG, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J 2022; 43: 3925-3946.
39. Ellis KL, Pang J, Chan DC, et al. Familial combined hyperlipidemia and hyperlipoprotein(a) as phenotypic mimics of familial hypercholesterolemia: frequencies, associations and predictions. J Clin Lipidol 2016; 10: 1329-1337.e3.
40. Trinder M, Paruchuri K, Haidermota S, et al. Repeat measures of lipoprotein(a) molar concentration and cardiovascular risk. J Am Coll Cardiol 2022; 79: 617-628.
41. Khera AV, Won HH, Peloso GM, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol 2016; 67: 2578-2589.
42. Mata P, Alonso R, Pérez de Isla L. Atherosclerotic cardiovascular disease risk assessment in familial hypercholesterolemia: does one size fit all? Curr Opin Lipidol 2018; 29: 445-452.
43. Perez de Isla L, Alonso R, Mata N, et al. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART Registry (Spanish Familial Hypercholesterolemia Cohort Study). Circulation 2017; 135: 2133-2144.
44. Sacks FM, Lichtenstein AH, Wu JHY, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation 2017; 136: e1-e23.
45. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111-188.
46. Cicero AFG, Fogacci F, Zambon A. Red yeast rice for hypercholesterolemia: JACC focus seminar. J Am Coll Cardiol 2021; 77: 620-628.
47. Barkas F, Nomikos T, Liberopoulos E, Panagiotakos D. Diet and cardiovascular disease risk among individuals with familial hypercholesterolemia: systematic review and meta-analysis. Nutrients 2020; 12: 2436.
48. Fulcher J, O’Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015; 385: 1397-1405.
49. Perez de Isla L, Alonso R, Watts GF, et al. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-Year SAFEHEART Registry follow-up. J Am Coll Cardiol 2016; 67: 1278-1285.
50. Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358: 1431-1443.
51. Gagné C, Gaudet D, Bruckert E. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation 2002; 105: 2469-2475.
52. Huijgen R, Abbink EJ, Bruckert E, et al. Colesevelam added to combination therapy with a statin and ezetimibe in patients with familial hypercholesterolemia: a 12-week, multicenter, randomized, double-blind, controlled trial. Clin Ther 2010; 32: 615-625.
53. Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med 2023; 388: 1353-1364.
54. Newman CB, Preiss D, Tobert JA, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2019; 39: e38-e81.
55. Tavori H, Fan D, Blakemore JL, et al. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation 2013; 127: 2403-2413.
56. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379: 2097-2107.
57. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713-1722.
58. Hovingh GK, Raal FJ, Dent R, et al. Long-term safety, tolerability, and efficacy of evolocumab in patients with heterozygous familial hypercholesterolemia. J Clin Lipidol 2017; 11: 1448-1457.
59. Farnier M, Hovingh GK, Langslet G, et al. Long-term safety and efficacy of alirocumab in patients with heterozygous familial hypercholesterolemia: an open-label extension of the ODYSSEY program. Atherosclerosis 2018; 278: 307-314.
60. Santos RD, Stein EA, Hovingh GK, et al. Long-term evolocumab in patients with familial hypercholesterolemia. J Am Coll Cardiol 2020; 75: 565-574.
61. Dufour R, Hovingh GK, Guyton JR, et al. Individualized low-density lipoprotein cholesterol reduction with alirocumab titration strategy in heterozygous familial hypercholesterolemia: results from an open-label extension of the ODYSSEY LONG TERM trial. J Clin Lipidol 2019; 13: 138-147.
62. Raal FJ, Hovingh GK, Blom D, et al. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol 2017; 5: 280-290.
63. Klevmoen M, Bogsrud MP, Retterstøl K, et al. Loss of statin treatment years during pregnancy and breastfeeding periods in women with familial hypercholesterolemia. Atherosclerosis 2021; 335: 8-15.
64. Graham DF, Raal FJ. Management of familial hypercholesterolemia in pregnancy. Curr Opin Lipidol 2021; 32: 370-377.
65. Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the Writing Committee of the American Society for Apheresis: the eighth special issue. J Clin Apher 2019; 34: 171-354.
66. Luirink IK, Wiegman A, Kusters DM, et al. 20-year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med 2019; 381: 1547-1556.
67. Vuorio A, Kuoppala J, Kovanen PT, et al. Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev 2019; 2019(11): CD006401.
68. Kusters DM, Caceres M, Coll M, et al. Efficacy and safety of ezetimibe monotherapy in children with heterozygous familial or nonfamilial hypercholesterolemia. J Pediatr 2015; 166: 1377-1384.e1-3.
69. Santos RD, Ruzza A, Hovingh GK, et al. Evolocumab in pediatric heterozygous familial hypercholesterolemia. N Engl J Med 2020; 383: 1317-1327.
70. Santos RD, Ruzza A, Hovingh GK, et al. Paediatric patients with heterozygous familial hypercholesterolaemia treated with evolocumab for 80 weeks (HAUSER-OLE): a single-arm, multicentre, open-label extension of HAUSER-RCT. Lancet Diabetes Endocrinol 2022; 10: 732-740.
71. Daniels S, Caprio S, Chaudhari U, et al. PCSK9 inhibition with alirocumab in pediatric patients with heterozygous familial hypercholesterolemia: The ODYSSEY KIDS study. J Clin Lipidol 2020; 14: 322-330.e5.
72. Blom DJ, Averna MR, Meagher EA, et al. Long-term efficacy and safety of the microsomal triglyceride transfer protein inhibitor lomitapide in patients with homozygous familial hypercholesterolemia. Circulation 2017; 136: 332-335.
73. Raal FJ, Rosenson RS, Reeskamp LF, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med 2020; 383: 711-720.
74. Ishigaki Y, Kawagishi N, Hasegawa Y, et al. Liver transplantation for homozygous familial hypercholesterolemia. J Atheroscler Thromb 2019; 26: 121-127.
75. Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med 2020; 382: 1520-1530.
76. Ray KK, Troquay RPT, Visseren FLJ, et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol 2023; 11: 109-119.
77. Tokgözoğlu L, Libby P. The dawn of a new era of targeted lipid-lowering therapies. Eur Heart J 2022; 43: 3198-3208.