Peer Reviewed
Gastroenterology clinic
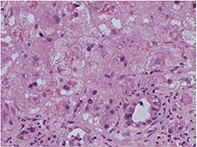
Amiodarone hepatoxicity
Abstract
Liver and lung toxicity associated with the use of amiodarone is uncommon but potentially life-threatening. Hepatotoxicity may be reversible if the drug is ceased as soon as toxicity is suspected.
Key Points
- Amiodarone hepatotoxicity can occur even on low-dose amiodarone therapy.
- There is a significant mortality and morbidity (approximately 1%).
- It can present as acute or chronic liver disease, early or late in the course of therapy (see the case studies in the box on page 58).
- The risk increases with higher doses and longer durations of therapy.
- The indication for amiodarone should be reviewed and other agents considered.
- Monitoring of liver function tests (one to three-monthly) is recommended throughout the duration of amiodarone therapy and for 12 months beyond cessation.
- Amiodarone should be ceased if the patient’s serum alanine aminotransferase (ALT) level is more than three times the normal for the testing pathology laboratory (i.e. is above about 150 U/L).
Remember
Purchase the PDF version of this article
Already a subscriber? Login here.