Metabolic dysfunction-associated fatty liver disease: the crucial link to CVD
Metabolic dysfunction-associated fatty liver disease (MAFLD), which is encountered every day in clinical practice, is a complex disorder that is linked to disease in almost every other organ system. Cardiovascular disease is principal among these, and the most likely cause of death in an individual with MAFLD, highlighting the need to ‘look beyond the liver’.
- The prevalence of metabolic dysfunction-associated fatty liver disease (MAFLD) is rising in Australia and worldwide. Cardiovascular disease (CVD) remains the single biggest cause of death in individuals with MAFLD.
- Liver fibrosis predicts poor outcomes such as development of cirrhosis, liver cancer and CVD. Liver fibrosis can be estimated with simple calculators using commonly available laboratory results. These tools also identify patients who need specialist referral.
- MAFLD is an independent risk factor for CVD. Commonly used risk calculators may underestimate the risk of CVD in people with MAFLD, especially those with liver fibrosis.
- CVD risk factors should be treated aggressively with pharmacological and nonpharmacological interventions.
- Medications such as statins are safe and tend to be under-prescribed in people with MAFLD.
- Several new medications are likely to become available over the next few years to treat the liver manifestations of MAFLD.
Metabolic dysfunction-associated fatty liver disease (MAFLD, previously known as nonalcoholic fatty liver disease [NAFLD]) is the most prevalent liver disease in Australia and also affects over a third of the global population.1 In Australia, incident cases of decompensated cirrhosis and primary liver cancer secondary to MAFLD are predicted to increase by 85% and 75%, respectively, this decade, rising in parallel with incident cases of obesity and the metabolic syndrome. Despite this, there is generally low awareness of MAFLD and, when present, it is easy to overlook or simply ignore it. This is likely because of the following factors:
- only a very small proportion of people with MAFLD will experience a liver-related adverse event (i.e. development of cirrhosis or liver cancer)
- there is a lack of effective drugs to improve liver fibrosis and inflammation or prevent liver-related adverse events (although this is hopefully soon set to change)
- the seemingly ubiquitous nature of MAFLD contributes to general desensitisation to the condition.
This therapeutic nihilism is illustrated by some guidelines that have explicitly recommended against routine screening for MAFLD, even in high prevalence settings, such as diabetes clinics, citing ‘uncertainties around diagnostic testing and treatment options’.2 Notably, other guidelines, including our local Asian Pacific Association for the Study of the Liver guidelines, recommend a more proactive approach to screening including in those with overweight or obesity, type 2 diabetes and the metabolic syndrome,3 whereas universal screening for MAFLD in people with type 2 diabetes is now endorsed by some diabetes guidelines, suggesting this lack of interest is shifting.4
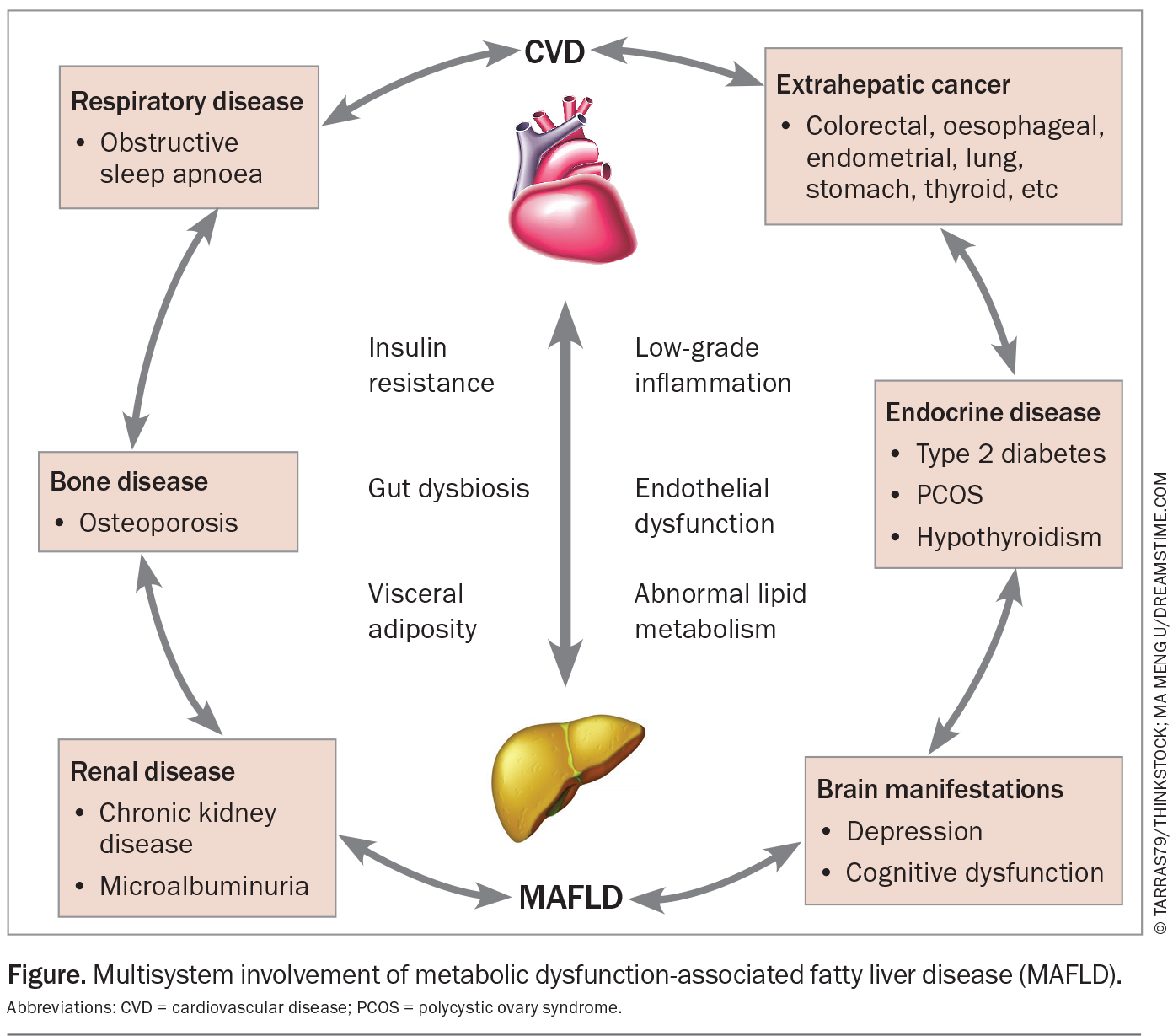
Importantly, however, MAFLD is not an isolated disorder of the liver, but rather the hepatic manifestation of systemic metabolic dysregulation, and in this regard, the liver can be thought of as a window into a person’s general metabolic health. This metabolic dysregulation state, which is integral to MAFLD, is characterised by insulin resistance, dysregulated lipid metabolism and chronic low-grade inflammation. Inter-organ cross-talk results in multiple other affected organ systems including the heart, brain, pancreas, blood vessels, adipose tissue and muscle. A large number of disease associations have been described, including sleep apnoea, cardiovascular disease (CVD), osteoporosis, chronic kidney disease, thyroid disease, polycystic ovary syndrome, mental health disorders and dementia (Figure). In keeping with this, several studies have demonstrated increased all-cause mortality in people with MAFLD compared with the general population, which tends to increase with increasing stages of liver fibrosis.5,6 However, most of this excess mortality is actually driven by nonliver-related events, most notably CVD, extrahepatic cancer and chronic kidney disease. In fact, despite a rapid rise in MAFLD-associated liver disease in recent decades, liver diseases still account for about one-third of the deaths caused by kidney disease in metabolically unhealthy people with type 2 diabetes in Australia.7
CVD warrants special mention as the already leading cause of death in Australia. MAFLD is associated with an increasing risk of fatal or nonfatal CVD events, and CVD is responsible for nearly a third of total mortality in individuals with MAFLD. In fact, upon diagnosis of MAFLD, a clinician’s first initial thought should be to consider CVD. In people with MAFLD, the risk of CVD increases along the MAFLD severity spectrum and fibrosis stage. Thus, just as assessing an individual’s degree of liver fibrosis predicts the risk of progression to cirrhosis or liver cancer, it likely also predicts the risk of a cardiovascular event.
MAFLD: screening, assessment and staging
Australian MAFLD guidelines were presented at the Australian Gastroenterology Week in 2023, and a concise version of the guideline document is expected to be published later this year.
People with obesity, type 2 diabetes or two or more metabolic risk factors (including hypertension, dyslipidaemia, increased waist circumference or prediabetes, regardless of bodyweight) are recommended to undergo screening for MAFLD, with liver ultrasound being the first-line diagnostic test. MAFLD should also be considered in people with unexplained abnormal liver enzyme levels.
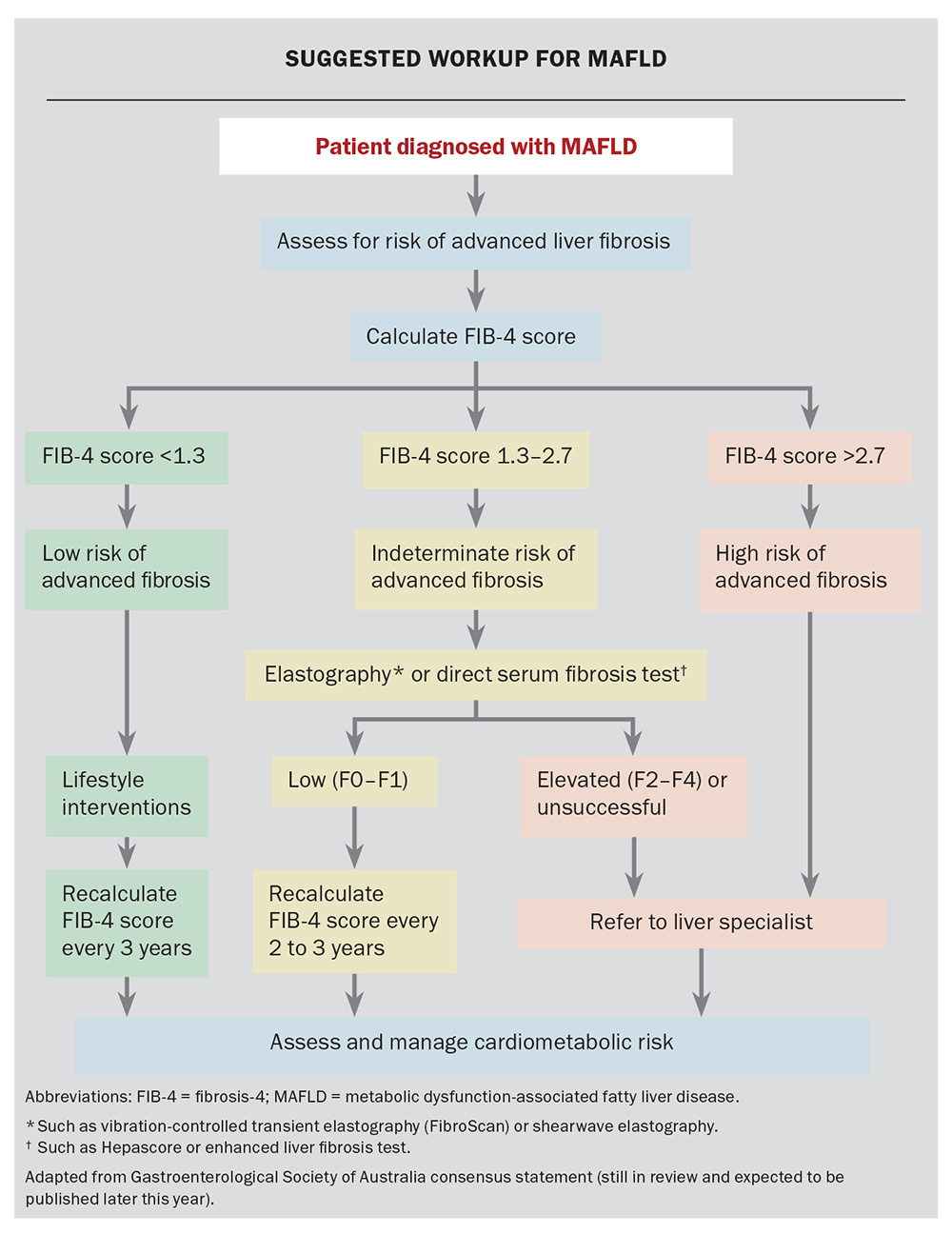
In individuals with MAFLD, the single most important prognostic factor is the presence and stage of liver fibrosis, which reflects chronic liver injury and inflammation. Fibrosis is typically reported on a scale of zero (F0; no liver fibrosis), one (F1; mild fibrosis), two (F2; significant fibrosis), three (F3; advanced fibrosis) and four (F4; cirrhosis). Individuals without significant fibrosis in F0 to F1 have a low risk of progressing to cirrhosis, liver decompensation or liver cancer in the short term. Individuals with more advanced fibrosis or cirrhosis (stage F3 to F4) are at significantly higher risk of adverse liver-related outcomes.
Although biopsy is the traditional gold-standard test for assessing liver fibrosis, this is rarely required in practice as several other noninvasive tests can predict the risk of liver fibrosis. The Fibrosis-4 (FIB-4) score is the recommended initial noninvasive test and can be calculated using commonly available clinical and laboratory results (patient age, alanine transaminase and aspartate transaminase levels and platelet count; test available online at: www.mdcalc.com/calc/2200/fibrosis-4-fib-4-index-liver-fibrosis). It stratifies individuals into low and high risk for significant fibrosis. Individuals with an intermediate FIB-4 score result have an indeterminate risk for advanced fibrosis and should have a second-line test. Second-line testing options include measurement of liver elastography or ‘stiffness’ (using vibration-controlled transient elastography [FibroScan] or shear wave elastography as an add on to standard ultrasound) or direct serum fibrosis tests, such as Hepascore or the enhanced liver fibrosis test. The choice of second-line test should take into account local availability and cost.
This risk stratification process is an integral part of the initial assessment of individuals diagnosed with MAFLD to ensure they end up on the appropriate care pathway, and that the clinician focus is aligned with treatment priorities. For example, in people without significant fibrosis, care is centred around management of obesity and addressing risk factors for comorbid conditions, including CVD, chronic kidney disease, diabetes and obstructive sleep apnoea. Those with significant fibrosis have an even higher risk of the above conditions, but also have a much higher competing risk of cirrhosis, decompensation and liver cancer and would benefit from liver specialist review. The suggested workup for MAFLD is shown in the Flowchart.
Mechanisms linking MAFLD to CVD
Atherosclerotic cardiovascular disease (ASCVD) is underpinned by the formation of atherosclerotic plaques, consisting of oxidised LDL accumulating within macrophages in the arterial intima in the setting of endothelial dysfunction. Inflammatory cytokines and vascular smooth muscle proliferation makes this plaque vulnerable to rupture and occlusion, particularly in prothrombotic states. MAFLD consists of multiple derangements that make this process more likely to occur.
MAFLD is associated with an atherogenic serum lipoprotein profile (consisting of increased very low LDL and small-dense LDL [the most atherogenic subtype of LDL] and decreased HDL levels), which has an increased propensity to form atherosclerotic plaques.8 Fat accumulation in hepatocytes induces oxidative stress and the upregulation of a number of proinflammatory signalling cascades. This contributes to the chronic low-grade systemic inflammatory state that mediates many of the extrahepatic complications of MAFLD including CVD. Endothelial dysfunction, referring to the loss of usual homeostatic mechanisms within vascular endothelial cells (which control functions such as regulation of vascular tone, platelet aggregation and immune cell migration), is also known to be a feature of MAFLD and is mediated in part by elevated serum levels of asymmetric dimethyl arginine,9 a nitric oxide synthase antagonist. Thus, in the milieu of chronic inflammation, endothelial dysfunction, haemostatic alteration and atherogenic dyslipidaemia, an environment primed for the formation of atherosclerotic plaques exists.
In this setting of chronic inflammation and the release of systemic and local proinflammatory mediators, MAFLD is associated with an increased risk of cardiac arrhythmias, including atrial fibrillation, ventricular arrhythmias and structural heart disease, including left ventricular hypertrophy accompanied by systolic and/or diastolic dysfunction.
MAFLD: an independent risk factor of CVD or an association?
Disentangling the effects of hepatic steatosis on CVD from other features of the metabolic syndrome that invariably accompany MAFLD is challenging. In other words, is hepatic steatosis an independent risk factor for CVD? Or does it simply tend to coincide with other established risk factors? Conflicting data exist reflecting heterogeneity between study populations in the published literature; however, a growing body of evidence suggests liver fat is indeed an independent risk factor. For example, one recent large prospective study with well-phenotyped participants found that baseline steatosis was associated with a 70% increased risk of major adverse cardiovascular events over a two-year follow-up period, even after adjusting for the presence of baseline coronary artery stenosis, ASCVD risk scores, obesity and the metabolic syndrome.10 Thus, it is likely that assessment of cardiovascular risk using ASCVD risk scores may underestimate risks in the MAFLD population, especially in those with more advanced liver inflammation or fibrosis; both of these factors could be considered when determining an individual’s CVD risk.
Management
Nonpharmacological interventions
Lifestyle measures including dietary changes, physical activity and weight loss interventions remain the mainstay of treatment of MAFLD.
Weight loss of a relatively small amount (5% bodyweight) is associated with a 30% improvement in liver fat content and improvement in metabolic parameters,11 although a greater degree of weight loss (>10%) may be required for regression of liver fibrosis.12 The benefits of sustained weight loss on cardiovascular risk can be dramatic, and this is well illustrated by bariatric surgery data. A recent large observational study of 650 obese individuals with MAFLD and steatohepatitis who underwent bariatric surgery significantly reduced their risk of major adverse cardiac events by 70% after a median of seven years follow up, compared with a matched control cohort.13 However, it is essential that weight loss measures be combined with other dietary and exercise interventions in people with MAFLD.
Exercise has beneficial effects on liver fat content and body composition, and promotes an antiatherogenic and anti-inflammatory state, independently of weight loss. Both aerobic exercise and resistance training have beneficial effects on liver fat reduction and, thus, a personalised approach is reasonable. Australian guidelines recommend 150 to 240 minutes of moderate intensity aerobic exercise per week, although as little as 135 minutes per week has been shown to be effective.14
High-calorie diets with excess saturated fat and refined carbohydrates are linked to obesity and MAFLD. Reducing caloric intake in line with the Mediterranean style diet is associated with reduced hepatic steatosis and improved cardiometabolic risk parameters.15,16 This consists of daily consumption of fruit and vegetables, unsweetened fibre-rich cereals, nuts, fish, white meat and olive oil with reduced consumption of saturated fat, processed food and simple sugars. Smoking cessation should also be promoted if relevant, and patients should be encouraged to abstain from alcohol. Coffee consumption appears protective against a variety of liver diseases and may be beneficial in MAFLD.
Pharmacological interventions
Blood pressure- and lipid-lowering therapy is recommended for individuals at high risk of CVD (>10% estimated five-year CVD risk, as determined by the Australian cardiovascular disease risk calculator, which uses age, sex, smoking status, blood pressure, total cholesterol to HDL-cholesterol ratio, diabetes status and use of CVD medications (see: www.cvdcheck.org.au/calculator). These therapies should be considered in people with intermediate (5 to 10%) five-year CVD risk as per Australian guidelines. It is important to bear in mind that existing algorithms do not yet incorporate MAFLD nor the severity of liver disease. Additionally, MAFLD is associated with an atherogenic lipid profile with the presence of highly atherogenic small-dense LDL, low HDL and high triglyceride levels, but often a normal (or only mildly elevated) total LDL-cholesterol level.
Statins are underprescribed in people with MAFLD (and in those with liver diseases more generally) despite substantial evidence to suggest no excess in the risk of hepatotoxicity with their use.17 Abnormal baseline liver chemistries are not a contraindication to use, and in addition to reducing the risk of cardiovascular events, they have pleiotropic antifibrotic effects on the liver and may reduce the risk of liver cancer (lipophilic statins such as atorvastatin and simvastatin may be more efficacious).18,19 Although statins are not yet recommended as a primary treatment for people with MAFLD, many of these patients present with other indications for statin use, although they are also underprescribed in these patients.20
Despite a previous paucity of pharmacotherapies that can improve liver histology in MAFLD, there are several other medications known to reduce cardiovascular risk that have, at worst, neutral (and possibly beneficial) effects on the liver. These include sodium-glucose cotransporter-2 inhibitors (empagliflozin, dapagliflozin), ACE inhibitors/angiotensin II receptor blockers (e.g. ramipril, perindopril, candesartan) and peroxisome proliferator-activated receptor-gamma agonists (e.g. pioglitazone). Although guidelines generally do not specifically recommend these medications for the sole indication of MAFLD (apart from considering pioglitazone), clinicians should have no hesitation prescribing these if otherwise indicated to do so.
Glucagon-like peptide-1 receptor agonists (liraglutide, dulaglutide, semaglutide) have been shown to improve cardiovascular risk factors, including improving blood pressure and lipid profiles and helping to achieve weight loss, as well as decreasing clinical events in cardiovascular outcome trials. A number of trials have also shown benefits in reducing liver fat, improving steatohepatitis and reducing fibrosis progression.21 A large phase 3 randomised control trial of semaglutide is currently underway with plans to enrol 1200 participants to assess the impact on liver histology and cardiovascular outcomes (clinicaltrials.gov NCT04822181).
Resmetirom is a new therapy that acts as an oral, liver-directed thyroid hormone receptor beta agonist. In a recent landmark study, the drug was shown to be superior to placebo in inducing steatohepatitis resolution and improving liver fibrosis after 52 weeks of use.22 On the basis of this study, the drug has just been granted FDA approval for use in the USA in people with noncirrhotic MAFLD with significant fibrosis (in conjunction with diet and exercise interventions), making it the first drug to receive approval for this indication after decades of failed attempts and disappointing trial results (no decision has been made on its approval in Australia at the time of writing this article). Although the trial was not powered to assess cardiovascular outcomes, it was notable that the drug was associated with significant improvements in atherogenic dyslipidaemia, including LDL-cholesterol, non-HDL-cholesterol, triglycerides, apolipoprotein B, apolipoprotein C-III, and lipoprotein(a) levels, thus long-term cardiovascular data are eagerly awaited. Other pharmacotherapies being studied for MAFLD are in late-stage trials, and it is likely that several of the compounds will be approved in Australia over the next five years.
Conclusion
The concept of MAFLD continues to evolve from a ‘siloed’ liver disease to that of a multi‑system disease in which most of the excess morbidity and mortality occurs from extra-hepatic events, with CVD being a crucial component. Although the development of drugs specifically targeting liver fibrosis has lagged behind, there are now several effective pharmacological and nonpharmacological interventions to reduce the risk of CVD. Thus, the screening for and identification of MAFLD is an important opportunity for intervention to improve an individual’s long-term outcome. MT
COMPETING INTERESTS: Dr Crane has received support for attending meetings from Novo Nordisk. Professor George has received consulting fees from Novo Nordisk, BI, AstraZeneca and payment or honoraria from Novo Nordisk and AstraZeneca.
References
1. Chan KE, Koh TJL, Tang ASP, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: a meta-analysis and systematic review of 10 739 607 individuals. J Clin Endocrinol Metab 2022; 107: 2691-700.
2. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatol 2018; 67: 328-357.
3. Eslam M, Sarin SK, Wong VWS, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020; 14: 889-919.
4. Elsayed NA, Aleppo G, Aroda VR, et al. Summary of revisions: Standards of Care in Diabetes—2023. Diabetes Care 2023; 46(Suppl 1): S5-S9.
5. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatol 2017; 65: 1557-1565.
6. Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 2021; 70: 1375-1382.
7. Tomic D, Salim A, George J, Magliano DJ, Shaw JE. Liver disease mortality and hospitalisations among people with type 2 diabetes mellitus: a population‐based study. Liver Int 2024; 44: 508-517.
8. Deprince A, Haas JT, Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab 2020; 42: 101092.
9. Dogru T, Genc H, Tapan S, et al. Elevated asymmetric dimethylarginine in plasma: An early marker for endothelial dysfunction in non-alcoholic fatty liver disease? Diabetes Res Clin Prac 2012; 96: 47-52.
10. Meyersohn NM, Mayrhofer T, Corey KE, et al. Association of hepatic steatosis with major adverse cardiovascular events, independent of coronary artery disease. Clin Gastroenterol Hepatol 2021; 19: 1480-1488.e14.
11. Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009; 49: 80-86.
12. Glass LM, Dickson RC, Anderson JC, et al. Total body weight loss of ≥10 % is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis. Dig Dis Sci 2015; 60: 1024-30.
13. Aminian A, Al-Kurd A, Wilson R, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy-proven nonalcoholic steatohepatitis. JAMA 2021; 326: 2031.
14. Keating SE, Sabag A, Hallsworth K, et al. Exercise in the management of metabolic-associated fatty liver disease (MAFLD) in adults: a Position Statement from Exercise and Sport Science Australia. Sports Medicine 2023; 53: 2347-2371.
15. Plauth M, Bernal W, Dasarathy S, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019; 38: 485-521.
16. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 2013; 59: 138-143.
17. Pastori D, Pani A, Di Rocco A, et al. Statin liver safety in non‐alcoholic fatty liver disease: a systematic review and metanalysis. Br J Clin Pharmacol 2022; 88: 441-451.
18. Pose E, Trebicka J, Mookerjee RP, Angeli P, Ginès P. Statins: old drugs as new therapy for liver diseases? J Hepatol 2019; 70: 194-202.
19. Simon TG, Duberg A-S, Aleman S, et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide swedish population. Ann Inter Med 2019; 171: 318-327.
20. Thomson MJ, Serper M, Khungar V, et al. Prevalence and factors associated with statin use among patients with nonalcoholic fatty liver disease in the TARGET-NASH Study. Clin Gastroenterol Hepatol 2022; 20: 458-460.e4.
21. Newsome PN, Ambery P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J Hepatol 2023; 79: 1557-1565.
22. Harrison SA, Bedossa P, Guy CD, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med 2024; 390: 497-509.