A man with a painless papule on the leg
Test your diagnostic skills in our regular dermatology quiz. What is this solitary lesion overlying the leg?
Case presentation
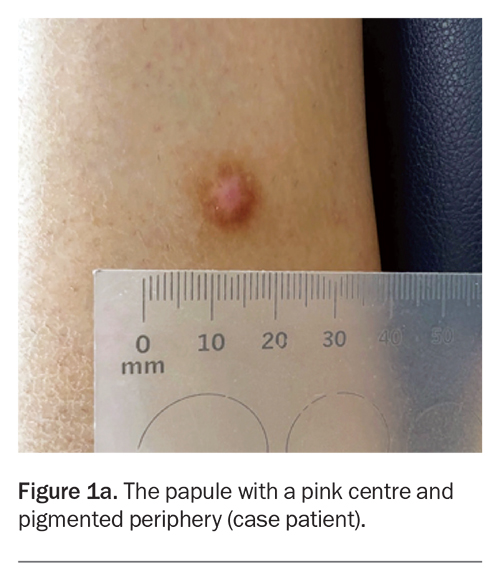
A 40-year-old man presents with a one-year history of a solitary papule on his leg (Figure 1a). The lesion is not tender but is mildly pruritic. The area has not been subjected to infection or trauma. He has no history of skin cancer but is concerned that the lesion may be malignant.
On examination, a centrally pink papule with a pigmented periphery is observed on the lateral aspect of the right leg. The lesion measures 8 mm in diameter, is firm on palpation and dimples inwards when lateral pressure is applied. The patient has Fitzpatrick skin type I.
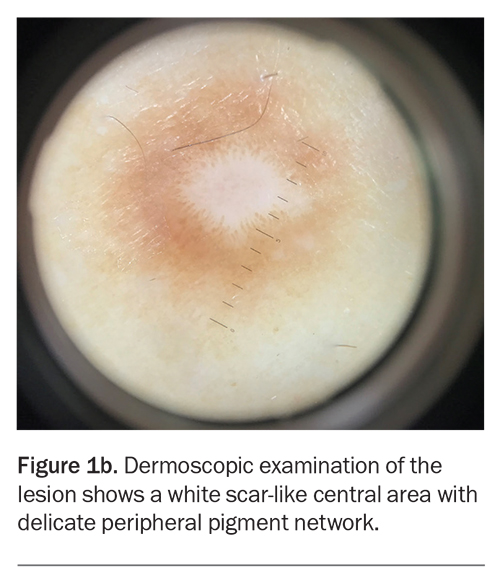
Dermoscopic examination shows a white scar-like centre with a delicate peripheral pigment network (Figure 1b).
Differential diagnoses
Conditions to consider among the differential diagnoses for a patient presenting with a solitary papule on the leg include the following.
Acquired melanocytic naevus
Acquired melanocytic naevus is a pigmented mole that appears after birth. It is the most common benign neoplasm, usually arising in the first three decades of life and occurring in greatest number in individuals of Caucasian background.1 Predisposing factors include lighter skin type and sun exposure, as well as a family history of high naevus count.2-4
Acquired melanocytic naevus is thought to be caused by mutations in the BRAF or NRAS genes, which activate the mitogen-activated protein kinase pathway leading to an increase in melanocyte proliferation.5BRAF V600 mutations have been found in over 80% of acquired melanocytic naevi.5,6
Acquired melanocytic naevus can be classified as common or dysplastic. There are three developmental stages, which are described according to the location of melanocyte nests:7,8
- junctional (proliferating melanocytes in the dermal-epidermal junction), which present as brown-black macules
- compound (melanocytes spanning the dermis and dermal-epidermal junction), which present as slightly raised, brown ovoid or round papules
- intradermal (melanocytes entirely within the dermis), which present as soft, light brown or flesh-coloured, dome-shaped papules with a smooth or papillomatous surface.
Typically, but not always, an acquired melanocytic naevus will progress through these three stages before senescence and involution later in life.8
For the case patient, the lesion was firm on palpation and showed a scar-like centre on dermoscopy. These features were not consistent with an acquired melanocytic naevus.
Nodular basal cell carcinoma
Basal cell carcinoma (BCC) is a malignant neoplasm of the basal cells. BBC is the most common type of skin cancer in Australia, accounting for up to 80% of nonmelanoma skin cancers, and its incidence is steadily increasing.9,10 The metastatic potential of BCC is very low and estimated to be between 0.05 and 0.1%.11-13
The pathogenesis of BCC involves aberrance of the Hedgehog signalling pathway and point mutation in the TP53 gene, which promotes neoplastic growth.14 The most significant risk factors include exposure to UV radiation, lighter skin type and increasing age.15,16 UV radiation is particularly important because of its mutagenic effects on keratinocyte progenitor cells.17
A BCC can arise anywhere on the body. The vast majority occur in sun-exposed areas, with 80% of lesions arising on the head and neck, but they are not uncommon on the arms and legs.18 Nodular BCC is the most common subtype (50 to 80% of BCCs) and presents as a papule or nodule with a pearly appearance and rolled edge.17-19 They are occasionally pigmented, with the most commonly observed pigment structures on dermoscopic examination being large blue-grey ovoid nests; usually arborising telangiectasias are visible on dermoscopy.20
For the case patient, the lesion did not have a pearly appearance or a rolled edge. The pigment was not that of blue-grey ovoid nests typical of a pigmented BCC and there were no arborising telangiectasias on dermoscopic examination.
Amelanotic melanoma
Amelanotic melanoma is a rare subtype of melanoma, comprising approximately 8% of all melanomas.21 Lacking the pigment classically associated with melanomas, they often masquerade as more benign entities.22,23 Although visually deceptive, amelanotic melanomas are malignant tumours of melanocytes and can occur as any histological subtype of melanoma.24 Risk factors are generally similar to those of melanoma (a history of sun exposure and a lighter, sun-sensitive phenotype); however, amelanotic melanomas are usually diagnosed in individuals over 50 years of age.24
The mechanism causing the lack of pigment in amelanotic melanoma remains unclear, but a few theories have been suggested. One theory is that amelanotic melanoma may be a poorly differentiated subtype of melanoma.25 Another theory is that amelanotic melanoma may result from de-differentiated melanocytes that have lost their normal phenotype.26 A third theory is that amelanotic melanoma may arise from multipotent melanocytes, resulting in cells that retain their melanocyte identity but form different phenotypes.27
Amelanotic melanomas usually present as pink, erythematous or flesh-coloured macules, papules or nodules. Dermoscopy is an important tool in identification because the vasculature offers valuable diagnostic clues – a predominance of central vessels, dotted vessels, linear irregular vessels or polymorphic vessels are concerning for amelanotic melanoma.28,29 Other concerning dermoscopic features include milky-red areas and, if the melanoma is hypomelanotic rather than amelanotic, it may have a blue-grey veil.28 A diagnosis of amelanotic melanoma is made on the basis of histological and immunohistochemical findings.
For the case patient, the lesion does not demonstrate any of these features of amelanotic melanoma macroscopically or under dermoscopic examination.
Dermatofibrosarcoma protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare, locally aggressive, cutaneous soft tissue sarcoma. It is predominantly diagnosed in adults between 30 and 50 years of age.30
DFSP is most often located on the trunk or limbs.31 The lesion starts as an asymptomatic, pink or skin-coloured indurated plaque that slowly enlarges and progresses to a red-brown or violaceous nodule.32 On palpation, it is firm and attached to the underlying subcutaneous tissue.
Histologically, DFSP is comprised of spindle-shaped cells in a dense but uniform arrangement.33 Immunohistochemistry greatly assists diagnosis, as CD34 positivity occurs in the majority of DFSP lesions.34
For the case patient’s lesion, the history does not support a diagnosis of DFSP because it has shown stability in a papular form since onset.
Dermatofibroma
This is the correct diagnosis. A dermatofibroma, also known as a cutaneous fibrous histiocytoma, is a benign lesion that occurs most frequently on the lower limbs in adults.35 It does not usually cause symptoms but can occasionally be pruritic. The precise aetiology is not known, but it is postulated to be a local tissue response to trauma or inflammation, such as an insect bite or a traumatised hair follicle.36
Dermatofibroma usually presents as a solitary pink, tan or brown papule or nodule.32 It is firm on palpation and produces a positive ‘dimple sign’ (downward dimpling upon application of lateral pressure on either side of the lesion). The most common dermoscopic findings are a scar-like white centre and a surrounding delicate pigment network.35 Histologically, dermatofibromas are composed of a nodular proliferation of fibroblasts and myofibroblasts in the dermis, with frequent hyperpigmentation and hyperplasia of the epidermis.32,36 If there is concern regarding the diagnosis, a biopsy can be performed to exclude the conditions discussed above.
For the case patient, the history, examination and dermoscopic findings of the lesion are consistent with a diagnosis of dermatofibroma.
Management
Dermatofibromas do not require intervention. For patients who have cosmetic concerns about their lesion, surgical excision can be offered, but they should be made aware of the possibility of recurrence and postsurgical scarring that can be more extensive and noticeable than the initial lesion. Other interventions, such as shave biopsy, cryotherapy and laser treatments, have been used, but the results have been variable and recurrence rates will likely be higher than for surgical removal.37
Outcome
The case patient was diagnosed with a dermatofibroma. He was reassured about the benign nature of the lesion, which did not undergo intervention. At follow-up appointments, the lesion was observed to be stable in size and appearance and did not cause the patient any issues. MT
COMPETING INTERESTS: None.
References
1. Balin SJ, Barnhill RL. Benign melanocytic neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018. pp. 1954-1988.
2. Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283: 2955-2960.
3. Duffy DL, Zhu G, Li X, et al. Novel pleiotropic risk loci for melanoma and nevus density implicate multiple biological pathways. Nat Commun 2018; 9: 4774.
4. Duffy DL, Iles MM, Glass D, et al. IRF4 variants have age-specific effects on nevus count and predispose to melanoma. Am J Hum Genet 2010; 87: 6-16.
5. Maher NG, Scolyer RA, Colebatch AJ. Biology and genetics of acquired and congenital melanocytic naevi. Pathology 2023; 55: 169-177.
6. Tan JM, Tom LN, Jagirdar K, et al. The BRAF and NRAS mutation prevalence in dermoscopic subtypes of acquired naevi reveals constitutive mitogen-activated protein kinase pathway activation. Br J Dermatol 2018; 178: 191-197.
7. Bolognia J, Schaffer JV, Duncan KO, Ko CJ. Dermatology essentials. 2nd ed. London: Elsevier; 2021.
8. Stefanaki I, Antoniou C, Stratigos A. Benign melanocytic proliferations and melanocytic naevi. In: Barker J, Bleiker TO, Chalmers R, Griffiths CEM, Creamer D, eds. Rook’s Textbook of dermatology. Oxford: John Wiley; 2016. pp. 3621-3668.
9. Perera E, Gnaneswaran N, Staines C, Win AK, Sinclair R. Incidence and prevalence of non-melanoma skin cancer in Australia: a systematic review. Australas J Dermatol 2015; 56: 258-267.
10. Olsen CM, Pandeya N, Green AC, Ragaini BS, Venn AJ, Whiteman DC. Keratinocyte cancer incidence in Australia: a review of population-based incidence trends and estimates of lifetime risk. Public Health Res Pract 2022; 32: 3212203.
11. Ganti AK, Kessinger A. Systemic therapy for disseminated basal cell carcinoma: an uncommon manifestation of a common cancer. Cancer Treat Rev 2011; 37: 440-443.
12. von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol 1984; 10: 1043-1060.
13. Weinstock MA, Bogaars HA, Ashley M, Litle V, Bilodeau E, Kimmel S. Nonmelanoma skin cancer mortality. A population-based study. Arch Dermatol 1991; 127: 1194-1197.
14. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med 2015; 88: 167-179.
15. Chen AC, Halliday GM, Damian DL. Non-melanoma skin cancer: carcinogenesis and chemoprevention. Pathology 2013; 45: 331-341.
16. Lovatt TJ, Lear JT, Bastrilles J, et al. Associations between ultraviolet radiation, basal cell carcinoma site and histology, host characteristics, and rate of development of further tumors. J Am Acad Dermatol 2005; 52(3 Pt 1): 468-473.
17. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol 2019; 80: 303-317.
18. Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med 2005; 353: 2262-2269.
19. Soyer HP, Rigel DS, McMeniman E. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018. pp. 1872-1893.
20. Reiter O, Mimouni I, Dusza S, Halpern AC, Leshem YA, Marghoob AA. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol 2021; 85: 653-664.
21. Thomas NE, Kricker A, Waxweiler WT, et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol 2014; 150: 1306-1314.
22. Garbe C, Bauer J. Melanoma. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018. pp. 1989-2019.
23. Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol 2000; 42(5 Pt 1): 731-734.
24. Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res 2019; 29: 221-230.
25. Bhawan J. Amelanotic melanoma or poorly differentiated melanoma? J Cutan Pathol 1980; 7: 55-56.
26. Roesch A, Berking C. Melanoma. In: Braun-Falco’s Dermatology. Berlin: Springer; 2020. pp. 1-17.
27. Cheung WL, Patel RR, Leonard A, Firoz B, Meehan SA. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol 2012; 39: 33-39.
28. Menzies SW, Kreusch J, Byth K, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol 2008; 144: 1120-1127.
29. Steglich RB, Meotti CD, Ferreira MS, Lovatto L, de Carvalho AV, de Castro CG. Dermoscopic clues in the diagnosis of amelanotic and hypomelanotic malignant melanoma. An Bras Dermatol 2012; 87: 920-923.
30. Oliveira-Soares R, Viana I, Vale E, Soares-Almeida LM, Picoto A. Dermatofibrosarcoma protuberans: a clinicopathological study of 20 cases. J Eur Acad Dermatol Venereol 2002; 16: 441-446.
31. Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol 2007; 56: 968-973.
32. Kutzner HH, Kamino H, Reddy VB, Pui J. Fibrous and fibrohistocytic proliferations of the skin and tendons. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018. p. 2068-85.e1.
33. Thway K, Noujaim J, Jones RL, Fisher C. Dermatofibrosarcoma protuberans: pathology, genetics, and potential therapeutic strategies. Ann Diagn Pathol 2016; 25: 64-71.
34. Weiss SW, Nickoloff BJ. CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am J Surg Pathol 1993; 17: 1039-1045.
35. Şenel E, Yuyucu Karabulut Y, Doğruer Şenel S. Clinical, histopathological, dermatoscopic and digital microscopic features of dermatofibroma: a retrospective analysis of 200 lesions. J Eur Acad Dermatol Venereol 2015; 29: 1958-1966.
36. Zelger B, Zelger BG, Burgdorf WHC. Dermatofibroma – a critical evaluation. Int J Surg Pathol 2004; 12: 333-344.
37. Parish LC, Yazdanian S, Lambert WC, Lambert PC. Dermatofibroma: a curious tumor. Skinmed 2012; 10: 268-270.