Deucravacitinib: a first-in-class oral treatment for psoriasis
The first-in-class, once daily oral therapy deucravacitinib has been PBS listed in Australia for adults with severe chronic plaque psoriasis. Deucravacitinib represents a new option for treating plaque psoriasis with its unique mechanism of action as a selective tyrosine kinase 2 inhibitor.
- Plaque psoriasis is an immune-mediated inflammatory condition with significant morbidity, often requiring long-term treatments to prevent flares of disease and maintain symptom control.
- Deucravacitinib is a first-in-class, oral, selective tyrosine kinase type 2 inhibitor that is listed on the PBS for adults with severe chronic plaque psoriasis who have failed to achieve an adequate response to methotrexate.
- Clinical trials have shown deucravacitinib to have a promising efficacy and safety profile for patients with moderate to severe chronic plaque psoriasis.
- GPs have a key role in identifying patients with plaque psoriasis who would benefit from systemic therapy.
- GPs can prescribe continued treatment of deucravacitinib following initiation by a dermatologist and monitor patients for treatment response or side effects.
Psoriasis is an immune-mediated inflammatory condition that results from the complex interplay of genetic and environmental factors. There are several clinical subtypes, the most common being plaque psoriasis, which presents with sharply demarcated salmon-pink plaques and overlying silvery-white scales typically on the extensor surfaces of the knees and elbows, scalp and intergluteal cleft. Other major subtypes include guttate, inverse, pustular and erythrodermic psoriasis.1
The prevalence of psoriasis in Australia is among the highest globally, estimated to be between 2.3 and 6.6%, with an estimated 50,000 people in Australia suffering from moderate to severe chronic plaque psoriasis.2,3 Psoriasis can significantly affect a patient’s quality of life and is also associated with several comorbidities, including psoriatic arthritis, inflammatory bowel disease, cardiovascular disease, obesity and diabetes.2
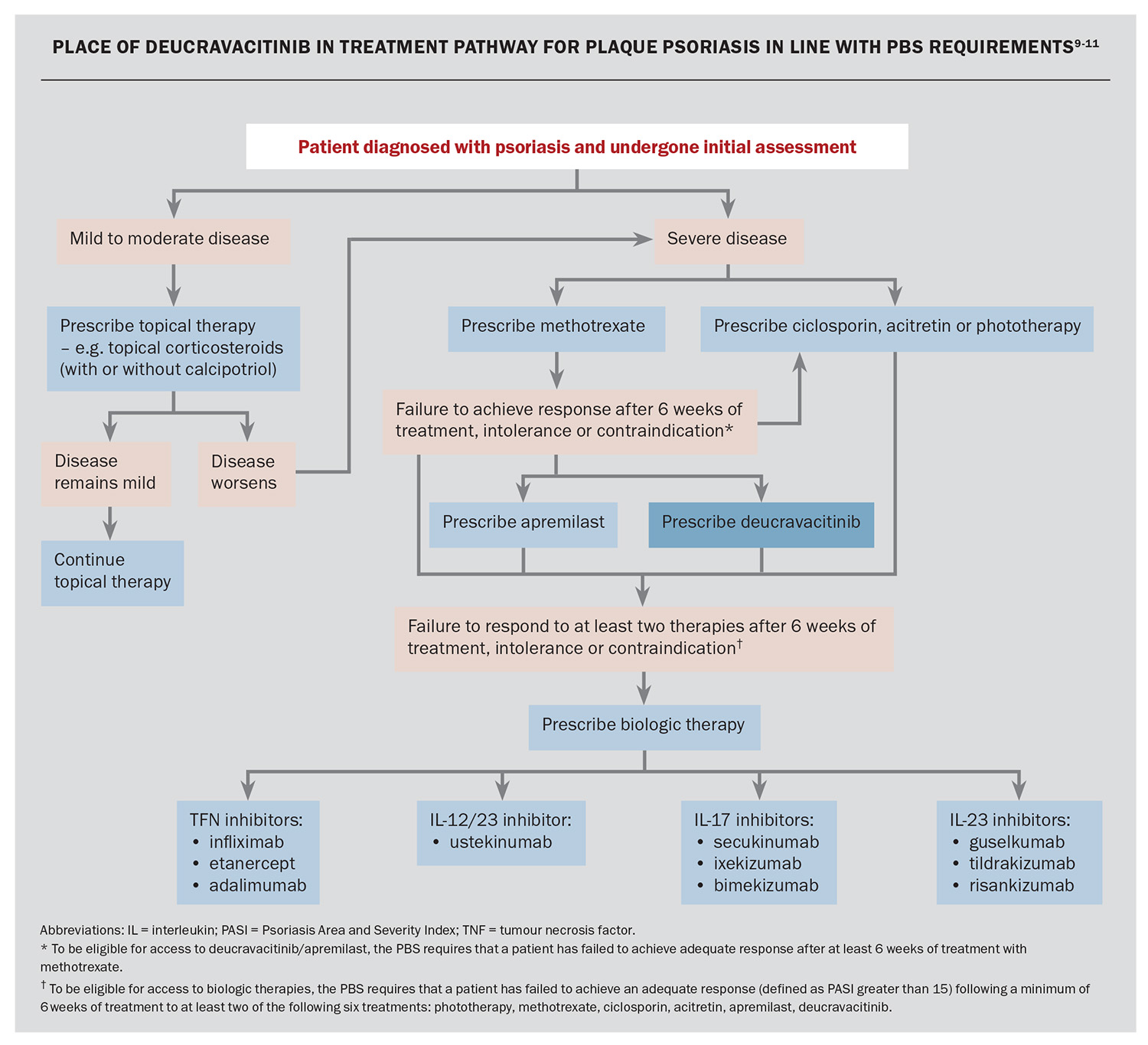
The severity of plaque psoriasis can be assessed objectively using the Psoriasis Area and Severity Index (PASI). Although mild or limited psoriasis is routinely managed by GPs using topical therapies such as topical corticosteroids (with or without calcipotriol), moderate to severe plaque psoriasis may require systemic treatment under the direction of a dermatologist. Patients can have variability in their response to different treatments, as well as different personal preferences and lifestyle factors, that makes it important to tailor therapies to patient-specific needs.4
In Australia, first-line therapy for moderate to severe chronic plaque psoriasis includes phototherapy or conventional oral agents, including methotrexate, ciclosporin and acitretin. These treatments, however, may be associated with significant side effects. More recently, apremilast has become available and is listed on the PBS for patients with severe psoriasis who have failed to achieve an adequate response to, or not tolerated, methotrexate. There are currently 10 biologic agents available on the PBS for patients with severe chronic plaque psoriasis who have failed to respond after six weeks of phototherapy, conventional oral agents and/or apremilast, defined by a PASI greater than 15 while still on treatment or no longer than four weeks after cessation of treatment. These biologics include:
- tumour necrosis factor (TNF) inhibitors (infliximab, etanercept and adalimumab)
- interleukin (IL)-12/23 inhibitor (ustekinumab)
- IL-17 inhibitors (secukinumab, ixekizumab and bimekizumab)
- IL-23 inhibitors (guselkumab, tildrakizumab and risankizumab).5
However, in Australia, patients with a PASI of 15 or less are unable to access these highly effective biologic treatments through the PBS. As many of the available agents for this cohort of patients have lower efficacy and/or higher risk of side effects, there has been an unmet need for an oral targeted agent with an acceptable safety and efficacy profile for the treatment of plaque psoriasis.
What is deucravacitinib?
Deucravacitinib is a first-in-class oral selective tyrosine kinase type 2 (TYK2) inhibitor. TYK2 is a member of the Janus associated kinase (JAK) family of intracellular kinases and has a role in regulating the signalling of key cytokines involved in the pathogenesis of psoriasis. TYK2 pairs with JAK1 and JAK2 to mediate multiple cytokine pathways for the IL-23/IL-12 and the type 1 interferon family.
The allosteric binding of deucravacitinib is selective for the TYK2 pseudokinase domain, differentiating it from inhibitors of the JAK1/2/3 kinase, which bind to the highly conserved active catalytic site of the kinase domain, resulting in their lower selectivity. Consequently, deucravacitinib inhibits the action of IL-23, IL-12 and type 1 interferon and reduces the downstream production and release of pro-inflammatory cytokines, including IL-17 and IL-22.5,6
Efficacy
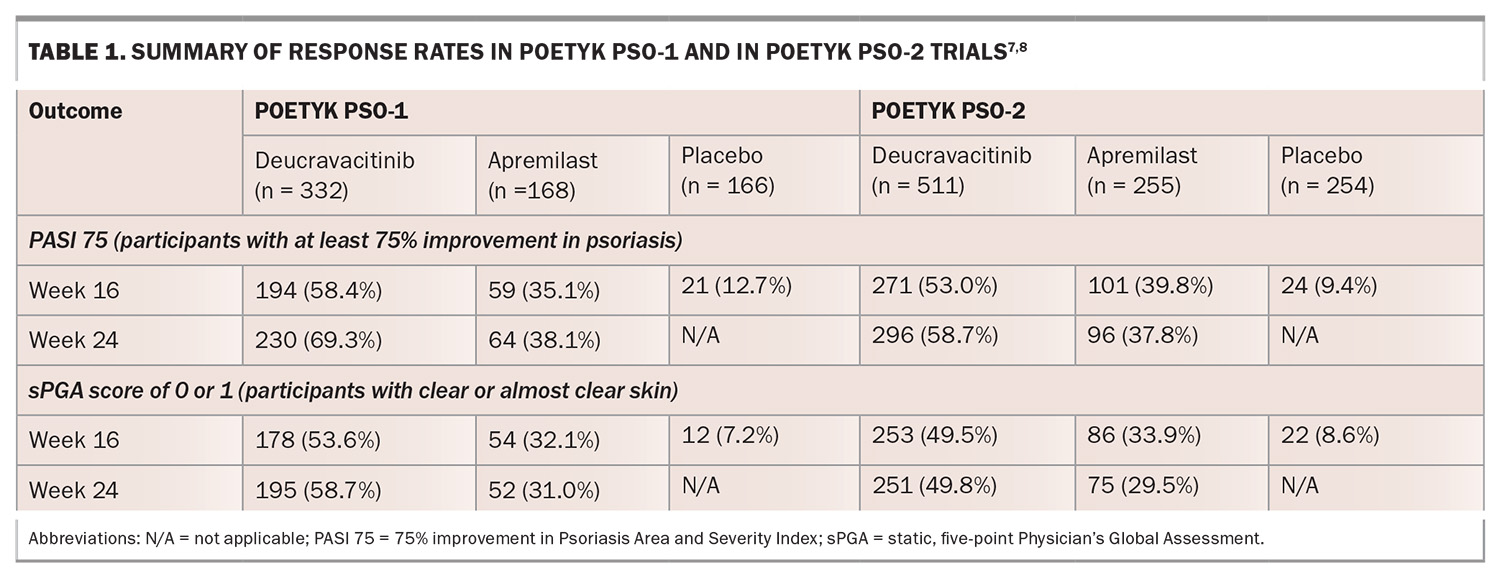
The evidence for deucravacitinib in adults with moderate to severe plaque psoriasis is supported by two pivotal phase 3 clinical trials: studies IM011046 (POETYK PSO-1) and IM011047 (POETYK PSO-2). Both studies were multicentred, randomised, double-blinded, placebo and active-comparator-controlled (apremilast 30 mg twice daily) for 52 weeks. In total, there were 1684 participants, 664 in POETYK PSO-1 and 1020 in POETYK PSO-2. All enrolled participants had moderate to severe plaque psoriasis, were candidates for phototherapy or systemic therapy, had a body surface area involvement equal to or greater than 10%, PASI equal to or greater than 12, and had a static, five-point Physician’s Global Assessment (sPGA) equal to or greater than 3 (moderate or severe disease).7,8
The results from both studies showed that deucravacitinib had superior efficacy compared with placebo at week 16 when assessing PASI 75 (at least 75% reduction in baseline PASI) and sPGA scores of 0 or 1 (clear or almost clear disease) (Table 1). Similarly, there were also significant PASI 75 and sPGA response rates for participants treated with deucravacitinib compared with apremilast at week 16, which continued to improve at week 24 and was maintained at week 52.7,8 In POETYK PSO-2, 31% of participants who were withdrawn from deucravacitinib at week 24 maintained PASI 75 response at week 52, suggesting some durability.8
With regard to patient-reported outcomes, there were significantly greater improvements in psoriasis symptoms (itch, pain, burning, stinging and skin tightness) based on the Psoriasis Symptoms and Signs Diary (PSSD) with deucravacitinib when compared with apremilast and placebo at week 16. This was observed through week 24 and maintained through week 52.7,8
PBS listing
Deucravacitinib was listed on the Australian PBS on 1 October 2023 for adults with severe chronic plaque psoriasis who have failed to achieve an adequate response to methotrexate (Box).9 The proposed use of deucravacitinib among the other treatment options for plaque psoriasis in Australia is shown in the Flowchart.9-11
The role of GPs in treatment
Greater access to effective treatments is essential to improving outcomes for patients with psoriasis. The PBS reimbursement of deucravacitinib represents a major milestone in helping to address the needs of patients with severe plaque psoriasis not met by previously available treatment options. GPs have a key role in identifying these patients who would benefit from systemic treatment. Awareness of deucravacitinib as a treatment option also allows GPs to prescribe continued treatment following initiation by a dermatologist, as well as to monitor for treatment response or side effects. If patients have inadequate response, loss of response or experience toxicity to deucravacitinib, GPs can refer them back to dermatologists for consideration of other treatment options.
Dosing
The recommended dose of deucravacitinib is 6 mg once daily, taken orally. Dosage adjustment is not needed in patients:
- aged 65 years and older
- with any form of renal impairment, including with end-stage renal disease
- with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment.
Safety
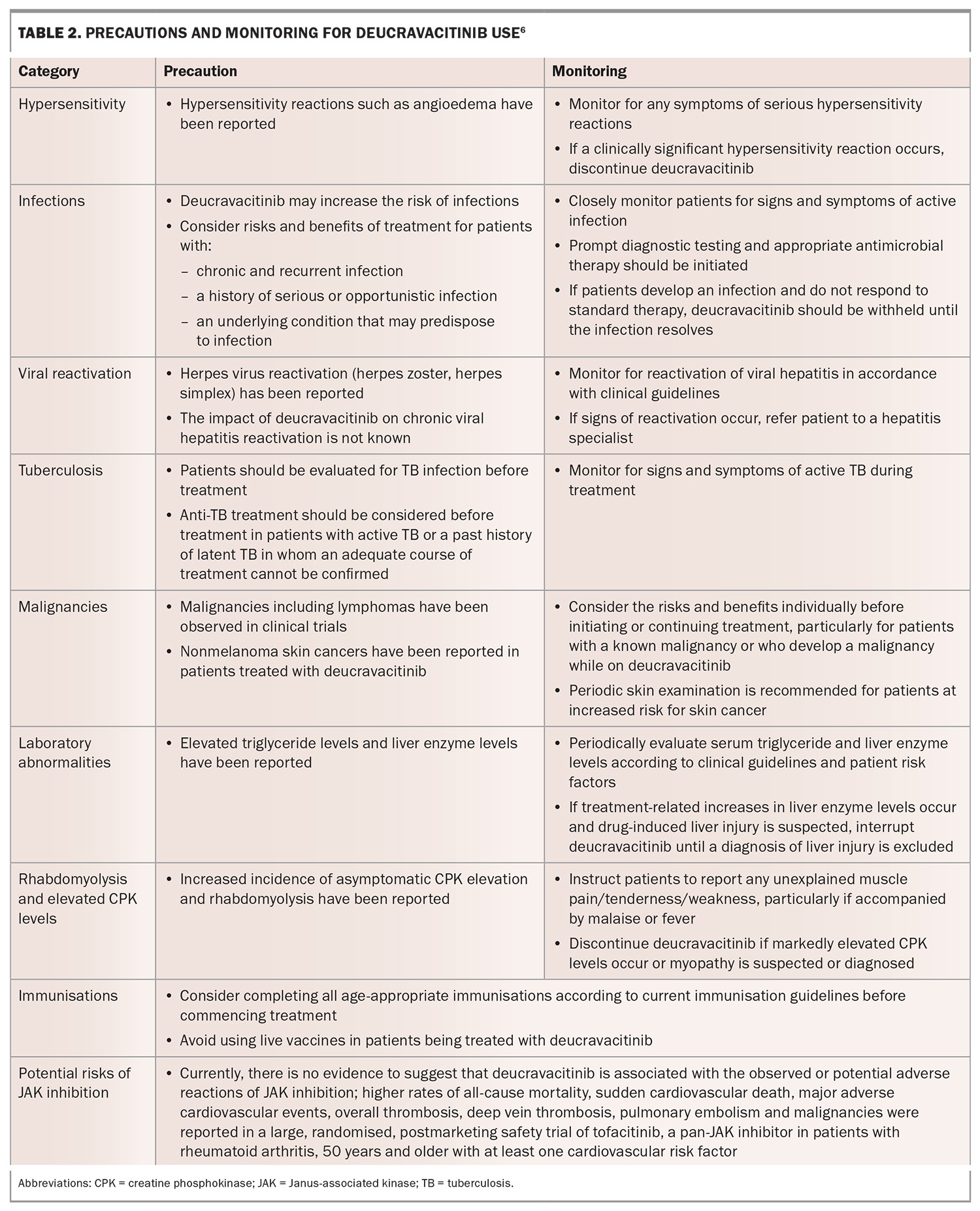
Contraindications
Deucravacitinib should not be used in patients with:
- a history of hypersensitivity reaction to deucravacitinib or to any of its excipients
- active or serious infections including hepatitis B, hepatitis C or active tuberculosis (TB)
- severe hepatic impairment (Child-Pugh Class C).
The safety and efficacy of deucravacitinib in patients less than 18 years of age have not been established.
Adverse reactions
The rate of adverse events overall with deucravacitinib from the two pivotal phase 3 trials was low, and similar to those of placebo and apremilast at 16 weeks.7,8 The incidence of serious adverse events for deucravacitinib was 1.8% compared with 2.9% for placebo and 1.2% for apremilast.6 Upper respiratory infections were considered a very common adverse drug reaction (frequency ≥1/10), and herpes simplex infections, oral ulcers, acneiform rash and folliculitis were considered common (≥1/100 to <1/10).6
Monitoring
Patients should undergo safety screening investigations before treatment with deucravacitinib. For patients following the PBS treatment pathway, many of the following pretreatment investigations may have occurred before treatment with methotrexate.12
Pretreatment evaluation can include:
- baseline full blood count, renal and liver function tests
- body mass index, weight and abdominal circumference
- glycated haemoglobin (HbA1c) and blood sugar levels
- fasting lipid levels
- hepatitis B surface antigen (HBsAg), surface antibody (Anti-HBs) and core antibody (Anti-HBc)
- hepatitis C antibody (Anti-HCV)
- human immunodeficiency (HIV) serology
- varicella zoster serology
- evaluation for latent and active TB: QuantiFERON-TB Gold test and a chest x-ray
- in patients in whom myopathy is suspected, creatine phosphokinase enzyme levels can be measured at baseline or during treatment; elevated levels may be clinically insignificant.6
Warnings, precautions and on-treatment monitoring
Warning and precautions with deucravacitinib are summarised in Table 2. Frequency of laboratory monitoring while on treatment with deucravacitinib can vary for patients depending on their comorbidities and should occur in line with clinical guidelines. Patients will need to be seen at least every 24 weeks for review of ongoing supply of deucravacitinib.9
Fertility, pregnancy and lactation
The effects of deucravacitinib on fertility have not been evaluated in humans. Animal studies have not shown direct or indirect harmful effects on fertility. There are no data on whether deucravacitinib or its metabolites are excreted in human milk; however, deucravacitinib is present in rat milk.6
Interactions
The safety and efficacy of deucravacitinib when coadministered with immunosuppressants and biologic agents have not been evaluated in patients with psoriasis. No dose adjustment for deucravacitinib is needed when used in combination with P-glycoprotein (Pgp) or breast cancer resistance protein (BCRP) inhibitors, strong CYP1A2 inhibitors, CYP1A2 inducers, UGT1A9 inhibitors, organic cation transporter 1 (OCT1) inhibitors or gastric pH modulators.6
Conclusion
Plaque psoriasis is a condition with significant morbidity, often requiring long-term treatments to prevent flares of disease and maintain symptom control. Deucravacitinib presents a new oral treatment option with a promising efficacy and safety profile for patients with severe chronic plaque psoriasis. The recent PBS reimbursement of deucravacitinib helps to provide people in Australia with timely and equitable access to a first-in-class treatment. MT
COMPETING INTERESTS: Dr Nguyen has received support for attending investigator meetings from AbbVie and Sanofi. Associate Professor Shumack has received research grants or honoraria from AbbVie, Amgen, Bristol Myer Squibb, Eli Lilly, Leo Pharma, Pfizer, Sanofi, Sun Pharma and UCB Pharma. Dr Daniel has served as a speaker for Eli Lilly and Bristol Myer Squibb. Associate Professor Foley has served as a consultant, investigator, speaker or advisor for, or received research or travel grants from AbbVie, Amgen, Apogee, Aslan Pharmaceuticals, Boehringer Ingelheim, Bristol Myer Squibb, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, Mayne Pharma, Novartis, Pfizer, Sanofi, Sun Pharma, Takeda and UCB Pharma.
This article is for general information purposes only, and the full product information should be consulted before prescribing any of the mentioned medications.
References
1. Langley RGB, Krueger GG, Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Annals Rheum Dis 2005; 64: 18-23.
2. Kovitwanichkanont T, Chong AH, Foley P. Beyond skin deep: addressing comorbidities in psoriasis. Med J Aust 2020; 212; 528-534.
3. Pharmaceutical Benefits Scheme. Post-market review: the use of biologics in the treatment of severe chronic plaque psoriasis. Report to PBAC. Canberra: Australian Government Department of Health and Aged Care; 2020.
4. Smith SD. Product review. Deucravacitinib use for adult plaque psoriasis. Research Review Sep 2023.
5. Therapeutic Goods Administration. Australian Public Assessment Report for Sotyktu. Canberra: Australian Government Department of Health and Aged Care; 2023.
6. Therapeutic Goods Administration. Sotyktu (deucravacitinib). Australian Product Information. Canberra: Australian Government Department of Health and Aged Care; 1 Dec 2022.
7. Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol 2023; 88: 29-39.
8. Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol 2023; 88: 40-51.
9. Pharmaceutical Benefits Scheme. Deucravacitinib. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://www.pbs.gov.au/medicine/item/13649J (accessed May 2024).
10. Sun HY, Keller E, Suresh H, Sebaratnam DF. Biologics for severe, chronic plaque psoriasis: An Australian cost-utility analysis. JAAD Int 2021; 5: 1-8.
11. Foley P, Gebauer K, Sullivan J, et al. Australian consensus: Treatment goals for moderate to severe psoriasis in the era of targeted therapies – adult patients. Australas J Dermatol 2023; 00: 1-12.
12. Rademaker M, Gupta M, Andrews M, et al. The Australasian Psoriasis Collaboration view on methotrexate for psoriasis in the Australasian setting. Australas J Dermatol 2017; 58: 166-170.