Type 1 diabetes – healthcare strategies for older people

Older people with type 1 diabetes represent a heterogeneous group, with varying ability to self-manage their condition. A multidisciplinary approach to management, tailored to the individual, and co-ordinated by the GP is recommended for older people with type 1 diabetes, with hypoglycaemia avoidance a priority. Regular review is essential as their health circumstances may change with time, requiring altered management, including simplified insulin regimens and de-escalating therapies.
- In Australia people with type 1 diabetes over the age of 60 years represent about one-third of all people living with the condition.
- Older people with type 1 diabetes vary widely in their burden of complications, functional state, cognitive abilities, social supports and access to material resources. Therefore, management should be tailored accordingly by a multidisciplinary team.
- In managing glucose levels, hypoglycaemia avoidance should be prioritised, although avoidance of hyperglycaemia is still important.
- Insulin regimens should be regularly assessed and may need to be simplified, but insulin should never be withheld.
- In managing and minimising the progression of complications, polypharmacy should be avoided and combined formulations considered to promote adherence.
- Older people with type 1 diabetes should be regularly reviewed and de-escalation of therapies considered according to their circumstances.
Of the 134,000 people in Australia living with type 1 diabetes, one-third are aged 60 years or older, which is a threefold higher prevalence than that among those aged under 20 years, due to longer life expectancy and advances in medical care. Although the incidence of type 1 diabetes peaks between 10 and 20 years of age, a secondary peak occurs between the ages of 60 and 64 years.1 Older people living with type 1 diabetes represent a heterogeneous group with regard to functional status, complication prevalence, frailty, cognition and access to material resources and social supports. For example, people living with the condition can range from being financially secure, cognitively intact, in good physical health and self-caring to living in supported accommodation, with limited financial resources and entirely dependent on others for the provision of their healthcare needs. It is also important to note an individual’s status in terms of these needs will almost certainly change over time.2
A multidisciplinary team, usually co-ordinated by a GP, and including an endocrinologist, a diabetes nurse educator, a practice nurse, a dietitian, support workers and family members best serves any older person with type 1 diabetes, regardless of where they fall on the spectrum of complexity. Other medical and surgical subspecialists may be required for the screening and management of complications. This support needs to be tailored to individual patient needs and will evolve with time. Therefore, the provision of health care for older people living with type 1 diabetes is relevant for all clinicians working in general practice.
The role of the GP as part of a multidisciplinary team
Few specialist clinics are tailored to support people living with type 1 diabetes as they grow older. GPs represent the healthcare frontline and are often an individual’s first point of contact within the health system.3 Older people living with type 1 diabetes are more likely to have multiple complex medical and psychosocial issues requiring multifaceted care. GPs provide comprehensive assessment and holistic care, which includes tailored treatment plans, co-ordination of specialist care and patient education. GPs will often develop an enduring, trusted relationship with their patients and are best placed for patient engagement. Primary care settings that use this patient-centred approach to systematically engage patients in decision making have improved patient outcomes. Additionally, GP input and communication between GPs and other healthcare providers are particularly important in the management of patients experiencing acute illness or hospitalisation, as their functional, cognitive and cardiorenal status may have changed and medication regimens may have been altered during this time. Patients may require close monitoring to ensure regular review and further therapeutic adjustments following hospitalisation and recovery.
Management of glucose levels
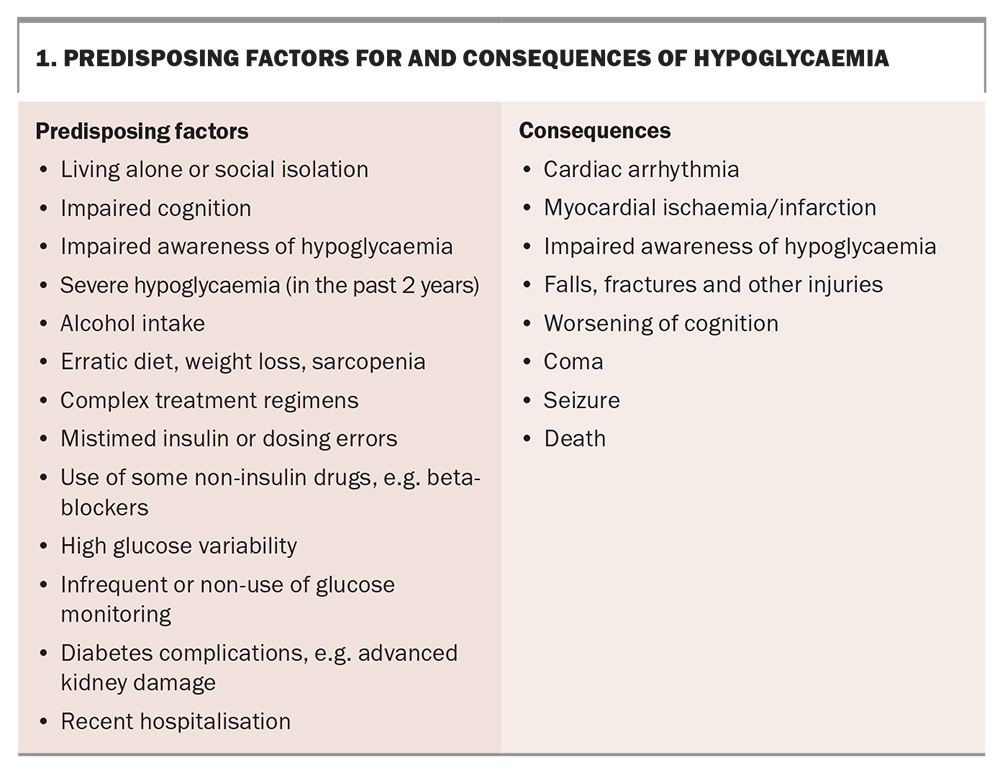
In managing glucose levels in the older person with type 1 diabetes, the prevention of acute complications, including diabetic ketoacidosis (DKA), hyperglycaemia and infections, is a priority as their consequences may be particularly catastrophic in this group. Both hypoglycaemia and hyperglycaemia can acutely impair cognition, and exposure to either over an extended period can contribute to the development of dementia.4,5 With increasing age, the gap in blood glucose levels between the sympathetic nervous system signalling awareness of hypoglycaemia (increased heart rate, sweatiness, and tremor) and the onset of neuroglycopenia impacting cognition decreases or, in the setting of impaired hypoglycaemia awareness, may be absent altogether. Impaired hypoglycaemia awareness is more prevalent in older people with diabetes.6,7 As a consequence, they are at greater risk of severe hypoglycaemia, which is associated with an altered conscious state and requiring third party assistance. Therefore, hypoglycaemia avoidance is the major priority (Box 1).
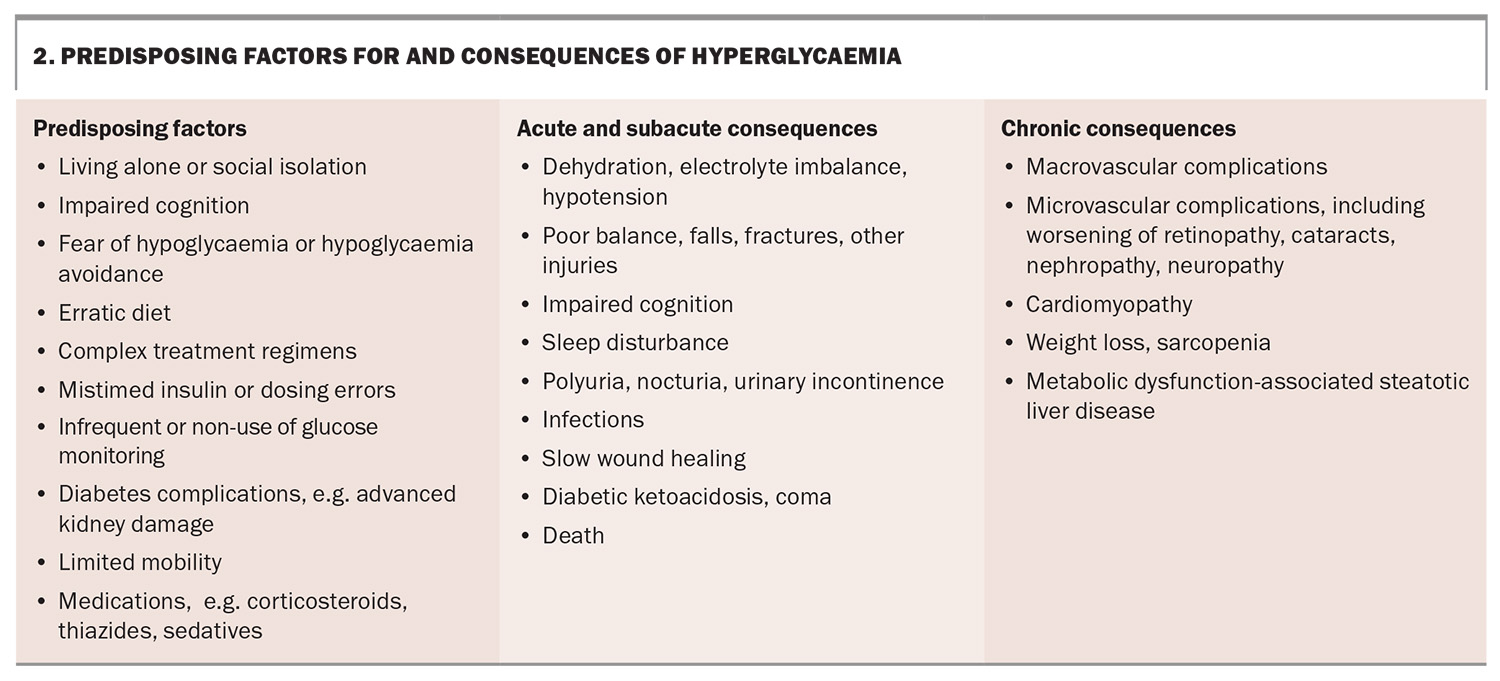
However, hyperglycaemia is also associated with risks, including infection, exacerbation of urinary incontinence, diminished cognitive function, dehydration and hypotension (Box 2), as well as the well-recognised complications of prolonged glycaemic exposure.8 Additionally, both high and low glucose levels can predispose patients to falls. We would caution against a nihilistic approach to chronic complication prevention based purely on a person’s age, as an older person who remains complication free may still have several years ahead of them and striving for the best possible glucose control will have its rewards. Ultimately, as with all other aspects of care, glucose management needs to be individualised, with the burden associated with management balanced against the benefits and risk reduction.
Glucose monitoring and target glucose levels
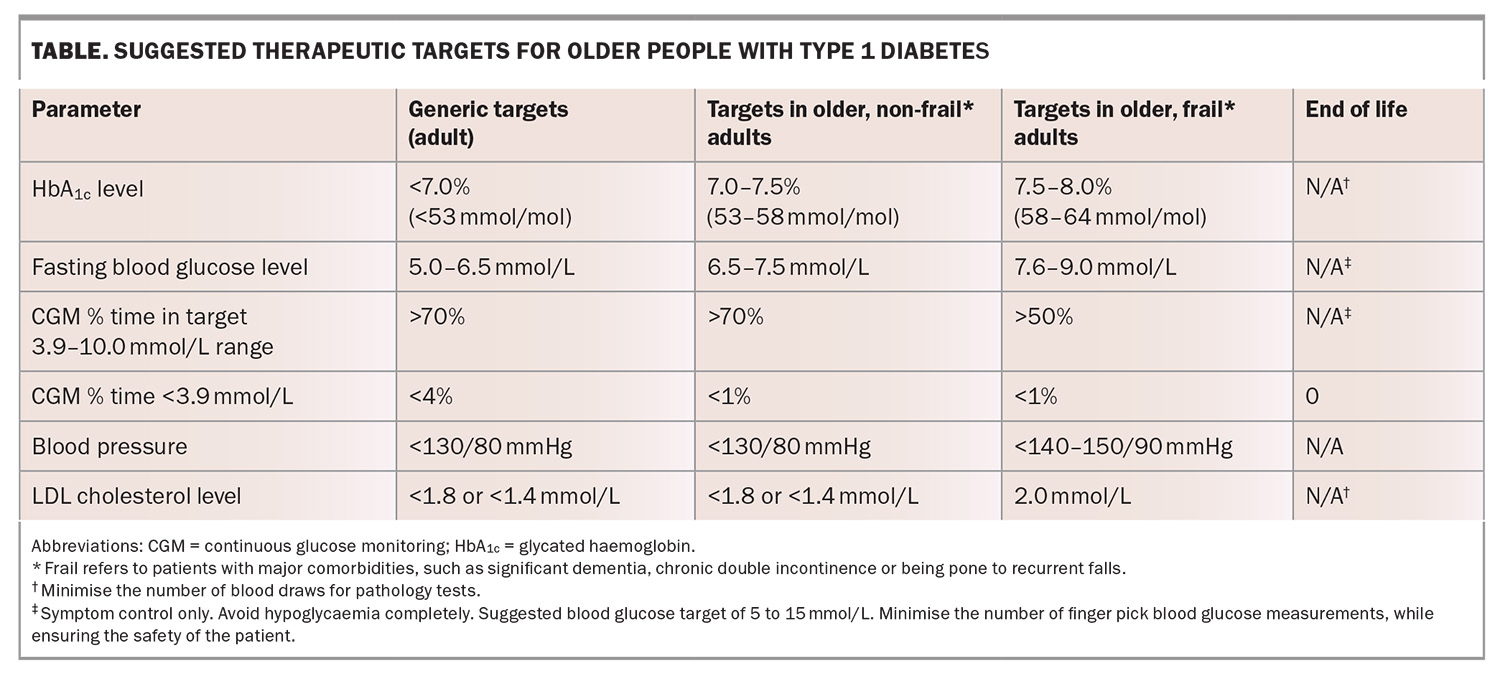
Historically, periodic glycated haemoglobin (HbA1c) measurements and finger-prick blood test glucose levels have been used for the assessment of glycaemia. In older people, the general target HbA1c level is between 7.0 to 7.5% (53 to 58 mmol/mol), although this may be liberalised to 7.5 to 8.0% (58 to 64 mmol/mol) in those with multiple comorbidities, and is even higher in people with a predicted limited life expectancy.7 Although an HbA1c level provides an assessment of glycaemic exposure and has a strong evidence base as a measure of chronic complication risk, it is less helpful as a guide for determining insulin dosing on a day-to-day basis.
Over 40 years ago, the care of people with diabetes was transformed with the advent of home blood glucose meters, which enabled self-monitoring. The general target for fasting blood glucose level is 5 to 6.5 mmol/L, whereas the target range for frail older adults and those with multiple comorbidities is 7.6 to 9.0 mmol/L to minimise hypoglycaemia risk and excessive hyperglycaemia.9 Finger-prick monitoring of blood glucose levels has its limitations. These include the need for the patient or their carer to proactively perform a test that is painful, with blood exposure, and which provides a single glucose reading without any trend information. For example, a glucose level of 4.0 mmol/L may have very different implications depending on whether the blood glucose levels are rising or falling and the rate at which this is occurring.
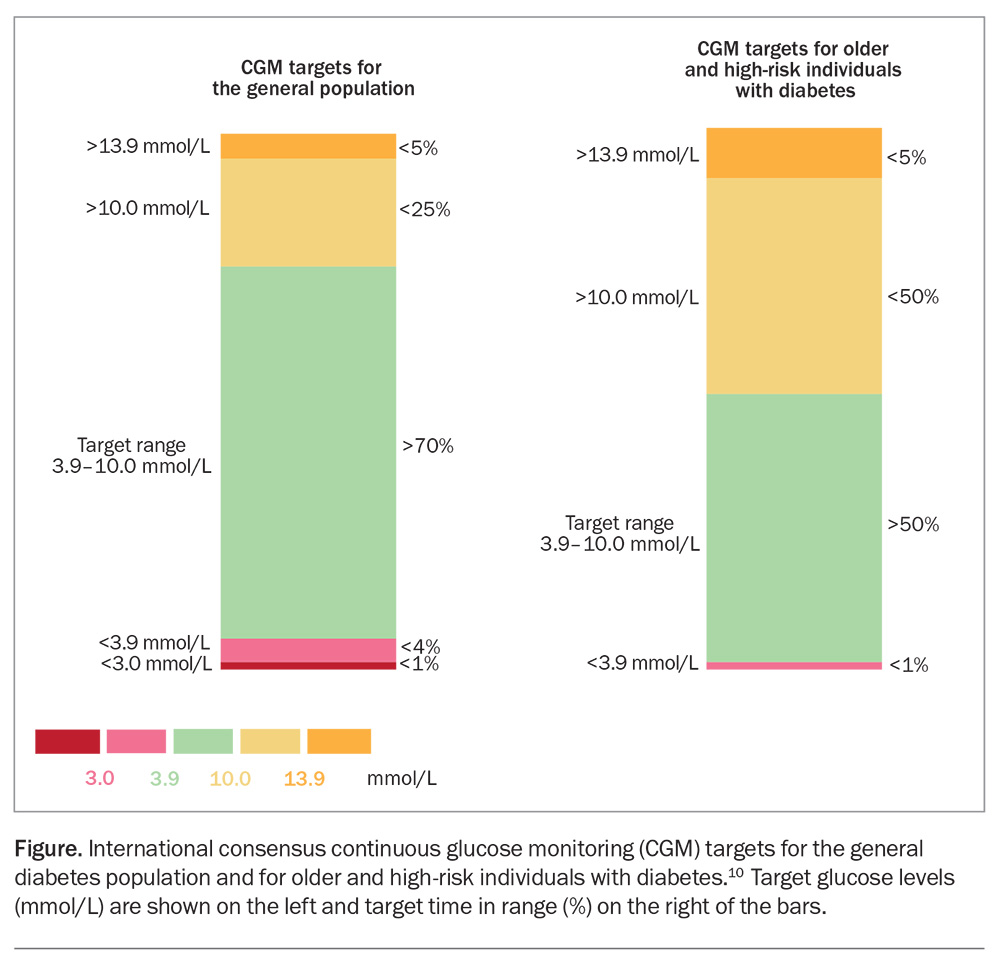
In July 2022, the Federal Government announced subsidised access to continuous glucose monitoring (CGM) devices for all Australian citizens with type 1 diabetes. CGM devices measure interstitial glucose levels on a near continuous basis using a disposable sensor inserted subcutaneously by the user and replaced every seven to 14 days. The devices do not require calibration with a finger-prick glucose measurement. Glucose readings can be transmitted to a smart phone, a dedicated reader or an insulin pump which automates insulin dosing. Unlike blood glucose meters, CGM devices provide trend information and can be programmed to raise an alarm if glucose levels reach user-specified high or low levels. Importantly, users, their family and clinicians can review historical data that have been uploaded to the cloud, processed and displayed in a standardised manner. Limitations of CGM include the dexterity to fit the device, the health literacy to operate the reader or connected smart device such as a phone or insulin pump, the need for a greater degree of engagement by the user, and a 5 to 7 minute lag between interstitial and blood glucose readings. The international consensus for CGM time in range (TIR) targets for the general population are over 70% at glucose level 3.9 to 10.0 mmol/L and TIR less than 4% below 3.9 mmol/L; for older and at-risk people the targets are TIR over 50% at 3.9 to 10.0 mmol/L and TIR less than 1% below 3.9 mmol/L (Figure).10
Diet
The dietary needs of older people with diabetes generally do not differ from a healthy diet in an older person without diabetes. Taste and appetite may diminish with age and dietary strategies may change from controlling weight gain to ensuring adequate nutrition and minimising frailty. Individuals should follow a tailored healthy eating plan developed in consultation with a dietitian, the patient and their family, which takes into account their taste preferences in the context of their culture, and is optimised for energy, protein and vitamin requirements.
Insulin regimens
Older people with type 1 diabetes have a wide-ranging ability to self-manage their glucose levels and availability of social and material resources. Most people with type 1 diabetes in Australia inject rapid-acting insulin with meals and basal insulin using an insulin pen as part of a multiple daily injection regimen.
Injected insulin regimens represent something of a trade-off. Simpler regimens, such as twice daily injections with biphasic insulins, minimise demands on the person with diabetes and their family. A twice-daily regimen may be a pragmatic choice for a person who requires supervision by a family member or a visiting nurse when injecting insulin and whose food intake and physical activity do not vary significantly on a daily basis. However, these regimens provide limited flexibility in glycaemic variability.
Conversely, many older people self-manage their glucose levels safely and live full and active lives. They may have been on a multiple daily injection regimen for most of their lives and are able to adjust their rapid-acting insulin level according to their food intake and physical activity levels. Such patients should continue their usual insulin regimens. For older people living in supported accommodation, the optimal insulin regimen will depend on the resources available in supported care, the number of daily injections acceptable to the person with diabetes and their family, and the target glucose levels.
A minority of people with type 1 diabetes use insulin pumps, which continuously deliver subcutaneous rapid-acting insulin at rates that can be varied. Current-generation pumps use automated insulin delivery (AID) systems to process CGM device information, which continuously adjusts insulin dosing to maintain glucose levels at target. There is a substantial body of evidence indicating that AID provides superior glucose levels compared with manual determination of insulin dosing.11 However, it should be noted that current commercial AID systems are by nature hybrid in their approach to automating insulin dosing because of limitations in the pharmacokinetics associated with the subcutaneous insulin delivery and require the user to announce meals and estimate their carbohydrate content and to announce exercise. Their use requires adequate cognition, numeracy and problem solving skills, manual dexterity and vision. Evidence for their use in older people, particularly those who are frail with multiple comorbidities, is limited. At present, although the Australian government subsidises CGM devices, insulin pumps, which are an essential component of any AID system, remain unsubsidised.
Screening and management of chronic complications
At a minimum, an annual risk factor assessment for chronic diabetes complications should be performed by a relevant member of the multidisciplinary team. This includes lying and standing blood pressure, a foot examination and an eye review. Relevant investigations include an HbA1c level, lipid profile, electrolytes and renal and liver tests, and a urine albumin creatinine ratio test. An annual assessment of thyroid function and nutritional parameters (pernicious anaemia and coeliac disease are more prevalent in older people with type 1 diabetes) may also be warranted given that type 1 diabetes is associated with other autoimmune conditions. Clinicians should have a low threshold for investigating the presence of coronary artery disease, which may be silent and manifest as shortness of breath.
Pharmacotherapy to minimise the development and progression of chronic complications remains important. The cost of medications and complex regimens may pose challenges. Combination preparations may facilitate adherence.
The consensus target blood pressure range for older frail people is less than 140 to 150/90 mmHg; however, can revert to less than 130/80 mmHg in those with no comorbidities.12 Angiotensin receptor blockers and angiotensin converting enzyme inhibitors remain first-line agents for blood pressure management, unless contraindicated. Blood pressure levels should be monitored closely in sitting and standing positions, and treatment should be tailored to prevent excessive postural drop in blood pressure, particularly with coexistent coronary artery and cerebrovascular disease.9,12 If the postural drop in blood pressure is significant, coexistent Addison’s disease (although rare) should be considered.
Statins for the optimisation of lipid profiles remain a cornerstone of cardiovascular risk reduction, with target LDL cholesterol levels of less than 1.8 mmol/L or, particularly if the person has known cardiovascular disease, 1.4 mmol/L, although this may be liberalised to 2.0 mmol/L in consideration of the person’s general health and circumstances.13 Fenofibrate has the dual benefits of increasing HDL cholesterol and reducing triglyceride levels, therby reducing the atherogenicity of the lipid profile and, although currently unproven in type 1 diabetes, may provide protection against renal and eye complications.
Older people with type 1 diabetes, especially if associated with poor glycaemic control or chronic complications, are at increased risk of infections and adverse related outcomes. Therefore, vaccination is recommended for all older people with type 1 diabetes. Many vaccines are funded for older people, including: COVID-19 vaccinations, which should be administered in accordance with current guidelines; influenza vaccinations, which are recommended to be administered yearly; pneumococcal vaccination, recommended as a single dose; and herpes zoster (shingles) vaccination as two doses about four months apart. Vaccination for respiratory syncytial virus is also advised and available under private prescription but not currently funded. Travel vaccines should be administered as needed. Therapeutic targets are summarised in the Table.
De-escalating therapy
Older people are more likely to face challenges with medication adherence as their physical wellbeing or cognition declines. Indeed, people with type 1 diabetes may have greater rates of cognitive decline and dementia than the general population.14 Medication adherence can be addressed by the provision of dosette boxes or Webster packs and third-party supervision, usually a family member. However, thought should also be given to de-escalating therapies that have become too complex and burdensome for the individual to adhere to or because the therapeutic goals targeted are no longer relevant.
De-escalation reduces polypharmacy, the risk of adverse side effects and the financial burden placed on patients and their families. Although guidelines exist for initiation, intensification or modification of treatments, including insulin regimens, lipid-lowering and antihypertensive therapies, evidence-based recommendations for de-escalating therapy in people with type 1 diabetes as they become older are lacking and research is needed in this area. Any de-escalation in therapy should be discussed with the patient and their family in a sensitive manner.
With regard to glucose monitoring, continuing with CGM is recommended as the cost is subsidised, it is less painful than blood glucose monitoring, it provides alerts if the person’s glucose levels are dropping too low or too high and it can be readily implemented by a family member if necessary. Readings from some devices can be followed remotely by a carer or family member, including with real-time alerts for risky low or high glucose levels.
Insulin regimens may need simplification, with reassessment of multiple daily injection regimens, particularly in the setting of cognitive decline, when insulin injections can be missed or double dosing – when the patient forgets that they have already given an injection – can occur. A guardian, family member or visiting nurse may only be available to supervise insulin injections once or twice daily. Although less aligned with the person’s insulin requirements, implementing a once or twice daily injection regimen can minimise risk and offer greater safety and certainty.
Current-generation insulin pumps providing AID functionality minimise the risk of double dosing and automatically adjust insulin delivery in response to changes in sensor glucose levels. However, they are complex devices and operation requires a definite minimum level of cognition, visual acuity and manual dexterity. Only rapid-acting insulin is delivered, and a failure in insulin delivery (e.g. because an insulin cannula has been left in situ too long and therefore failed) can result in DKA. Therefore, continued use of the insulin pump should be reviewed regularly and changes in the person’s cognitive and functional status considered. If the patient or family member is not able to operate their pump safely, consideration should be given to reverting to supervised insulin injections.
Ceasing lipid-lowering therapies used for primary prevention may be advisable for patients without high cardiovascular risk, particularly if their life expectancy is limited in the setting of cognitive decline, multiple comorbidities unrelated to cardiovascular disease and polypharmacy. Continued use of lipid-lowering therapies in the setting of secondary prevention is recommended.
The prescription of antihypertensive agents should be reviewed. If the upright blood pressure is at or below target, reducing the number of medications or using combined preparations should be considered to simplify regimens.
End of life care
For patients at end of life, the highest priority is given to ensure their comfort and minimise distress. Ensuring optimal glucose control to prevent chronic complications is no longer a priority. The frequency of finger-prick monitoring may be reduced or, preferably, eliminated altogether with the use of CGM. Loss of subcutaneous fat may impact the insertion of sensors and insulin delivery cannulas. The management of glucose levels should focus on avoiding hypoglycaemia, symptomatic hyperglycaemia and DKA. A blood glucose level ranging 5 to 15 mmol/L is desirable. Asymptomatic elevated glucose levels are acceptable in the interests of liberalising the person’s diet and reducing the burden associated with therapies. Insulin should not be withdrawn completely, but dosing should be reviewed, accounting for factors such as weight loss and anorexia associated with a terminal illness. For those on injections, long-acting insulin should never be stopped, but rapid-acting insulin administered with meals may be omitted. Statins may be withdrawn, and antihypertensive agents may be reduced or ceased altogether.15
Conclusion
Older people constitute a substantial proportion of those with type 1 diabetes and the prevalence is expected to increase. They represent a heterogeneous group with a wide range of cognitive, functional and health status, which should be periodically evaluated. Older people with type 1 diabetes also require regular review of treatment targets and regimens, including at end of life, to optimise their health outcomes and quality of life. MT
COMPETING INTERESTS: Associate Professor Kilov: None. Professor O’Neal has received lecture fees from, and is on the Advisory Boards of, Abbott, Insulet and Medtronic; and received grants from Abbott, Insulet, Medtronic and Sanofi-Aventis. Professor Jenkins has received grant funding from Mylan/Abbott; is on the Advisory Boards of Abbott, CSL and GSK; and is Honorary President for Insulin For Life Global and International Diabetes Federation Western Pacific Region Chair Elect.
References
1. National Diabetes Services Scheme (NDSS). Type 1 Diabetes. NDSS; Canberra, March 2024. Available online at: https://www.ndss.com.au/wp-content/uploads/Type-1-Diabetes-Snapshot-202403.pdf (accessed June 2024).
2. Yu R, Wong M, Chong KC, et al. Trajectories of frailty among Chinese older people in Hong Kong between 2001 and 2012: an age-period-cohort analysis. Age Ageing 2018; 47: 254-261.
3. Australian Institute of Health and Welfare (AIHW). General practice, allied health and other primary care services. Australian Government, 2024. Available online at: https://www.aihw.gov.au/reports/primary-health-care/general-practice-allied-health-primary-care (accessed June 2024).
4. Lacy ME, Gilsanz P, Karter AJ, Quesenberry CP, Pletcher MJ, Whitmer RA. Long-term glycemic control and dementia risk in type 1 diabetes. Diabetes Care 2018; 41: 2339-2345.
5. Husain KH, Sarhan SF, AlKhalifa HKAA, Buhasan A, Moin ASM, Butler AE. Dementia in diabetes: the role of hypoglycemia. Int J Mol Sci 2023; 24: 9846.
6. Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care 2005; 28: 2948-2961.
7. Weinstock RS, DuBose SN, Bergenstal RM, et al; T1D Exchange Severe Hypoglycemia in Older Adults With Type 1 Diabetes Study Group. Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care 2016; 39: 603-610.
8. Vischer UM, Bauduceau B, Bourdel-Marchasson I, et al. A call to incorporate the prevention and treatment of geriatric disorders in the management of diabetes in the elderly. Diabetes Metab 2009; 35: 168-177.
9. Dhaliwal R, Weinstock RS. Management of type 1 diabetes in older adults. Diabetes Spectr 2014; 27: 9-20.
10. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019 Aug; 42: 1593-1603.
11. Pease A, Lo C, Earnest A, Kiriakova V, Liew D, Zoungas S. The efficacy of technology in type 1 diabetes: a systematic review, network meta-analysis, and narrative synthesis. Diabetes Technol Ther 2020; 22: 411-421.
12. Solini A, Grossman E. What should be the target blood pressure in elderly patients with diabetes? Diabetes Care 2016; 39 Suppl 2: S234-S243.
13. Lan NSR, Bell DA, Watts GF, Fegan PG. Lipid-lowering therapies and cardiovascular risk-stratification strategies in adults with type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 2023; 30: 103-112.
14. Shalimova A, Graff B, Gąsecki D, et al. Cognitive Dysfunction in Type 1 Diabetes Mellitus. J Clin Endocrinol Metab 2019; 104: 2239-2249.
15. Dunning TL. Palliative and end-of-life care: vital aspects of holistic diabetes care of older people with diabetes. Diabetes Spectr 2020; 33: 246-254.