Therapies for psoriasis. Beyond lesion control
Conventional therapies for psoriasis that aim to decrease skin turnover have not met the needs of all patients. Novel biologic therapies that target the underlying immune mechanisms of psoriasis can be effective and well tolerated. They include the cytokine modulators infliximab, adalimumab, etanercept, ustekinumab, guselkumab, tildrakizumab, risankizumab, secukinumab and ixekizumab, and the enzyme inhibitor apremilast.
- Psoriasis is a common disease involving chronic inflammation of the skin, nails or joints that often decreases quality of life.
- Traditional management aims to decrease skin turnover and control disease, rather than cure; options include topical medications, phototherapy and, for patients with resistant or widespread disease, systemic acitretin, methotrexate or ciclosporin.
- Moderate-to-severe psoriasis or involvement of special sites such as the scalp, genitals, palms or soles warrants specialist referral.
- Recent improved understanding of the immune mechanisms underlying psoriasis has led to novel biologic therapies that specifically target cytokines and enzymes involved in its pathophysiology.
- Targeted therapies for psoriasis include inhibitors of tumour necrosis factor-alpha (infliximab, adalimumab, etanercept), interleukin-23 (ustekinumab, guselkumab, tildrakizumab, risankizumab), interleukin-17 (secukinumab, ixekizumab), phosphodiesterase 4 (apremilast) and janus kinase (deucravacitinib and tofacitinib, which are not currently approved in Australia).
- Targeted therapies are effective, well tolerated and may have a better long-term safety profile than conventional systemic therapies.
Psoriasis is a common condition, affecting 2 to 4% of the population, in which alterations in immune regulation manifest as skin disease.1 Psoriasis also causes morbidity through associated systemic diseases and can have a major impact on patients, decreasing quality of life.2 It is recognised that psoriasis is linked to psoriatic arthritis, inflammatory bowel disease, vascular inflammation and cardiac disease.3 Long-term therapy for psoriasis also has social and quality of life implications and an economic impact on the healthcare system.
In recent decades, insight into the immunological mechanisms underlying psoriasis has increased, and the range of therapies has expanded to include novel biologic treatments that specifically target these mechanisms. These changes have revolutionised the treatment of psoriasis, decreasing morbidity and impact on the healthcare system.
This article reviews the pathophysiology of psoriasis and conventional and novel treatments available for management of patients with psoriasis in Australia. Current treatments for psoriasis are summarised in the Box.
Clinical features
The main types of psoriasis are plaque-type psoriasis, guttate psoriasis, inverse psoriasis, localised pustular psoriasis and generalised pustular psoriasis.4 Plaque-type psoriasis is the most common of these, and pustular or erythrodermic types are the least common.5
Pathologically, psoriasis is characterised by:
- parakeratosis – hyperproliferative epidermis and premature maturation of keratinocytes with retention of cell nuclei in the stratum corneum
- acanthosis – thickened epidermis due to an increased mitotic rate of basal keratinocytes
- dermal inflammatory infiltrate.6
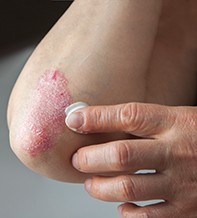
Clinically, psoriasis manifests as raised, well-demarcated, erythematous and scaly plaques that typically affect extensor surfaces (Figure 1 and Figure 2). Visible areas and special sites such as the scalp, genitals, palms and soles can pose a therapeutic challenge and have a high impact on quality of life. Psoriasis can also cause psoriatic nail dystrophy, which is often unsightly and can lead to psychological distress, pain and functional impairment.2 Recognised comorbidities with psoriasis include psoriatic arthritis, metabolic syndrome and depression. Part of the GP’s role is to recognise and manage comorbidities to improve patient outcomes.
Pathophysiology
The pathophysiology of psoriasis is underpinned by a complex interplay between environmental and genetic factors that act as disease initiating triggers. Environmental triggers include stress, bacterial products, drugs, trauma and smoking. Genetic studies have identified an association of psoriasis with several specific chromosomal loci, including psoriasis susceptibility locus 1 (PSORS1), and also with variants in the genes encoding the interleukin-23 (IL-23) and interleukin-12 (IL-12) receptors. Activation of a dysregulated immune system and the interplay between the immune system, skin epithelium and connective tissue shape and maintain the inflammatory disease process.3
Over the past two decades, it has been recognised that the immune process in psoriasis results from the functional role of dendritic cells, T cells and cytokines (Figure 3).7 After an initial trigger, plasma-cytoid dendritic cells become activated and secrete interferon-alpha, activating myeloid dendritic cells. Subsequent secretion of IL-12 and IL-23 induces naïve T cells to differentiate into effector cells, such as type 1 and type 17 T-helper cells and cytotoxic T cells. The type 1 T cells release interferon-gamma and tumour necrosis factor (TNF)-alpha, and the type 17 T cells produce interleukins-17A, 17F and 22 (Figure 4).7 These interleukins and TNF-alpha lead to keratinocyte activation and proliferation and subsequent production of antimicrobial peptides and chemokines. T cells migrate from the dermis to the epidermis in psoriasis. The microvasculature in psoriasis is also seen as leaky, and dendritic cells and T cells form perivascular clusters around blood vessels.2,7
Conventional treatments
The traditional aim of psoriasis therapy has revolved around decreasing skin turnover. Medications used include topical corticosteroids, coal tar (with or without salicylic acid), vitamin D derivatives, vitamin A derivatives and dithranol. Phototherapy with narrow-band ultraviolet B (UVB) or psoralen plus ultraviolet A (PUVA) is also used. These therapies may be used as monotherapy or in combinations.
Patients with more severe or generalised psoriasis may require systemic therapy with the oral vitamin A derivative acitretin or an immunosuppressant such as methotrexate or ciclosporin. Referral to a dermatologist for management is encouraged for this group.2,3
Other patients with psoriasis who require specialist referral because of the therapeutic challenge include:
- patients with severe nail psoriasis or pustular psoriasis
- children
- pregnant women
- patients with acquired immunodeficiency.8
Nonpharmacological treatments
Patient education and nonpharmacological management should be explored in all patients with psoriasis. This includes avoidance of triggers such as stress, infection, trauma, xerosis and potential culprit medications.
Topical corticosteroids
Topical corticosteroids are used to reduce inflammation. Low-potency corticosteroids should be used on the face, axillae and groin, where the skin is more sensitive, and concomitantly higher potency agents are typically needed on thicker plaques and patches on the palms and soles. Adverse effects of inappropriate use may include thinning of skin and the development of striae; caution is required to avoid a rebound flare following cessation. Topical corticosteroids are often used in conjunction with other treatments listed below that have complementary effects.9
Coal tar with or without salicylic acid
Coal tar therapy is available in various forms and combinations with other agents such as salicylic acid. Coal tar in its natural form is thick and black and can be obtained over the counter. Coal tar in combination with agents such as salicylic acid requires a prescription.
The exact mechanism of action of tar in patients with psoriasis is not completely understood, but it is believed to suppress DNA synthesis and thereby to inhibit keratinocyte proliferation.10 As well as this keratoplastic effect, tar is proposed to have antimicrobial and antipruritic effects.11 Salicylic acid is a keratolytic that causes shedding of the epidermis and scale, improving penetration of other topical medications.
The benefit of tar therapy is its low cost, but it may cause local irritation, has an unpleasant smell and can stain skin and clothing.12
Vitamin D derivatives (calcipotriol)
Natural vitamin D3 (calcitriol) requires activation through skin exposure to ultraviolet light. Calcipotriol is an already active form of vitamin D. Calcipotriol is included in topical treatments that are used once or twice daily, often in combination with corticosteroids. A fixed combination of calcipotriol with betamethasone dipropionate can be used daily.9 Adverse effects of calcipotriol include local irritation and disturbances of calcium metabolism. However, only 1% of calcipotriol is absorbed through the skin and the risk of hypercalcaemia is significantly lower than for natural vitamin D3 (100 to 200 times lower risk).13,14 As salicylic acid deactivates calcipotriol, the two medications should not be used concurrently.
Vitamin A (retinol) and synthetic derivatives
Vitamin A is needed for normal skin growth. Vitamin A (retinol) and synthetic vitamin A derivatives bind to nuclear retinoic acid receptors in the skin and reduce the rate at which skin cells develop and renew themselves; they also reduce inflammation. Tazarotene is the first topical receptor-selective retinoid approved for use to treat psoriasis. It binds only to the cell receptors in the epidermis and hence has a lower adverse effect profile.3 However, local irritation is still common, and it is usually used along with topical corticosteroids to counteract this.
Oral retinoids such as acitretin are usually reserved for patients with severe psoriasis and are particularly useful for those with the pustular or erythrodermic type of psoriasis.15 Adverse effects can include more significant local irritation, with redness and dry skin, and emollients should be used. Caution is needed with oral retinoids in patients with liver abnormalities, and they must not be used by women who are pregnant or for at least two years before pregnancy, because of potential teratogenic effects.
Topical and oral retinoids are typically used in combination with other therapies, such as topical corticosteroids and phototherapy.16
Dithranol
Dithranol (also known as anthralin) is a natural anthraquinone derivative that controls skin growth by reducing DNA synthesis and mitotic activity in the hyperplastic epidermis. This restores a normal rate of cell proliferation and keratinisation. Dithranol also has anti-inflammatory effects. It is available as a cream, ointment or paste, but is less commonly used today.17
Phototherapy (narrow-band UVB or psoralen plus UVA)
Patients with psoriasis that is widespread or difficult to control should be referred to a dermatologist. Phototherapy, methotrexate and ciclosporin are reserved for these groups of patients.
In phototherapy, UV light reduces inflammation and slows the production of skin cells. Treatment involves exposing the skin to UV light for a set duration on a regular schedule, usually three times a week. The most common modality is narrow-band UVB (311nm). UV light seems effective in combination with topical medications. Psoralen increases the skin’s responsiveness to UVA light. For this reason, psoralen plus UVA is generally more effective than UVB but has a greater risk of adverse effects. Phototherapy can be time consuming for patients and may cause phototoxicity, photoageing and increased risk of skin malignancy.3 As the risk of skin malignancy increases with greater DNA damage, PUVA carries a greater risk than narrow-band UVB therapy.4 All patients receiving phototherapy should use standard sun protection measures.
Methotrexate
Systemic options such as methotrexate and ciclosporin are useful to treat psoriasis as they prevent T cell activation. Methotrexate is a folic acid antagonist that inhibits DNA synthesis and cell replication by competitively inhibiting the conversion of folic acid to folinic acid. It has cytotoxic, immunosuppressive and anti-inflammatory actions that are beneficial in psoriasis. A low-dose weekly regimen of either oral or subcutaneous methotrexate is recommended. Adverse effects include hepatic impairment, bone marrow suppression with neutropenia or pancytopenia, renal impairment and pulmonary toxicity.18
Ciclosporin
Ciclosporin is a calcineurin inhibitor that blocks the action of calcineurin in activated T cells, hence downregulating inflammatory interleukins and T-cell proliferation. As with methotrexate, care should be taken with toxicity and adverse effects such as renal impairment and hypertension.19,20
Novel targeted therapies
Improved understanding of the complex immune process underlying psoriasis has suggested a therapeutic role for agents that target the immune system. This pathogenesis-based approach to therapy for psoriasis is effective and overall well tolerated, validating the theories about the underlying immune mechanisms.
The biologic therapies in current use target cytokines. Therapies that target T cells, such as alefacept, are no longer used as more effective targeted therapies have become available. The initial cytokine-targeted therapies targeted TNF. Therapies that target interleukins were then explored; these are delivered subcutaneously. Janus kinase (JAK) inhibitors and phosphodiesterase-4 (PD4) inhibitors are oral targeted therapies that may also have a role in changing the face of psoriasis treatment.
Biologic therapies for psoriasis are available on the PBS for patients who fulfil specific criteria, which typically include failure of several other systemic treatments. This includes failure to achieve an adequate response, intolerance or a contraindication to two of the following four treatments for psoriasis: acitretin, phototherapy, methotrexate or ciclosporin (with specific doses and durations applicable). In addition, biologic therapies for psoriasis must be prescribed by a dermatologist.21
Anti-tumour necrosis factor-alpha
Anti-TNF-alpha therapies are cytokine modulators that bind to TNF-alpha and inhibit its activity. They include infliximab, adalimumab and etanercept. Anti-TNF-alpha therapies therefore reduce activation of myeloid dendritic cells and downstream inflammatory pathways. Adverse effects include potential infections, exacerbation of psoriasis (because of blocking of other anti-inflammatory effects), triggering of autoimmune processes, cutaneous malignancies and blood dyscrasias.22,23 Cardiac failure is also reported but is infrequent.
Anti-interleukin-23
Anti-IL-23 therapies include ustekinumab, guselkumab, tildrakizumab and risankizumab. Anti-IL-23 therapies bind to the p40 subunit of IL-12 and IL-23 or to the p19 subunit of IL-23, preventing these interleukins binding to cell-surface receptors. This prevents IL-23 or IL-12-mediated activation and differentiation of T cells, interrupting signalling and cytokine cascades involved in psoriasis pathology.7 Side effects to consider include reactivation of infections such as inactive hepatitis B or latent tuberculosis.
Anti-interleukin-17
Anti-IL-17 therapies include secukinumab and ixekizumab. Like anti-IL-23 therapies, anti-IL-17 therapies act as cytokine modulators by selectively binding and neutralising cytokine IL-17A. This blocks IL-17A from forming a complex with the IL-17 receptor, and thereby inhibits activation of keratinocytes and release of proinflammatory cytokines, chemokines and mediators of tissue damage.3 Adverse effects are similar to those of IL-23 agents; ixekizumab can cause site reactions. Another anti-IL-17 agent, brodalumab, is available in other countries but has not been taken to market in Australia because of a black box warning following a study that reported two suicides, despite its impressive efficacy in clinical trials.
Janus kinase inhibitors
JAK inhibitors include tofacitinib and deucravacitinib. JAK enzymes include four types: JAK 1, JAK 2, JAK 3 and Tyk2. They act in pairs on the intracytoplasmic portion of cytokine receptors. Each JAK pair can be activated by different cytokines and in turn activates different signal transducer and activator of transcription (STAT) proteins. Activated STAT proteins control the expression of nuclear gene targets, inducing the transcription of pro-inflammatory genes. Hence, JAK inhibition decreases production of inflammatory cytokines.7 Adverse effects are determined by the selectivity profile but can include bone marrow dysfunction and increased risk of gastrointestinal perforation.24 Other drugs metabolised by the cytochrome P450 3A4 (CYP3A4) enzyme should be avoided. None of the JAK inhibitors are currently approved in Australia for use in psoriasis.
Phosphodiesterase-4 inhibitors
PD4 inhibitors act as cytokine modulators by inhibiting PD4 in many cells, including T cells and keratinocytes. This in turn reduces production of pro-inflammatory cytokines, including TNF-alpha, interferon-gamma, IL-17 and IL-23, and also increases anti-inflammatory cytokines such as IL-10. PD4 inhibitors include apremilast, which is available on the PBS for patients who have not responded to methotrexate, and is prescribed by a dermatologist. Adverse effects include gastrointestinal upset, renal impairment and depression.25
Treatment monitoring
Psoriasis causes significant discomfort and has associated comorbidities, which can affect patients’ quality of life. Treatment monitoring should include not only skin assessment but also a measure of quality of life.2
The skin can be assessed with the Psoriasis Area and Severity Index (PASI). This rates severity on a scale of 0 to 72 based on area of coverage and plaque appearance (erythema, thickness and scaling) for each body region. A pro forma is available at: www.humanservices.gov.au/organisations/health-professionals/forms/pb115 (Figure 5).26 Quality of life can be assessed with the Dermatology Life Quality Index (DLQI), a 10-question questionnaire that measures the impact of a skin problem on the patient’s life over the previous week (http://sites.cardiff.ac.uk/dermatology/quality-of-life-dermatology-quality-of-life-index-dlqi).
The 2013 Australian consensus on treatment goals for psoriasis agreed that treatment success is a reduction in PASI score of 75% or more at the end of the induction phase of treatment.27 Treatment failure was defined as a reduction in PASI score of 50% or less at the end of induction, indicating the need to modify the treatment regimen. It was also considered highly relevant to assess quality of life when assessing psoriasis severity and treatment, because an absolute PASI score and skin appearance may not be clinically meaningful measures if they do not correlate with quality of life.27
Since the introduction of anti-interleukin therapies, greater improvements in PASI score are expected. A reduction in PASI score of 90% or more has been considered a more appropriate standard.28 Some studies include a 100% reduction in PASI score as a secondary endpoint. This change highlights our higher expectations; as new therapies evolve, we can aim for patients with psoriasis to have clear, or almost clear, skin. This is a shift from the traditional view that only lesion control is achievable.4
Conclusion
Psoriasis is a chronic inflammatory disease that has not only physical but also psychological, social and economic implications. Conventional topical and systemic immunosuppressants have not met the treatment needs of all patients with psoriasis. However, newer pathogenesis-targeted therapies that act as cytokine modulators have shown their effectiveness. Although these biologic therapies must be prescribed by specialists, it is important for the wider medical community to understand their role in the evolving management of psoriasis. These newer therapies have shown appropriate long-term risk-benefit and cost profiles. An exciting frontier includes further research into the role of these therapies in treating patients with associated inflammatory diseases.29 MT
COMPETING INTERESTS: None.
References
1. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Identification and Management of Psoriasis and Associated Comorbidity (IMPACT) project team. J Invest Dermatol 2013; 133: 377-385.
2. Berth-Jones J. Psoriasis. Medicine 2009; 37: 235-241
3. Ayala-Fontanez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckl) 2016; 6: 7-32.
4. Pardasani PAC, Feldman SR, Clark AR. Treatment of psoriasis: an algorithm-based approach to primary care physicians. Am Fam Physician 2000; 61: 725-733.
5. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263-271.
6. Helwig EB. Pathology of psoriasis. Ann N Y Acad Sci 1958; 73: 923-935.
7. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496-509.
8. Schön MP, Boehncke W-H. Psoriasis. N Engl J Med 2005; 352: 1899-1912.
9. Segaert S, Shear NH, Chiricozzi A, et al. Optimizing anti-inflammatory and immunomodulatory effects of corticosteroid and vitamin D analogue fixed-dose combination therapy. Dermatol Ther (Heidelb) 2017; 7: 265-279.
10. Smith CH, Jackson K, Chinn S, Angus K, Barker JN. A double blind, randomized, controlled clinical trial to assess the efficacy of a new coal tar preparation (Exorex) in the treatment of chronic, plaque type psoriasis. Clin Exp Dermatol 2000; 25: 580-583.
11. Paghdal KV, Schwartz RA. Topical tar: back to the future. J Am Acad Dermatol 2009; 61: 294-302.
12. Ngan V. Coal tar treatment. DermNet NZ; 2005. Available online at: https://www.dermnetnz.org/topics/coal-tar (accessed June 2018).
13. Oakley A. Calcipotriol. DermNet NZ; 2001. Available online at: https://www.dermnetnz.org/topics/calcipotriol (accessed June 2018).
14. Menne T, Larsen K. Psoriasis treatment with vitamin D derivatives. Semin Dermatol 1992; 11: 278-283.
15. Ofranos CE, Pullmann H, Runne U, et al. Treatment of psoriasis using vitamin A, vitamin A acid and oral retinoids. Hautarzt 1979; 30: 124-133.
16. Arechalde A, Saurat JH. Management of psoriasis: the position of retinoid drugs. BioDrugs 2000; 13: 327-333.
17. Oakley A. Dithranol. DermNet NZ; 1998. Available online at: https://www.dermnetnz.org/topics/dithranol (accessed June 2018).
18. Montaudié H, Sbidian E, Paul C, et al. Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J Eur Acad Dermatol Venereol 2011; 25(Suppl 2): 12.
19. Fellström B. Cyclosporine nephrotoxicity. Transplant Proc 2004; 36(Suppl 2): 220S-223S.
20. Curtis JJ, Luke RG, Jones P, Diethelm AG. Hypertension in cyclosporine-treated renal transplant recipients is sodium dependent. Am J Med 1988; 85: 134-138.
21. Australian Government Department of Health. PBS Schedule: ixekizumab. Available online at: http://www.pbs.gov.au/medicine/item/11032P-11033Q (accessed June 2018).
22. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat 2009; 20: 100-108.
23. Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatolog Treat 2004; 15: 280-294.
24. Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68: 2612-2617.
25. Forchhammer S, Ghoreschi K. Update on the treatment of psoriasis and psoriatic arthritis – role of apremilast. Psoriasis (Auckl) 2015; 5: 117-124.
26. Australian Government Department of Human Services. PASI calculation and body diagram – whole body form. Canberra: Australian Government; 2018. Available online at: https://www.humanservices.gov.au/organisations/health-professionals/forms/pb115 (accessed June 2018).
27. Baker C, Mack A, Cooper A, et al. Treatment goals for moderate to severe psoriasis: an Australian consensus. Australas J Dermatol 2013; 54: 148-154.
28. Puig, L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 645-648.
29. Siegel M. Promising new directions in psoriasis research. Psoriasis updates from the 76th annual meeting of the American Academy of Dermatology. National Psoriasis Foundation. 2018 April. Available online at: https://www.psoriasis.org/advance/promising-new-directions-psoriasis-research (accessed June 2018).