Psoriasis: systemic treatment options for adults
Mild psoriasis can usually be managed using topical agents, but moderate-to-severe psoriasis typically requires systemic therapies. There are several nonbiologic and biologic therapies available in Australia for psoriasis.
- Chronic plaque psoriasis can be classified as mild, moderate or severe disease based on the body surface area affected, psoriasis area severity index, effect on quality of life and location on high-impact sites.
- Psoriasis is not purely a cutaneous disease and has multiple associated comorbidities, including psoriatic arthritis, metabolic syndrome, cardiovascular disease, diabetes, obesity and inflammatory bowel disease.
- GPs play a key role in identifying and managing psoriasis and any disease or treatment complications, as well as any associated comorbidities in patients.
- Nonbiologic systemic treatment options for patients with moderate-to-severe psoriasis include methotrexate, ciclosporin, acitretin, apremilast, deucravacitinib and ultraviolet B phototherapy.
- Patients must fulfil specific criteria for severe chronic plaque psoriasis to qualify for treatment with a biologic agent in Australia through the PBS.
- Biologic systemic treatment options available in Australia include tumour necrosis factor-alpha inhibitors (infliximab, adalimumab, certolizumab), interleukin (IL)-12/23 inhibitors (ustekinumab), IL-17 inhibitors (secukinumab, ixekizumab, bimekizumab) and IL-23 inhibitors (risankizumab, guselkumab, tildrakizumab).
Chronic plaque psoriasis affects about 2% of the population. The severity can be determined based on the percentage of body surface area (BSA) affected, psoriasis area severity index (PASI) and effect on quality of life. A BSA of less than 3% is considered mild disease, 3 to 10% is considered moderate and greater than 10% is considered severe disease.1 Plaque psoriasis with a PASI score greater than 10 is considered at least moderate in severity. Newer definitions include high- and low-impact sites. High-impact sites include the face, scalp, palms, soles and genital areas, and are associated with a poorer quality of life.2 This article provides an overview of nonbiologic and biologic systemic treatment options for psoriasis in adults.
Comorbidities of psoriasis
Psoriasis is a systemic inflammatory condition, rather than a purely cutaneous disease, and is associated with multiple comorbidities. It is important to identify these comorbidities, as they can be reversible and, if untreated, can result in significant morbidity and mortality.
Psoriatic arthritis (PsA) affects up to 42% of all patients with psoriasis.3 There are different types of PsA including distal interphalangeal, asymmetric oligoarticular, symmetric polyarthritis, spondylitis and arthritis mutilans types. PsA questionnaires, such as the Psoriasis Epidemiology Screening Tool, Psoriatic Arthritis Screening and Evaluation, and Early Psoriatic Arthritis Screening Questionnaire, can help detect early symptoms of PsA and help clinicians in deciding whether a rheumatology referral is required. Clinical signs that can indicate an increased risk of developing PsA include enthesitis, dactylitis (sausage digits), scalp or flexural psoriasis and nail changes, such as pitting, onycholysis and subungual hyperkeratosis.
Psoriasis is also associated with metabolic syndrome, cardiovascular disease, diabetes and obesity. Psoriasis is an independent risk factor for myocardial infarction, stroke and peripheral vascular disease because of the involvement of common inflammatory mediators and cytokines. Patients with severe psoriasis are twice as likely to develop diabetes and three times more likely to develop heart failure than those without psoriasis. For every 10% increase in the BSA affected by psoriasis, there is an additional 20% risk of developing diabetes.4 Obesity is also an independent risk factor for psoriasis and PsA. Weight loss improves pre-existing psoriasis and prevents new-onset psoriasis.5 Psoriasis also occurs in almost 10% of patients with Crohn’s disease. Patients with psoriasis are almost 2.5 times more likely to have Crohn’s disease and have 1.7 times the risk of developing ulcerative colitis.6
Role of GPs in managing moderate-to-severe psoriasis
GPs play a key role in managing psoriasis. They are in the unique position of knowing the clinical guidelines for different comorbidities and have often established longer-term relationships with patients.
Prompt referral to a dermatologist is suggested if the psoriasis is unresponsive to topical therapies, there is a significant impact on the patient’s quality of life or there is moderate to severe disease with a greater than 10% BSA involvement. In the context of moderate-to-severe psoriasis, GPs can:
- identify and manage any comorbidities
- assess patients for potential complications of psoriasis, including metabolic syndrome, cardiovascular disease, arthritis and mental health disease
- ensure patients are up to date with their malignancy screens and vaccinations.
GPs can also prescribe certain continuing treatments for psoriasis following initiation by a dermatologist, as well as monitoring for treatment response and treatment complications. Live vaccines should not be given to patients who are taking immunosuppressive or immunomodulatory agents.
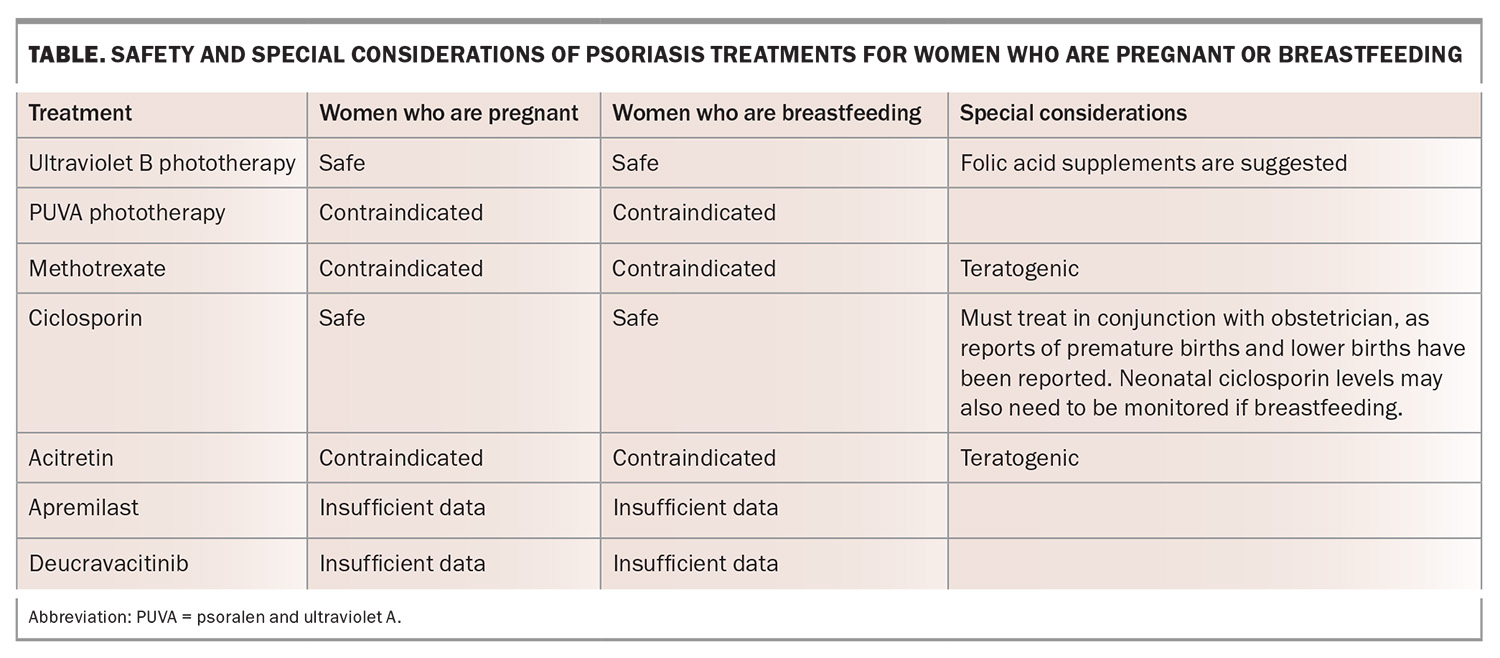
Pregnancy and breastfeeding
Psoriasis typically improves in pregnancy but can worsen postpartum. Owing to a lack of data, there is no international guideline on the safety of administering biologics in pregnant and breastfeeding women, as biologics can be actively transported across the placenta, especially in the late second and third trimesters. A multidisciplinary approach with the treating dermatologist and obstetrician is recommended, as untreated severe psoriasis can result in negative pregnancy outcomes. Live vaccines should not be administered to a newborn exposed to a biologic in utero (particularly in the second half of gestation) because of the risk of dissemination. The Table provides an overview of the safety of psoriasis treatments in pregnant and breastfeeding women.
Systemic medications for psoriasis
Most patients can manage their psoriasis using topical agents, but moderate-to-severe disease typically requires systemic therapies.1 There are a range of nonbiologic and biologic therapies for psoriasis available in Australia.
Methotrexate, ciclosporin, apremilast and deucravacitinib can be used for PsA. Tumour necrosis factor (TNF)-alpha inhibitors and interleukin (IL)-17 inhibitors have been approved and used successfully in patients with both psoriasis and PsA. Some of the IL-23 inhibitors (risankizumab and guselkumab) have been approved for the treatment of PsA, although they have limited efficacy in axial disease. Patients with a high body mass index (BMI) are less likely to respond to biologics and more likely to withdraw from the drug because of their lower efficacy.7 Overweight and obese patients may require higher doses or an increased frequency of injections to achieve the same effect as that in patients of a healthy weight.
Patients with chronic plaque psoriasis must fulfil certain criteria to qualify for a biologic agent in Australia through the PBS. The patient must be at least 18 years of age and be treated by a dermatologist. The patient must have had psoriasis for at least six months and, at the time of application, must have severe chronic plaque psoriasis affecting the whole body (PASI score >15), or have severe chronic plaque psoriasis of the face, palm of a hand or sole of a foot (at least 30% involvement, or at least two of the three PASI symptom subscores indicating severe or very severe erythema, thickness and scaling).
Patients must have tried and failed (alternatively, have a contraindication or toxicity to) two of six systemic treatments for a minimum of six weeks, as listed in Box 1.
Nonbiologic systemic treatment options for adults with psoriasis
Methotrexate
Methotrexate is a dihydrofolate reductase inhibitor that is widely available and affordable, with anti-inflammatory and antiproliferative properties.8 It can be used for psoriasis and PsA as either monotherapy or in conjunction with biologics.9 Methotrexate can be administered as an oral, intramuscular or subcutaneous preparation for psoriasis and PsA. The usual oral dose is between 10 and 25 mg/week, although in clinical practice, the dose ranges from 2.5 to 25 mg/week. Folic acid (e.g. 5 mg/week, although the dosing varies in clinical practice) is commonly prescribed at least 24 hours after the methotrexate dose to reduce gastrointestinal and immunosuppressive effects. Parenteral administration of methotrexate reduces the common gastrointestinal symptoms.
A minimum of 10 mg/week for at least six weeks is the dose as per PBS criteria to be eligible for a biologic agent. A test dose of 5 mg/week is sometimes used to identify early methotrexate toxicity, such as bone marrow failure, although this practice may only be beneficial in patients at increased risk of methotrexate toxicity, such as older individuals or those with impaired renal function.10
About 45% of patients achieve PASI 75 (75% reduction in PASI score from baseline) at weeks 12 to 16 with methotrexate treatment.11 Other studies have shown similar outcomes, with a range of 7.5 mg/week to 15 mg/week achieving PASI 75 at week 12 in 39 to 75% of patients.8
Common side effects of methotrexate include nausea and vomiting (18%), oral ulcers (11.1%), upper respiratory tract infections (10.2%), abnormal liver function test results (10%), leucopenia (3.4%), infections and pneumonitis.11
Ciclosporin
Ciclosporin is an oral calcineurin inhibitor that reduces T-cell function and inhibits the synthesis of interleukin (IL)-2. It is used to treat dermatological conditions such as psoriasis, eczema and lupus. The typical dose ranges between 2 and 5 mg/kg/day; however, the optimal dose to achieve disease control is 5 mg/kg/day with 58 to 71% achieving PASI 75 at weeks 12 to 16. Failure to achieve an adequate response to a lower dose (2 mg/kg/day) is required to be eligible for a biologic agent.
A significant benefit of ciclosporin is it can quickly bring psoriasis under control, as it has a rapid onset of action. It is used for a short treatment course (e.g. six months, although this can vary among clinicians), as long-term use is associated with hypertension and nephrotoxicity. Some common side effects of ciclosporin include hypertension, renal dysfunction, headaches, gastrointestinal issues (nausea, diarrhoea and abdominal discomfort) and paraesthesia.9 Regular renal function testing and blood pressure monitoring are essential with ciclosporin, with deterioration of at least 10% from baseline necessitating at least temporary cessation of the drug. Other potential side effects include hyperkalaemia, headache, hirsutism, gingival hyperplasia, malignancy and risk of infection. Ciclosporin is metabolised and, therefore, is associated with drug interactions through the cytochrome P450 pathway, including CYP3A4.12
Acitretin
Acitretin is a second-generation retinoid used in plaque psoriasis, pustular psoriasis and palmoplantar psoriasis. It reduces the proliferation of epidermal keratinocytes, inhibiting the IL-6-induced T helper (Th)1/Th17 inflammatory cell pathways while downregulating the expression of interferon-gamma.9
At week 12, PASI 75 was shown to be achieved in 47% and 69% of patients treated with doses of 25 mg and 35 mg, respectively.13 The effect of acitretin is augmented with concomitant phototherapy. Acitretin is contraindicated in women of childbearing potential because of the drug’s teratogenicity. Acitretin can be esterified into the lipophilic etretinate in the presence of alcohol; therefore, women should avoid pregnancy for at least three years after the discontinuation of acitretin. The drug is, therefore, commonly only prescribed in men and postmenopausal women. An advantage of acitretin is that it is not an immunosuppressive agent and can be used in patients with a history of infections or malignancies, especially skin cancers. Common side effects of acitretin include photosensitivity, transient liver function test abnormalities and hyperlipidaemia, as well as mucocutaneous side effects (e.g. xerosis, cheilitis, epistaxis, fragile and brittle skin and nails) and telogen effluvium.14
Apremilast
Apremilast is an oral small-molecule inhibitor of phosphodiesterase 4 that does not suppress the immune system and does not need blood monitoring. It reduces the production of proinflammatory cytokines (including TNF, IL-17, IL-23 and IL-22) by reducing the intracellular concentration of cyclic adenosine monophosphate. It is PBS listed for patients with severe psoriasis who have failed to respond to, a contraindication to or a severe intolerance to methotrexate. The initial dose is 10 mg, which is titrated up over six days to a maximum of 30 mg twice daily. Dosing adjustment may be required in patients with severe renal impairment.
The common side effects are gastrointestinal issues (diarrhoea, nausea), headache and nasopharyngitis, with some reports of depressive symptoms and suicidal ideation. Apremilast is effective for chronic plaque psoriasis, as well as scalp and genital psoriasis.15
More patients achieved PASI 75 at week 8 when treated with apremilast and ultraviolet B (UVB) phototherapy compared with UVB phototherapy alone (p<0.03), with no statistically significant difference in PASI 90.16 Randomised controlled trials have shown that 28.8 to 33.1% of patients taking apremilast achieved PASI 75 compared with 5.3 to 5.8% taking placebo at week 16.17,18
GPs can prescribe apremilast if directed to continue treatment (not initiate treatment) by a dermatologist or a dermatology registrar in consultation with a dermatologist.
Deucravacitinib
Deucravacitinib is an oral, tyrosine kinase 2 inhibitor that inhibits the IL-23 signalling pathway, which is effective in treating psoriasis and PsA. It has been shown to be more effective than apremilast and placebo in achieving PASI 75 and 90 at weeks 16 and 24, respectively. PASI 75 was achieved at week 16 in 53% of patients treated with deucravacitinib compared with 39.8% treated with apremilast and 9.4% with placebo.19
Similar to the PBS criteria for apremilast, deucravacitinib can be prescribed to patients with severe psoriasis who have failed to respond to, a contraindication to or a severe intolerance to methotrexate. The dose is 6 mg once daily. Common side effects include nasopharyngitis, upper respiratory tract infections, headache, skin infections (e.g. herpes simplex virus, varicella zoster virus), acne and hypertension. Given it is an immunosuppressive agent, it is important to perform an immunosuppressive screen prior to starting treatment and monitor for infections including herpes zoster disease.
As with apremilast, GPs can prescribe deucravacitinib if directed to continue treatment (not initiate treatment) by a dermatologist or a dermatology registrar in consultation with a dermatologist.
Ultraviolet B phototherapy
Phototherapy consists of treatments with ultraviolet A (UVA) or UVB wavelengths. UVA machines are no longer commonly used in Australia because of the associated increased risk of skin cancers. UVA can be combined with psoralens (topical or oral) (PUVA) for more effective clearance of psoriasis and other skin conditions, but there is a risk of the onset of cutaneous and systemic side effects including nausea, headache, hypertrichosis, peripheral oedema, lentigines and skin cancers.
Narrow-band UVB phototherapy is an effective and frequently used treatment for psoriasis, typically administered three times per week, with few adverse events at a wavelength of 311 to 313 nm. The effect is equivalent or near equivalent to that of PUVA, as it clears psoriasis and results in long-term remission.20 To date, narrow-band UVB phototherapy is not associated with an increased risk of skin cancer.21
Biologic systemic treatment options for adults with psoriasis
Screening for biologics
There is no international consensus on screening tests that should be performed before prescribing a biologic, but it is suggested to perform a full blood count, measurement of electrolyte levels, renal and liver function tests, measurement of fasting serum lipid levels and antinuclear antibody (ANA) positivity testing if prescribing a TNF inhibitor. An immunosuppressive screen can consist of tuberculosis testing and HIV, hepatitis B and C, varicella, measles and Strongyloides serology. A chest x-ray is required to exclude latent tuberculosis. Box 2 lists some common immunosuppressive screening assessments to be performed before initiating biologics in adults with psoriasis.
There is no consensus regarding the exact timing of live vaccines; however, a patient taking an immunosuppressive agent, including a biologic, should not receive a live vaccine during treatment with the immunosuppressant and for three months after the last dose. An immunosuppressant or biologic can be administered one month after the live vaccine dose.
Tumour necrosis factor-alpha inhibitors
The TNF-alpha inhibitors, including etanercept, infliximab and adalimumab, were among the original biologics for psoriasis and are still used today. In general, they are effective for psoriasis and PsA, but they also have other indications such as inflammatory bowel disease, hidradenitis suppurativa, rheumatoid arthritis and ankylosing spondylitis. TNF-alpha inhibitors have been used for psoriasis at specific sites including scalp, nail and palmoplantar psoriasis. Side effects of TNF-alpha inhibitors include paradoxical pustular psoriasis, palmoplantar psoriasis or pustulosis, lupus, opportunistic infections, reactivation of tuberculosis or hepatitis, nonmelanoma skin cancer or lymphomas, demyelinating disease and worsening heart failure.
Newer biologics are preferred to the TNF-alpha inhibitors as first-line treatment because they are more effective and are associated with fewer side effects. Etanercept is used for paediatric and adult chronic plaque psoriasis, or those with scalp, nail, pustular, erythrodermic and inverse psoriasis at doses of 50 mg twice per week for 12 weeks, followed by a maintenance dose of either 50 mg/week or 50 mg twice per week. Etanercept is a recombinant TNF-alpha receptor protein fused with the Fc portion of immunoglobulin (Ig)G1. In terms of efficacy, at week 12, PASI 75 has been shown to be achieved in 33% and 49% of patients taking the drug at doses of 50 mg/week and 50 twice per week, respectively.1
Infliximab is a chimeric monoclonal antibody consisting of a murine variable region and human IgG1-alpha constant region, which neutralizes the effects of TNF-alpha and is administered as an intravenous infusion of 5 mg/kg at weeks 0, 2 and 6 as an induction, followed by every eight weeks as maintenance, although higher doses (e.g. up to 10 mg/kg) or an increased frequency (e.g. every four weeks) is sometimes used for better disease control. With rapid onset, 80% and 57% of patients receiving 5 mg/kg intravenously have been shown to achieve PASI 75 and PASI 90 at week 10, respectively.22 Subcutaneous infliximab (120 mg every two weeks, regardless of weight) has recently become available for patients who have had two or more doses of intravenous infliximab.
Adalimumab is given as a subcutaneous injection for psoriasis, PsA and hidradenitis suppurativa. The dose is 80 mg subcutaneously in week 0, 40 mg in week 1 and followed by 40 mg every fortnight as maintenance. At week 16, PASI 75 is achieved in 71% of patients treated with adalimumab compared with 7% of those treated with placebo.23
Certolizumab is a PEGylated, humanised monoclonal antibody, which is TGA approved, but not PBS listed, for psoriasis. Given its molecular structure, it is not actively transported across the placenta and is safe in pregnancy. It is administered subcutaneously at a dose of 400 mg every two weeks.
Interleukin-12/23 inhibitors
Ustekinumab is a human monoclonal antibody targeting the common p40 subunit of IL-12 and IL-23. The dose is weight dependent: 45 mg if the patient’s body weight is 100 kg or less, or 90 mg if the patient’s body weight is greater than 100 kg, administered subcutaneously at week 0 and 4, and then every 12 weeks as maintenance. At week 12, PASI 75 was shown to be achieved in 66.7 to 67.1% of patients at a dose of 45 mg and 66.4 to 75.7% of patients at a dose of 90 mg.1
The IL-17 inhibitors, secukinumab and ixekizumab, have been shown to be more effective than ustekinumab. Secukinumab 300 mg was found to be more effective than ustekinumab in achieving PASI 75 at week 16 (79% vs 57.6% of patients). In another randomised controlled trial, those treated with ixekizumab were more likely to achieve PASI 90 at week 12 (72.8%) compared with the ustekinumab group (42.2%).1
The durability of the response of biologics is important, as a loss of effect over time may reduce treatment adherence and affect a patient’s quality of life. Ustekinumab has a longer drug survival than TNF-alpha inhibitors, with lower rates of discontinuation.24
Interleukin-17 inhibitors
Secukinumab is a human IgG1 monoclonal antibody that binds to IL-17A and inhibits its interaction with the IL-17 receptor. It is used to treat psoriasis, PsA, hidradenitis suppurativa and ankylosing spondylitis. The initiation dose for psoriasis is 300 mg via subcutaneous injection weekly for five weeks, followed by 300 mg monthly. Phase III randomised controlled trials assessing the efficacy of secukinumab in moderate-to-severe psoriasis have shown that PASI 75 was achieved at week 12 in 77.1 to 81.6% of patients receiving the 300 mg dose, 67 to 71.6% of patients receiving the 150 mg dose and 4.5 to 4.9% of patients receiving placebo.25
Secukinumab has also been found to be effective for palmoplantar psoriasis. At week 16, 33.3% of patients taking 300 mg and 22.1% of those taking 150 mg achieved a palmoplantar Investigator’s Global Assessment response of 0 or 1.26
Ixekizumab is a humanised IgG4 monoclonal antibody that binds to IL-17A and prevents it from interacting with its receptor. It is administered as a subcutaneous dose of 160 mg in week 0, followed by 80 mg every fortnight for the first 12 weeks and then 80 mg every four weeks. At week 12, 84.2% of those treated with ixekizumab achieved PASI 75 compared with 53.4% with etanercept and 7.4% in the placebo group. Ixekizumab is also more effective in achieving PASI 90 at week 12 than ustekinumab.27
Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits both IL-17A and IL-17F. It is administered subcutaneously via a prefilled pen at a dose of 360 mg (2×160 mg) at weeks 0, 4, 8, 12 and 16, and thereafter every eight weeks. Bimekizumab has been shown to be more effective for moderate-to-severe psoriasis than secukinumab (which selectively inhibits IL-17A alone). In a trial, 62% of participants in the bimekizumab group and 49% of participants in the secukinumab group achieved PASI 100 at week 16. At week 48, 67% of participants treated with bimekizumab had a PASI 100 response, compared with 46% of participants treated with secukinumab.28 Similarly, bimekizumab has been shown to be more effective for moderate-to-severe psoriasis than placebo, adalimumab and ustekinumab. At week 16, 86.2% of participants in the bimekizumab group achieved PASI 90, compared with 47.2% of participants with adalimumab.29 At week 16, 85% of those treated with bimekizumab achieved PASI 90 compared with 50% of those treated with ustekinumab.30
Il-17 inhibitors are associated with mucocutaneous candidiasis and are not effective for treating inflammatory bowel disease. Most cases of candidiasis affect the oral cavity, are mild in nature and can be treated with topical clotrimazole, miconazole, amphotericin lozenges or nystatin for seven to 14 days. More moderate to severe infections may require oral fluconazole for seven to 14 days or even cessation of treatment. It may be prudent to avoid IL-17 inhibitors in patients with a personal history of inflammatory bowel disease.
Interleukin-23 inhibitors
IL-23 inhibitors, including risankizumab, guselkumab and tildrakizumab, specifically target and inhibit the p19 subunit of IL-23 and are more effective than placebo.
Risankizumab is a humanised IgG1 monoclonal anti-IL-23, administered as 150 mg subcutaneously at weeks 0 and 4, and then every 12 weeks, the effects of which are superior to those of placebo at week 16.31 Risankizumab was also shown to be more effective than adalimumab at weeks 16 and 44, ustekinumab at weeks 16 and 52 and secukinumab at week 52.32 At week 16, 73% of participants achieved PASI 90 compared with 2% receiving placebo.33 At week 16, 55.9% of those treated with risankizumab achieved PASI 90 compared with 5.1% receiving apremilast.34
Risankizumab has demonstrated durability and safety. An ongoing phase III open-label extension study designed to evaluate the long-term safety and efficacy of risankizumab has shown that more than 75% of patients achieved PASI 90 and more than 40% achieved PASI 100 after 16 weeks of risankizumab treatment.35 After 52 weeks, more than 86% of patients achieved PASI 90 and more than 58% of patients achieved PASI 100. Data up to 172 weeks showed that 85.5% of patients achieved PASI 90 and 54.4% achieved PASI 100 with risankizumab treatment. The most common adverse effects include nasopharyngitis (31%) and upper respiratory tract infections (20%).33
Tildrakizumab is an IL-23 inhibitor administered as 100 mg subcutaneously at weeks 0 and 4, and then every 12 weeks. Clinical studies have shown that at week 12, 64% of participants treated with 100 mg achieved PASI 75 compared with 6% receiving placebo.36
Guselkumab is an IL-23 inhibitor administered as 100 mg subcutaneously at weeks 0 and 4, and then every eight weeks. A trial has shown that 86.3 to 91.2% of participants treated with guselkumab achieved PASI 75 at week 16 compared with 68.5 to 73.1% with adalimumab and 5.3 to 8.1% with placebo.1
Common side effects of IL-23 inhibitors include nasopharyngitis, upper respiratory tract infection, headaches and fatigue.
Future directions
The cost of biologics can burden the healthcare system and economy. When drugs lose their patent protection, the introduction of biosimilars can reduce drug costs and improve accessibility while providing similar efficacy rates to those of the originators.37 A biosimilar is a biological product with an active product that is derived from a biological source. It is structurally similar and should have the same efficacy as the originator biological medication. Due to the complexity of biological molecules, biosimilars are not identical to the originator biologic but undergo testing to ensure the safety, efficacy and immunogenicity are similar.
Janus kinase inhibitors are small-molecule inhibitors that affect intracellular signalling and may have potential beneficial effects in psoriasis. Trials of topical and oral Janus kinase inhibitor agents are ongoing.
Conclusion
In summary, there are a range of nonbiologic and biologic systemic treatments available for patients with moderate-to-severe psoriasis in Australia. GPs play an important role in identifying patients who would benefit from systemic treatments from a dermatologist, as well as in managing any disease complications and comorbidities. MT
COMPETING INTERESTS: Dr Daniel: None. Dr Nguyen has received support from Sanofi and AbbVie for attending investigator meetings. Associate Professor Shumack has received speaker payment from BMS. Associate Professor Foley has received research grants to the institution from AbbVie, Amgen, BMS, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, Sun Pharma and UCB Pharma; is or has been a consultant for Apogee, Aslan Pharmaceuticals, Eli Lilly, Galderma, Janssen, LEO Pharma, Pfizer; has received payment or honoraria for lectures, presentations, speakers’ bureaus and educational events from AbbVie, Amgen, Boehringer-Ingelheim, BMS, Eli Lilly, Janssen, Novartis, LEO Pharma, Pfizer, Sanofi, Sun Pharma and UCB; has received personal payments from Pfizer; has received support to attend meetings from Eli Lilly, Sun Pharma and Boehringer-Ingelheim; is or has been a (paid) Advisory Board member for AbbVie, Amgen, Boehringer-Ingelheim, BMS, Eli Lilly, Galderma, GSK, Janssen, Novartis, LEO Pharma, Mayne Pharma, Pfizer, Sanofi, Sun Pharma and UCB; and has received drug samples for patients and medical writing services from AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Sanofi, Sun Pharma and UCB.
References
1. Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol 2019; 80: 1029-1072.
2. Onorati, H. Reassessing psoriasis severity. Portland, OR: National Psoriasis Foundation; 2024. Available online at: https://www.psoriasis.org/advance/psoriasis-severity-high-impact-sites/?utm_source=Research+and+Medical+Master&utm_campaign=7272a187bd-ADVANCE_PRO_1_18_24&utm_medium=email&utm_term=0_27658c37df-7272a187bd-184182164 (accessed August 2024).
3. Daniel BS. The multiple comorbidities of psoriasis: the importance of a holistic approach. Aust J Gen Pract 2020; 49: 433-437.
4. Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol 2017; 77: 657-666.e8.
5. Mahil SK, McSweeney SM, Kloczko E, McGowan B, Barker JN, Smith CH. Does weight loss reduce the severity and incidence of psoriasis or psoriatic arthritis? A critically appraised topic. Br J Dermatol 2019; 181: 946-953.
6. Whitlock SM, Enos CW, Armstrong AW, et al. Management of psoriasis in patients with inflammatory bowel disease: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol 2018; 78: 383-394.
7. Pirro F, Caldarola G, Chiricozzi A, et al. Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig 2021; 41: 917-925.
8. van Huizen AM, Sikkel R, Caron AGM, Menting SP, Spuls PI. Methotrexate dosing regimen for plaque-type psoriasis: an update of a systematic review. J Dermatolog Treat 2022; 33: 3104-3118.
9. Hsieh TS, Tsai TF. Combination therapy for psoriasis with methotrexate and other oral disease-modifying antirheumatic drugs: a systematic review. Dermatol Ther (Heidelb) 2023; 13: 891-909.
10. Herrmann T, Buhnerkempe M, Wilson ML. Initiation of methotrexate with or without a test dose: a retrospective toxicity study. J Am Acad Dermatol 2019; 80: 1160-1162.
11. West J, Ogston S, Foerster J. Safety and efficacy of methotrexate in psoriasis: a meta-analysis of published trials. PLoS One 2016; 11: e0153740.
12. Balak DMW, Gerdes S, Parodi A, Salgado-Boquete L. Long-term safety of oral systemic therapies for psoriasis: a comprehensive review of the literature. Dermatol Ther (Heidelb) 2020; 10: 589-613.
13. Dogra S, Jain A, Kanwar AJ. Efficacy and safety of acitretin in three fixed doses of 25, 35 and 50 mg in adult patients with severe plaque type psoriasis: a randomized, double blind, parallel group, dose ranging study. J Eur Acad Dermatol Venereol 2013; 27: e305-e311.
14. Guenther LC, Kunynetz R, Lynde CW, et al. Acitretin use in dermatology. J Cutan Med Surg 2017; 21: 2s-12s.
15. Stein Gold LS, Papp K, Pariser D, et al. Efficacy and safety of apremilast in patients with mild-to-moderate plaque psoriasis: results of a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 2022; 86: 77-85.
16. Morita A, Yamaguchi Y, Tateishi C, et al. Efficacy and safety of apremilast and phototherapy versus phototherapy only in psoriasis vulgaris. J Dermatol 2022; 49: 1211-1220.
17. Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol 2015; 173: 1387-1399.
18. Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol 2015; 73: 37-49.
19. Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol 2023; 88: 40-51.
20. Lapolla W, Yentzer BA, Bagel J, Halvorson CR, Feldman SR. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol 2011; 64: 936-949.
21. Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2012; 26: 22-31.
22. Reich K, Nestle FO, Papp K, et al.; EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005; 366: 1367-1374.
23. Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58: 106-115.
24. No DJ, Inkeles MS, Amin M, Wu JJ. Drug survival of biologic treatments in psoriasis: a systematic review. J Dermatolog Treat 2018; 29: 460-466.
25. Langley RG, Elewski BE, Lebwohl M, et al.; ERASURE Study Group; FIXTURE Study Group. Secukinumab in plaque psoriasis results of two phase 3 trials. N Engl J Med 2014; 371: 326-338.
26. Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol 2017; 76: 70-80.
27. Reich K, Pinter A, Lacour JP, et al.; IXORA-S investigators. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol 2017; 177: 1014-1023.
28. Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med 2021; 385: 142-152.
29. Warren RB, Blauvelt A, Bagel J, et al., Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med 2021; 385: 130-141.
30. Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021; 397: 487-498.
31. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018; 392: 650-661.
32. Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol 2021; 184: 50-59.
33. Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol 2020; 156: 649-658.
34. Stein Gold LF, Bagel J, Tyring SK, et al. Comparison of risankizumab and apremilast for the treatment of adults with moderate plaque psoriasis eligible for systemic therapy: results from a randomized, open-label, assessor-blinded phase IV study (IMMpulse). Br J Dermatol 2023; 189: 540-552.
35. Papp KA, Lebwohl MG, Puig L, et al., Long-term efficacy and safety of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial beyond 3 years of follow-up. Br J Dermatol 2021; 185: 1135-1145.
36. Reich K, Papp KA, Blauvelt A, et al., Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017; 390: 276-288.
37. Lee HJ, Kim M. Challenges and future trends in the treatment of psoriasis. Int J Mol Sci 2023; 24; 1-12.