What’s the diagnosis?
A man with a rapidly evolving, widespread pruritic rash
Case presentation
A 21-year-old man presents with a two-week history of a widespread rash that is moderately pruritic. The rash started as separate papules and plaques but progressed to a more widespread state and, in some areas, the lesions have coalesced. He reports an episode of tonsillitis four weeks earlier that was managed with oral phenoxymethylpenicillin. He has no other significant medical history and is systemically well.
Prior to presenting, the patient had tried over-the-counter 0.5% hydrocortisone ointment, sorbolene cream as an emollient and oral loratadine 10 mg daily, without significant improvement.
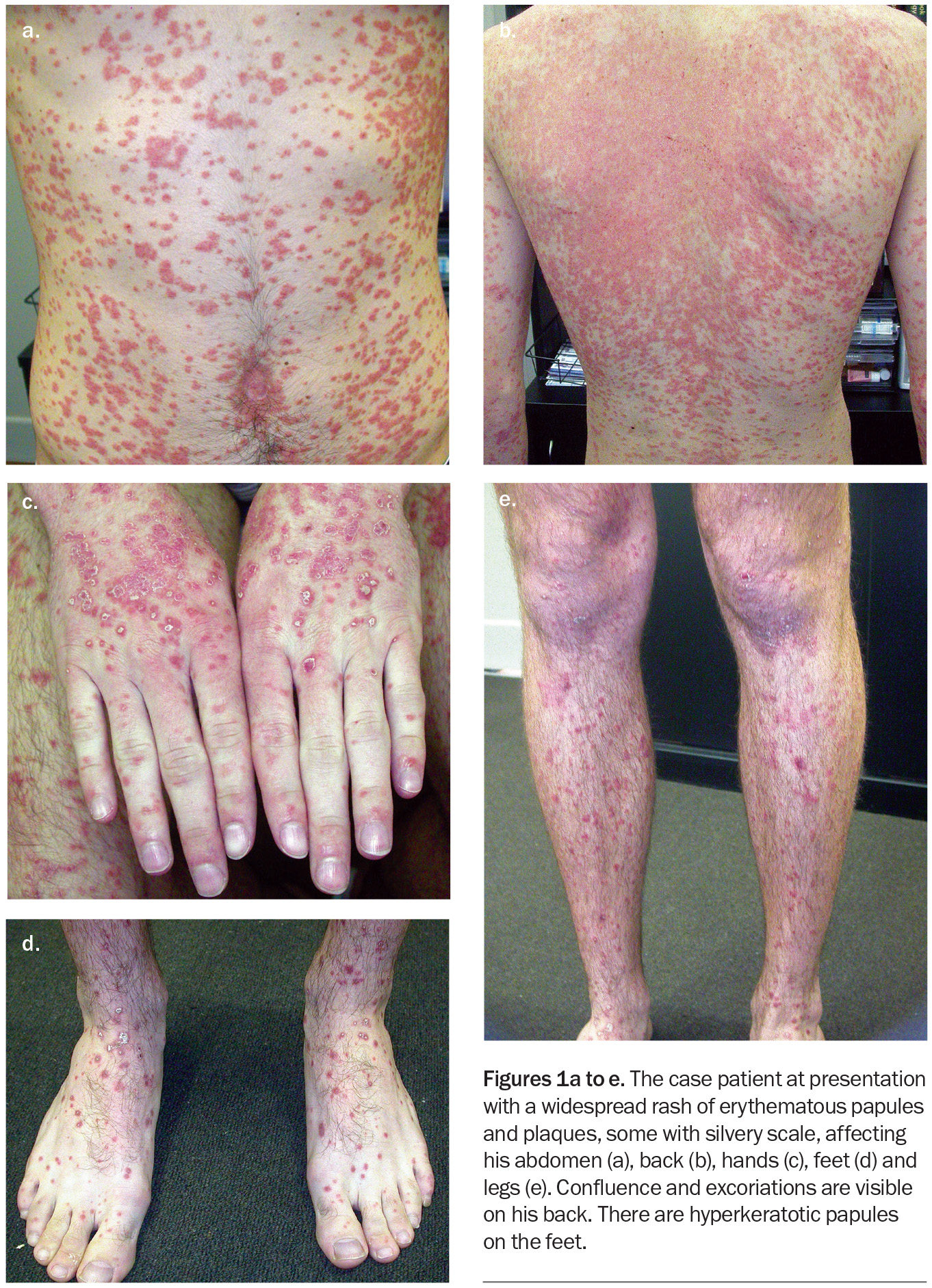
On examination, the patient is observed to have a widespread rash affecting his abdomen, back, hands, feet and legs (Figures 1a to e). Discrete, erythematous papules and plaques, some with silvery scale, are noted. The papules have coalesced into plaques on his upper back. His scalp is involved. Hyperkeratotic papules are observed on the dorsum of the feet. There is sparing of his palms, soles, face and mucosal surfaces.
Differential diagnoses
Conditions to consider among the differential diagnoses include the following.
Pityriasis rosea
Pityriasis rosea, a self-limiting cutaneous exanthem that typically lasts for six to 10 weeks, can generally be diagnosed clinically on the basis of its history and presentation. Typically there will be an initial herald patch (up to 10 cm), followed by the development of numerous smaller, scaly, ovoid patches (2 to 5 cm) within a two-week period.1 The trunk and proximal extremities are most commonly affected. The lesions present along Langer’s lines, giving a characteristic ‘Christmas tree’ appearance.2 Generally, the patches have a fine scale that is most apparent at the peripheries of the lesions (collarette scaling). The patches may be asymptomatic but can be mildly or moderately pruritic.1 The exact aetiology for pityriasis rosea is unknown but it is thought to be associated with reactivation of latent human herpesvirus-6 and herpesvirus-7 infection.3
The case patient had no herald patch. Additionally, the history of recent tonsillitis, which is not a feature of pityriasis rosea, gives clues to the true diagnosis.
Viral exanthems
Viral exanthems, which are most commonly seen in infants and young children, are usually associated with benign and self-limiting diseases.4 Significant morphological and distribution variation may occur, including presentations that are maculopapular, vesicular or even petechial in nature.5 Specific viral exanthems may exhibit a distinctive pattern – for instance, those caused by viruses such as rubella, roseola (herpesvirus-6B), erythema infectiosum (parvovirus B19), measles (morbillivirus) or chickenpox (varicella).6 However, many viral exanthems lack specificity and a clear causative virus may not be apparent.7 The cutaneous features of viral exanthems are typically accompanied by flu-like symptoms, including fever and malaise.
Viral exanthems can usually be identified by considering the age of the patient and the distribution of the rash and its morphology.6 For the case patient, a careful history regarding his recent illness was helpful – viral exanthems usually present with a rash around the same time as systemic symptoms, but he experienced a two-week latent period between infective symptoms and the onset of the skin eruption. Furthermore, he falls outside the age range in which viral exanthems are usually observed.
Tinea corporis
Tinea corporis (‘ringworm’) is a superficial fungal infection of the skin predominantly caused by Trichophyton or Microsporum species. Zoonotic fungal species may be transmitted from animals, including dogs, cats, horses and cattle.8 Risk factors include immunosuppression, diabetes mellitus and hyperhidrosis.
Tinea corporis typically presents as a mildly pruritic, polycyclic or annular solitary plaque, but multiple plaques may be present. The plaques often have an erythematous, scaly ‘active edge’ that is sometimes papular or pustular and central clearing.8 Commonly affected areas are the groin, buttocks and feet.9
Tinea corporis can generally be diagnosed clinically. However, if there is diagnostic uncertainty then taking skin scrapings from the active edge for microscopy may be valuable.
Secondary syphilis
Although rare, secondary syphilis is important to consider because of the potential public health ramifications. The dermatological presentations are varied and encompass a wide variety of mucocutaneous lesions. Typically, however, there will be an initial macular rash followed by a symmetrical papular eruption. Generally, this involves the entire trunk and extremities, including the hands and soles of the feet. Papules usually have a scale but may also have follicular, smooth or pustular morphology.10 The rash is not commonly pruritic. The disease generally presents as a systemic illness characterised by malaise, headaches, weight loss, fevers and myalgias, alongside dermatological manifestations. Lymphadenopathy is present in most cases.10
Secondary syphilis generally occurs four to 10 weeks after the primary infection. About 22% of cases of syphilis present at the secondary stage.11 In Australia, cases of syphilis have been steadily rising among men who have sex with men, Aboriginal and Torres Strait Islander people and women of reproductive age.12 Secondary syphilis can be diagnosed through a positive cardiolipin antibody result, which can be detected through the rapid plasma reagin or venereal diseases research laboratory serological tests.10
Guttate psoriasis
This is the correct diagnosis. Guttate psoriasis is an acute variant of psoriasis that is typically triggered by acute streptococcal infection of the tonsils.13 The term ‘guttate’ refers to the classical drop-like appearance of the papules and plaques, which are typically well demarcated and about 2 to 6mm in size. The plaques and papules are salmon-pink to erythematous in colour and may also have a silvery scale. Koebnerisation phenomenon is associated with guttate psoriasis, wherein lesions may develop in areas of friction or irritation.14
Guttate psoriasis typically develops one to three weeks after a streptococcal infection.15 Rarely, the condition may be triggered by TNF-alpha inhibitor therapy or streptococcal infection to another location of the body (such as a perianal streptococcal infection).16,17
Guttate psoriasis accounts for 2% of psoriasis presentations.18 Children and adolescents are most commonly affected; the incidence is equal across sexes.13 It is generally a self-limiting condition, but about one-third of patients will go on to develop long-term chronic plaque psoriasis.19
Clinically, secondary syphilis may resemble guttate psoriasis but can be distinguished by evaluating for systemic symptoms, lymphadenopathy and assessing for palmoplantar involvement. When multiple plaques are present in tinea corporis, these are usually more sparsely distributed than in guttate psoriasis.
Pathophysiology
The exact pathophysiological correlation between streptococcal infection and guttate psoriasis remains elusive, but there is a complex interplay between genetic and environmental factors. It has been postulated that the guttate eruption might result from an immune reaction to the streptococcal infection in individuals who have susceptible genotypes. These include associations with human leukocyte antigen (HLA) genes, with the HLA-Cw*0602 allele in particular appearing to increase risk for guttate psoriasis.20
Diagnosis
Guttate psoriasis can usually be diagnosed clinically on the basis of a careful history and examination. If there is diagnostic uncertainty, testing for antistreptolysin O, anti-DNase B or streptozyme antibody titres may be beneficial – these are often elevated, indicating recent streptococcal infection.15 Skin biopsy is rarely indicated unless the diagnosis is still uncertain.21
Management
Guttate psoriasis typically responds well to topical management measures and phototherapies. A moderate to potent topical corticosteroid may be beneficial, such as methylprednisolone aceponate 0.1% fatty ointment or mometasone furoate 0.1% ointment, applied once or twice daily until symptoms settle. Topical application of calcipotriol and betamethasone foam can also be beneficial for symptom management. For some patients, several weeks of graded sun exposure to affected areas can be helpful.
For patients with more severe cases of guttate psoriasis, referral to a dermatologist for consideration of phototherapy may be beneficial. Broadband or narrowband ultraviolet B (UVB) phototherapy may be used, typically two or three times per week for six to 12 weeks or until remission occurs.15
Although guttate psoriasis is often triggered by streptococcal infection, there is currently no compelling evidence of a role for tonsillectomy or antibiotic treatment in management.22
Outcome
The case patient was managed with methylprednisolone aceponate 0.1% ointment twice-daily, emollients and narrowband UVB therapy (three times weekly for six weeks). His rash responded well to treatment and cleared completely. MT
COMPETING INTERESTS: None.
References
1. Litchman G, Nair PA, Le JK. Pityriasis rosea. StatPearls [Internet]: StatPearls Publishing; 2022.
2. Stulberg DL, Wolfrey J. Pityriasis rosea. Am Fam Physician 2004; 69: 87-91.
3. Yildirim M, Aridogan BC, Baysal V, Inaloz HS. The role of human herpes virus 6 and 7 in the pathogenesis of pityriasis rosea. Int J Clin Pract 2004; 58: 119-121.
4. Fölster-Holst R, Kreth HW. Viral exanthems in childhood – infectious (direct) exanthems. Part 1: Classic exanthems. J Dtsch Dermatol Ges 2009; 7: 309-316.
5. Keighley CL, Saunderson RB, Kok J, Dwyer DE. Viral exanthems. Curr Opin Infect Dis 2015; 28: 139-150.
6. Ting S, Nixon R. Clinical features of viral exanthems. Aust J Gen Pract 2021; 50: 231-236.
7. Knöpfel N, Noguera-Morel L, Latour I, Torrelo A. Viral exanthems in children: a great imitator. Clin Dermatol 2019; 37: 213-226.
8. Leung AK, Lam JM, Leong KF, Hon KL. Tinea corporis: an updated review. Drugs Context 2020; 9: 2020-5-6.
9. Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician 1998; 58: 163-174, 177-178.
10. Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev 2005: 18: 205-216.
11. Melbourne Sexual Health Centre. Syphilis treatment guidelines. Melbourne: MSHC; 2021. Available online at: https://www.mshc.org.au/health-professionals/treatment-guidelines/syphilis-treatment-guidelines (accessed May 2023).
12. NSW Health. NSW sexually transmissible infections strategy 2022-2026: data report January to December 2021. Sydney: NSW Health; 2021. Available online at: https://www.health.nsw.gov.au/Infectious/Reports/Publications/sti/nsw-2021-sti-report.pdf (accessed May 2023).
13. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci 2019; 20: 1475.
14. Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2020.
15. Saleh D, Tanner LS. Guttate psoriasis. StatPearls [Internet]: StatPearls Publishing; 2018.
16. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis 2019; 4: 70-80.
17. Ulger Z, Gelenava T, Kosay Y, Darcan S. Acute guttate psoriasis associated with streptococcal perianal dermatitis. Clin Pediatr (Phila) 2007; 46: 70-72.
18. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58: 826-850.
19. Ko HC, Jwa SW, Song M, Kim MB, Kwon KS. Clinical course of guttate psoriasis: long-term follow-up study. J Dermatol 2010; 37: 894-899.
20. Mallon E, Bunce M, Savoie H, et al. HLA-C and guttate psoriasis. Br J Dermatol 2000; 143: 1177-1782.
21. Shahid N, Bawany MZ, Rafiq E, Sodeman T. Guttate psoriasis: a rare cause of diffuse rash. BJMP 2013; 6(4): a627.
22. Owen CM, Chalmers R, O’Sullivan T, Griffiths CE. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev 2019; 3(3): CD001976.