What’s the diagnosis?
Painful ulceration with necrosis on a finger
Case presentation
A 38-year-old man presents with acute painful ulceration of his right ring finger that has developed over the past five days. He denies any trauma or chemical exposure to the affected skin. He does not have any allergies and is not taking regular medications. He works in information technology and is right-hand dominant.
The patient reports a six-week history of intermittent bloody diarrhoea with abdominal pain that preceded the rash to his finger. He denies fevers or weight loss. He returned from a trip to India three months earlier, and denies any issues with diarrhoea during this time.
On examination, distal circumferential ulceration of the finger with necrosis is observed. The skin involving the other digits and forearm is normal. Multiple erythematous plaques with a pustular rim and central ulceration are also noted on the patient’s anterior trunk. There are no masses observed on abdominal or rectal examination.
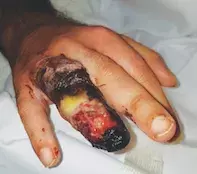
A provisional diagnosis of cellulitis is made and the patient is admitted to hospital. He commences treatment with empirical intravenous antibiotics. He remains haemodynamically stable and afebrile, although there is progressive worsening pain and proximal extension of the ulceration towards his metacarpophalangeal joint (Figure 1). A surgical plan is made for terminalisation of the finger to avoid proximal extension and systemic dissemination of suspected infection.
Differential diagnoses
Conditions to consider among the differential diagnoses for a patient who has ulcerative lesions to the distal digits include the following.
- Necrotising fasciitis. This is a rapidly progressive and potentially fatal infection of the skin and the subcutaneous and underlying fascia. Risk factors include age over 60 years, immunosuppression, diabetes mellitus, chronic kidney disease, malnutrition, intravenous drug use, obesity and malignancy.1 There may be a history of trivial antecedent trauma to the affected area. The most common causative organisms are streptococcal species but most cases are polymicrobial.1 Necrotising fasciitis can involve any part of the body but the most common sites are the trunk, distal extremities and also the perineum (Fournier’s gangrene). Initially it appears with macular erythema, with associated oedema and pain that is disproportionate to the clinical examination findings. As the infection progresses, the margins of affected skin expand and become indistinct, with accompanying oedema and palpable crepitus of the soft tissue. Eventually, the skin turns dusky blue, which indicates imminent necrosis, before developing bullae that may become haemorrhagic. Concomitant septic shock is common, indicated by mental obtundation, hypotension and fevers. This state of systemic compromise is reflected in a metabolic acidosis with coagulopathy and raised inflammatory markers. Imaging of the affected area may demonstrate gas in the soft tissue compartments. Biopsy performed at the time of surgical debridement reveals necrosis from the epidermis to the subcutis, a florid neutrophilic infiltrate and occasional occlusive thrombosed vessels.2
- Neutrophilic dermatosis of the hands. This localised variant of Sweet syndrome was previously known as pustular vasculitis of the hands.3,4 Lesions appear as painful pustules that may evolve into large bullous, ulcerated lesions that involve the dorsal, lateral or palmar surfaces of the hands and digits (Figure 2).3,4 It is more common in the seventh decade of life and beyond, and has a predominance in women.3 Between 40 and 54% of cases occur as a result of underlying haematological malignancy (e.g. myelodysplastic syndrome); cases caused by solid-organ tumours are less common (8% of new diagnoses).3 Other associations include inflammatory bowel disease (IBD) and seropositive arthritides.3 Accompanying systemic features reflect the underlying cause – these include fever, malaise, unintentional weight loss, abdominal pain and diarrhoea. Biopsy is diagnostic and reveals a predominantly neutrophilic dermatosis with associated dermal oedema and leukocytoclasia without evidence of vasculitis.4
- Burn injury. Burn injuries can be caused by thermal (heat/freezing), chemical, electrical or radiation-induced trauma to the skin.5 Assessment requires determination of the body surface area affected (Wallace’s rule of nine) as well as the extent of soft tissue damage. There are four categories of devitalised tissue: superficial (involving the epidermis only), superficial partial (superficial dermis), deep partial (deeper layer dermis) and deep (full thickness of skin and into subcutaneous fat or deeper).5 The most common causes of burn injury are thermal – scalds caused by hot liquids are more common in children whereas flame burns are more common in adults.5
- Metastatic Crohn’s disease (MCD). This is a rare cutaneous manifestation of Crohn’s disease where skin lesions appear at cutaneous sites that are anatomically noncontiguous with the gastrointestinal tract.6 The morphological features of MCD are variable – presentations include asymptomatic papules, nodules or areas of ulceration that involve the extremities, intertriginous surfaces (axillae, inguinal folds), face and genital skin. In about a quarter of cases, MCD precedes the diagnosis of Crohn’s disease, with active gastrointestinal disease occurring between nine months and 14 years after the onset of MCD.6 Biopsy is diagnostic and shows diffuse sterile noncaseating granulomas composed of giant cells, histiocytes, lymphocytes and plasma cells involving the dermis and, occasionally, adipose tissue.6
- Pyoderma gangrenosum (PG). This is the correct diagnosis. PG is a chronic neutrophilic dermatosis of unknown aetiology. The lesions are characterised by progressively enlarging painful ulcers with undermined, dusky violaceous borders on a pustular base that worsen following trauma (pathergy).7 PG is rare (3 to 10 new cases per million individuals annually) and can occur at any age but is more common between the third and sixth decades of life.7 Up to 50% of cases of PG occur in the setting of antecedent or coincident systemic disease, which includes IBD, rheumatological disease (e.g. rheumatoid arthritis, seronegative arthritis, polychondritis) and haematological dyscrasia (e.g. acute myeloid leukaemia, myelodysplasia, myeloma and lymphoma).7 Clinical variants of PG include classic, pustular, bullous, vegetative, peristomal, genital, superficial, infantile and extracutaneous. The classic ulcerative form begins as a tender papule/ pustule, most commonly involving the distal extremities, that enlarges and undergoes necrosis, developing a central ulcer on a purulent base with undermined borders. There is rapid centrifugal extension.7 PG lesions are destructive and can extend to the fascial compartment. Serum investigations show increased inflammatory markers (erythrocyte sedimentation rate, C-reactive protein) with a leukocytosis. Biopsy features of PG are nonspecific and include dermal oedema, predominantly neutrophilic infiltration and vascular thrombosis involving small- and medium-sized vessels.7
Diagnosis and investigations
PG is a clinical diagnosis of exclusion. The initial management approach includes excluding other causes of ulceration, investigating for underlying systemic disease or malignancy, and ensuring that there are no contraindications for immunosuppressive treatment (e.g. latent tuberculosis, strongyloidiasis, HIV disease, hepatitis B, hepatitis C). Biopsy is used to exclude other diagnoses, such as infection (bacterial, fungal, parasitic), cutaneous malignancy (primary or metastatic) and ulcers secondary to vascular insufficiency (arterial and/or venous).7 Further investigations to reveal the underlying causes are guided by the clinical picture and may include colonoscopy, bone marrow aspirate and serum protein electrophoresis.
Management
PG can be a difficult condition to treat. It runs an unpredictable course and has a reported mortality rate of up to 30%, with poorer prognosis reserved for patients with PG secondary to malignancy.7 Effective management of the underlying condition will result in a concomitant improvement in PG lesions. The mainstay of treatment for PG is immunosuppressive treatment, initially high-dose systemic corticosteroids, antineutrophilic agents (e.g. dapsone), ciclosporin, methotrexate and azathioprine. Regular wound care in combination with wound dressing and elevation of the limb encourage healing. Wounds should be monitored closely for signs of infection, and surgical debridement is discouraged to avoid pathergy and inducement of new PG lesions.7 Tumour necrosis factor-alpha inhibitors such as infliximab or adalimumab have the dual benefits of treating both IBD and PG; these agents should also be considered for treatment of recalcitrant PG even in the absence of IBD.7
Outcome
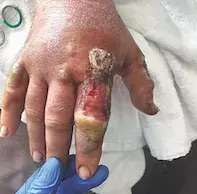
In this patient, bacterial swabs of the affected finger showed polymicrobial growth. Skin biopsy was taken from the edge of the ulcerated surface, showing an irregular acanthotic and hyperkeratotic epidermis with adjacent full-thickness ulceration. There was also accompanying epidermal necrosis with bacterial colonisation, and a predominantly neutrophilic dermal infiltrate without evidence of vasculitis or malignancy. A new diagnosis of ulcerative colitis was made on colonoscopy, which showed multiple ulcers from the rectum involving the sigmoid and descending colon with evidence of chronic active proctocolitis. Stool cultures returned negative results, and faecal calprotectin level was elevated.
Plans for further surgical debridement were abandoned and the patient commenced treatment on immunosuppressive therapy: intravenous hydrocortisone 100mg every six hours, azathioprine 100mg daily and oral ciclosporin 5mg/kg/day. This resulted in rapid improvement. He was discharged from hospital on oral ciclosporin, azathioprine, prednisone 50mg daily and mesalazine 4g daily; in addition, weekly wound care was arranged for his finger. His condition has continued to improve on follow up as an outpatient.
References
1. Hasham S, Matteucci P, Stanley PRW, Hart NB. Necrotising fasciitis. BMJ 2005; 330: 830-833.
2. Barker FG, Leppard BJ, Seal DV. Streptococcal necrotising fasciitis: comparison between histological and clinical features. J Clin Pathol 1987; 40: 335-341.
3. Weenig RH, Bruce AJ, McEvoy MT, Gibson LE, Davis MD. Neutrophilic dermatosis of the hands: four new cases and review of the literature. Int J Dermatol 2004; 43: 95-102.
4. Galaria NA, Junkins-Hopkins JM, Kligman D, James WD. Neutrophilic dermatosis of the dorsal hands: pustular vasculitis revisited. J Am Acad Dermatol 2000; 43(5 Pt 1): 870-874.
5. Evers LH, Bhavsar D, Mailänder P. The biology of burn injury. Exp Dermatol 2010; 19: 777-783.
6. Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136: 329-332.
7. Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol 2009; 23: 1008-1017.

