What’s the diagnosis?
A photodistributed rash and muscle weakness
Case presentation
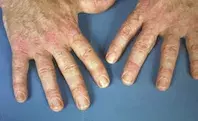
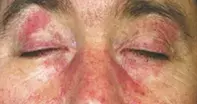
A 52-year-old farmer presents with a three-week history of a pruritic erythematous rash and associated pruritic papules on the extensor surfaces of his hands and elbows (Figure 1a). The rash, which is affecting his face (Figure 1b), chest and back, is violaceous and maculopapular and has a symmetrical distribution affecting photoexposed skin. He has also noticed progressive difficulty in performing farm duties, and especially walking uphill, due to weakness without pain or dyspnoea.
The patient was previously well. His only comorbidity is hypertension, for which he takes amlodipine 5 mg once daily; he does not have any known allergies. There is a family history of colorectal and prostate cancer.
Differential diagnoses
Conditions to consider among the differential diagnoses include the following.
- Psoriasis. This is a very common inflammatory dermatosis, affecting between 2 and 3% of individuals worldwide.1,2 It most commonly occurs before the age of 50 years and there is a bimodal onset with peaks in the second and sixth decades of life, although it can occur at any age, including during childhood. Males and females are affected equally.2 Psoriasis has many presentations; however, the classic ‘psoriasis vulgaris’ is characterised by pruritic, well-circumscribed salmon-pink plaques with overlying silvery scale affecting the extensor surfaces and scalp.1 Concomitant nail changes, which include nail pitting, oil spots, splinter haemorrhages and dystrophic change, are seen in 50% of patients with psoriasis.2 In adults, psoriasis rarely affects the face, but it is commonly seen over the metacarpophalangeal joints and on extensor surfaces. Morphologically, psoriasis plaques are scaly and raised eruptions, rather than macular. Arthralgia and stiffness may indicate psoriatic arthritis, the most common extracutaneous manifestation, affecting a quarter of individuals with psoriasis and preceding cutaneous disease in 10% of cases.2 Psoriatic arthritis usually manifests with arthralgia and stiffness and is not usually associated with muscle weakness. Although psoriasis is usually a clinical diagnosis, it has a characteristic histopathology, with histological features of an active plaque showing dermal lymphocytic (CD4 and CD8) infiltrate and dilated vascular changes with overlying epidermal hyperplasia.2
- Mixed connective tissue disease (MCTD). This idiopathic systemic autoimmune disease has clinical and serological features shared between systemic sclerosis, systemic lupus erythematosus (SLE), dermatomyositis and polymyositis.3 It is characterised by high titres of antibodies to spliceosomal protein U1 ribonucleoprotein (U1 RNP) and systemic complications, which include oesophageal dysmotility, chronic kidney disease and pulmonary arterial hypertension.4 MCTD is rare and has a predominance in females, with peak onset between 20 and 30 years of age.3 The earliest signs are Raynaud’s phenomenon, hand and digit swelling and proximal myopathy – Raynaud’s phenomenon is often the first and is followed by polyarthritis, sclerodactyly and dysphagia.3 Cutaneous manifestations include truncal poikiloderma, photosensitive erythematous facial malar eruption, small vessel vasculitis and livedoid vasculitis.5 The diagnosis of MCTD is made on clinical and serological grounds, with anti-U1 RNP antibodies being the most specific serological marker of disease.4 Histopathological features of cutaneous lesions are often nonspecific and variable, such as lichenoid interface dermatitis and keratinocyte necrosis, although the demonstration of complement membrane attack complex (C5b-9) mediated vasculitis has been reported in MCTD but not cutaneous lupus erythematosus skin lesions.6
- Systemic lupus erythematosus (SLE). This multisystem autoimmune disease predominantly affects women, with a peak onset in the third to fifth decades of life.7 Its cause is unknown, although several known triggers such as ultraviolet exposure, medications (e.g. isoniazid, statins), smoking and viral infections can initiate an autoinflammatory cascade resulting in renal, cardiopulmonary and CNS disease.7 Cutaneous involvement is common and often the earliest sign of disease, which can be SLE-specific (see below) or SLE-nonspecific (e.g. leukocytoclastic vasculitis, urticarial, livedo reticularis). Constitutional symptoms such as fever, fatigue, arthralgia, arthritis and weight loss are common. Localised acute cutaneous lupus erythematosus presents with the classic photosensitive symmetrical malar ‘butterfly’ erythema that extends over the bridge of the nose and spares the nasolabial folds, and is seen in 50% of patients with SLE on initial presentation.7 Generalised acute cutaneous lupus erythematosus is more diffuse and presents with an erythematous maculopapular eruption that may be accompanied by hand swelling involving the skin overlying the proximal and distal interphalangeal joints but spares the skin over the metacarpophalangeal joints.7 The diagnosis of SLE is made by a combination of clinical and serological criteria, with anti-double-stranded DNA (dsDNA) and anti-Smith (anti-Sm) antibodies being the most specific, having specificities of 70% and 25%, respectively, for SLE.7 Histopathological features of acute cutaneous lupus erythematosus include interface dermatitis at the dermoepidermal junction, vacuolar basal degeneration, and dermal mucin deposition; immunofluorescence demonstrates IgG and C3 deposition at the dermoepidermal junction.7
- Dermatomyositis (DM). This is the correct diagnosis. DM is an idiopathic inflammatory myopathy. Other types include polymyositis and inclusion body myositis. DM has a predominance in females and a bimodal distribution, with the juvenile form affecting those in the first decade of life whereas adult-onset DM commonly occurs in the fifth decade.8 The pathogenesis of DM is unknown but overlaps that of other connective tissue diseases (scleroderma and MCTD). The cardinal cutaneous signs of DM are an erythematous or violaceous ‘heliotrope rash’ on the eyelids (Figure 1b) and Gottron’s papules, which are erythematous papulosquamous lesions found over bony prominences of the hand, elbows and knees that may closely resemble psoriasis (Figure 1a).9 Other cutaneous features of DM include nail-fold telangiectasia, alopecia and photodistributed poikiloderma, which is known as the ‘Shawl sign’ when distributed over the upper back and the ‘V-neck sign’ when involving the chest wall.9 The cutaneous manifestations of DM may precede muscle weakness and sometimes occur without it. Muscle involvement develops as a symmetrical proximal myopathy, with accompanying fatigue and arthralgia.9
Diagnosis
A diagnosis of DM is made after evaluation of evidence of compatible cutaneous disease, symmetrical proximal myopathy and abnormal muscle enzymes (creatine kinase, lactate dehydrogenase), as well as electromyogram and muscle biopsy findings.9 Histopathological features of cutaneous DM lesions are nonspecific and difficult to distinguish from acute cutaneous lupus erythematosus, such as interface dermatitis and vacuolar basal degeneration, although increased dermal mucin deposition is typical of DM.10
Management
DM is a multisystem disease and assessment should be directed at identifying gastrointestinal, cardiac (cardiomyopathy) or pulmonary (interstitial pneumonitis) involvement, as well as identifying any underlying metachronous malignancy or concomitant CTD. Age-appropriate malignancy screening should be performed, particularly in patients with DM over the age of 50 years, because 25% of cases occur in conjunction with occult malignancy, which if treated results in resolution of DM.9 Dysphagia due to oesophageal dysmotility occurs in up to 50% of patients, reflecting myopathy of the oesophagus and oropharyngeal muscles.9
Systemic corticosteroids are first-line treatment, although a quarter of patients will not respond to this. Steroid-sparing agents are usually started to achieve or maintain remission and to avoid side effects, including steroid myopathy, which may complicate assessment of an already myopathic process, thus mimicking DM.11 Common steroid-sparing agents include methotrexate, azathioprine, cyclophosphamide, mycophenolate and ciclosporin.9 Cutaneous DM does not respond as well to treatment as myopathy, and can persist. Topical treatment options include corticosteroids and tacrolimus. Oral methotrexate and hydroxychloroquine have shown efficacy but are limited by their side-effect profile.9 Refractory DM can be managed with intravenous immunoglobulin, with an added benefit of clearing persistent DM skin lesions that often do not respond to other immunomodulators.9
Outcome
For this patient, blood tests returned negative results for anti-dsDNA, anti-U1 RNP and anti-Sm antibodies. Histopathological examination of a cutaneous lesion showed interface dermatitis and negative results for direct immunofluorescence. Electromyography was performed, which showed characteristic myopathic pattern changes of DM. He responded well to initial systemic corticosteroids. The patient returned a positive faecal occult blood test and was referred for a colonoscopy, which showed occult adenocarcinoma of the sigmoid colon, and he was subsequently referred to a colorectal surgeon. He successfully underwent a sigmoid colectomy, with subsequent resolution of his DM.
References
1. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496-509.
2. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263-271.
3. Ortega-Hernandez OD, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol 2012; 26: 61-72.
4. Burdt MA, Hoffman RW, Deutscher SL, Wang GS, Johnson JC, Sharp GC. Long-term outcome in mixed connective tissue disease: longitudinal clinical and serologic findings. Arthritis Rheum 1999; 42: 899-909.
5. Pope JE. Other manifestations of mixed connective tissue disease. Rheum Dis Clin North Am 2005; 31: 519-533.
6. Magro CM, Crowson AN, Regauer S. Mixed connective tissue disease. A clinical, histologic, and immunofluorescence study of eight cases. Am J Dermatopathol 1997; 19: 206-213.
7. Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol 2009; 10: 365-381.
8. Koler RA, Montemarano A. Dermatomyositis. Am Fam Physician 2001; 64: 1565-1572.
9. Callen JP, Wortmann RL. Dermatomyositis. Clin Dermatol 2006; 24: 363-373.
10. Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for dermatomyositis. American Academy of Dermatology. J Am Acad Dermatol 1996; 34(5 Pt 1): 824-829.
11. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003; 362: 971-982.
Muscle disorders

