Idiopathic inflammatory myopathies – an overview

Idiopathic inflammatory myopathies include several subtypes with varying clinical and pathological features. Clinical assessment in combination with selected investigations can help distinguish between subtypes and differential diagnoses.
- Idiopathic inflammatory myopathies (IIM) should be considered in patients with unexplained weakness, myalgia, rash, dyspnoea or dysphagia.
- Most patients with IIM present with progressive muscle weakness, with or without other systemic features.
- Serum creatine kinase testing is a useful screening tool but the results can be normal in some cases, independent of muscle weakness or disease severity.
- There is an association between IIM and malignancy, particularly within the first three years after diagnosis; all patients with newly diagnosed IIM should undergo age- and sex-appropriate malignancy screening.
- The management of patients with IIM requires a holistic approach, with input from GPs, various specialists and allied health practitioners.
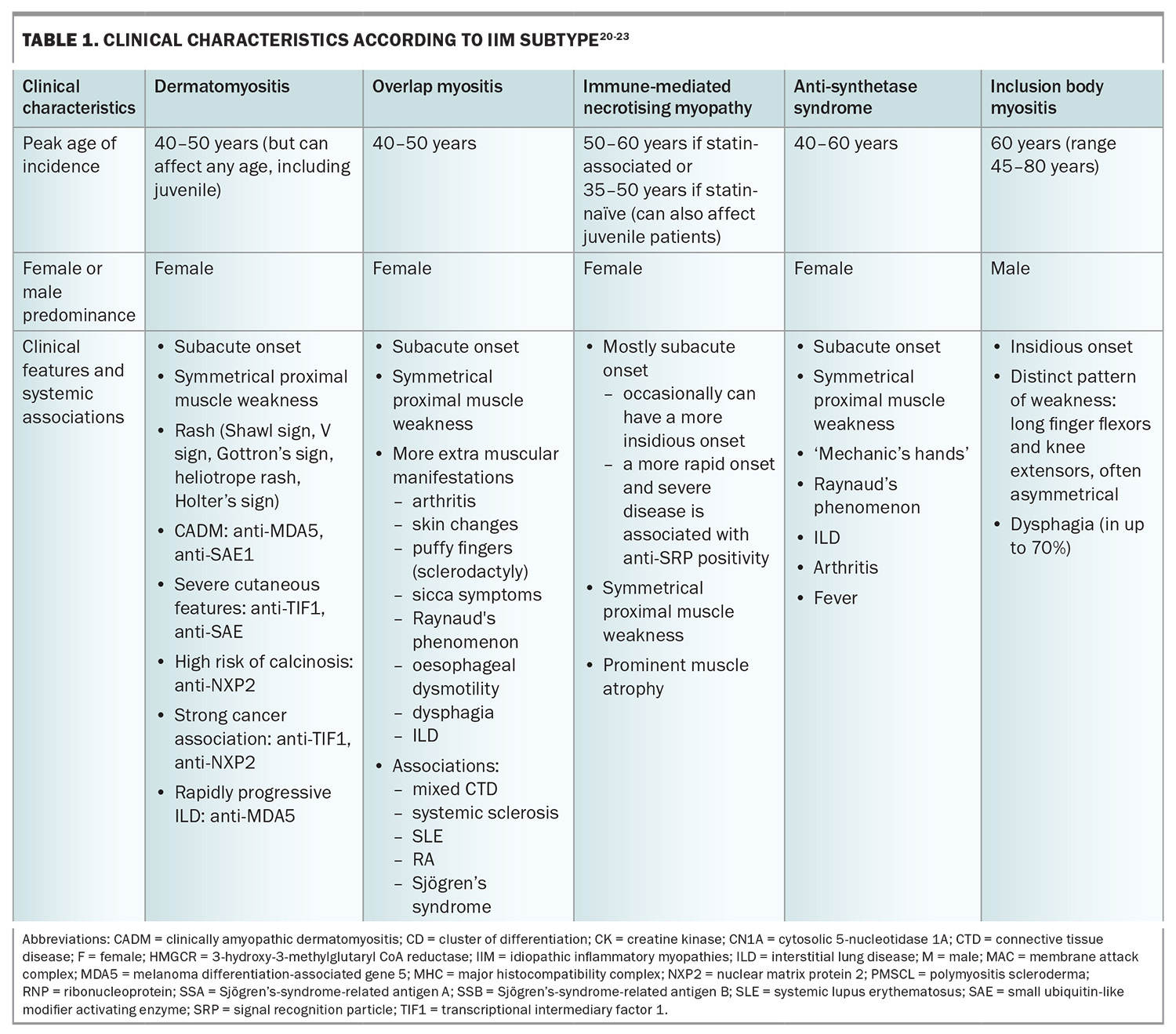
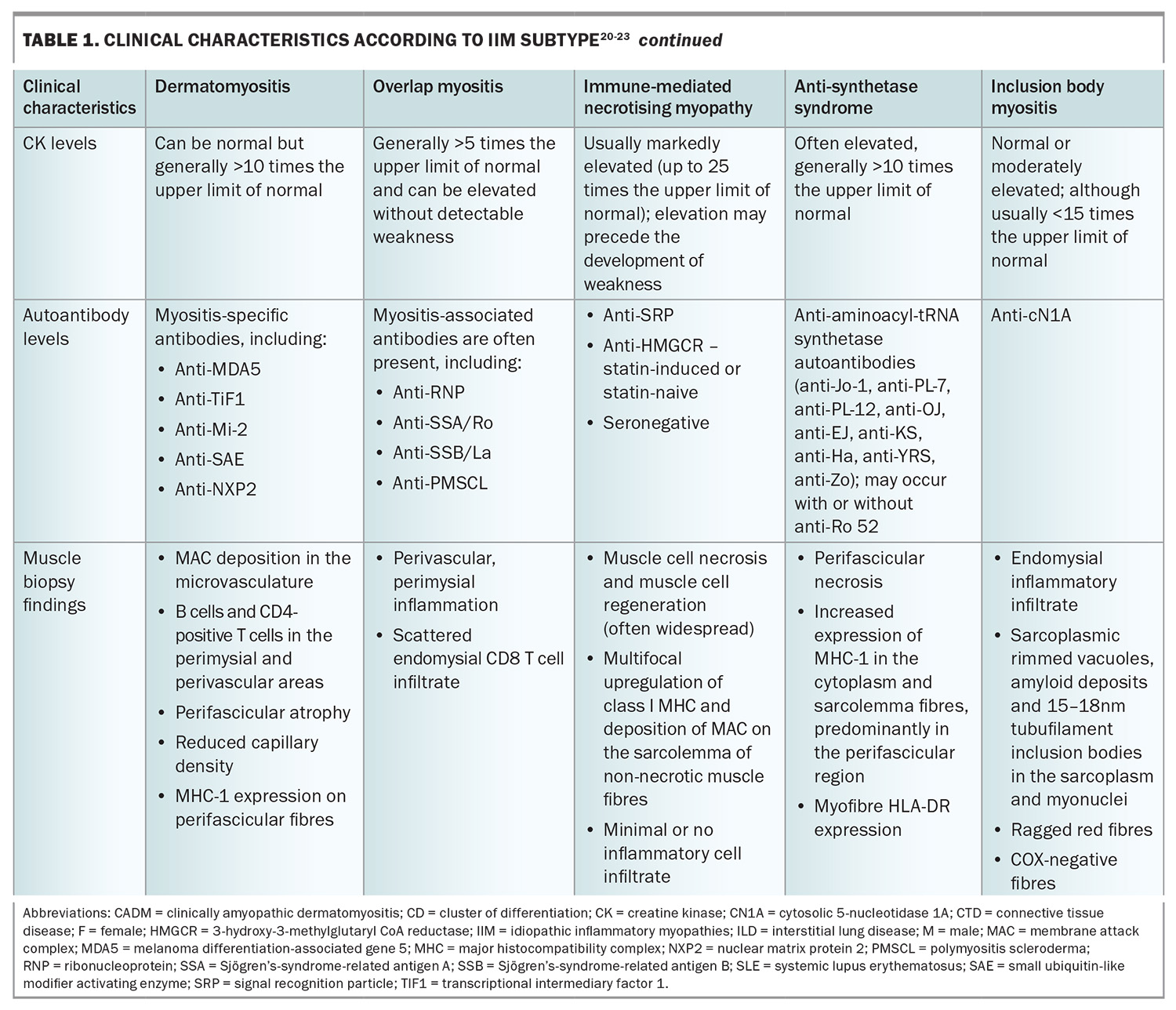
Idiopathic inflammatory myopathies (IIM) are heterogeneous disorders characterised by muscle weakness and muscle inflammation. The subtypes include dermatomyositis (DM), polymyositis (PM), antisynthetase syndrome (ASyS), overlap myositis (OM), inclusion body myositis (IBM) and immune-mediated necrotising myopathy (IMNM). PM is now thought to be a diagnosis of exclusion as more cases are now being reclassified as IBM or IMNM. ASyS was traditionally classified as either DM, PM or OM, but has more recently gained recognition as a separate entity.
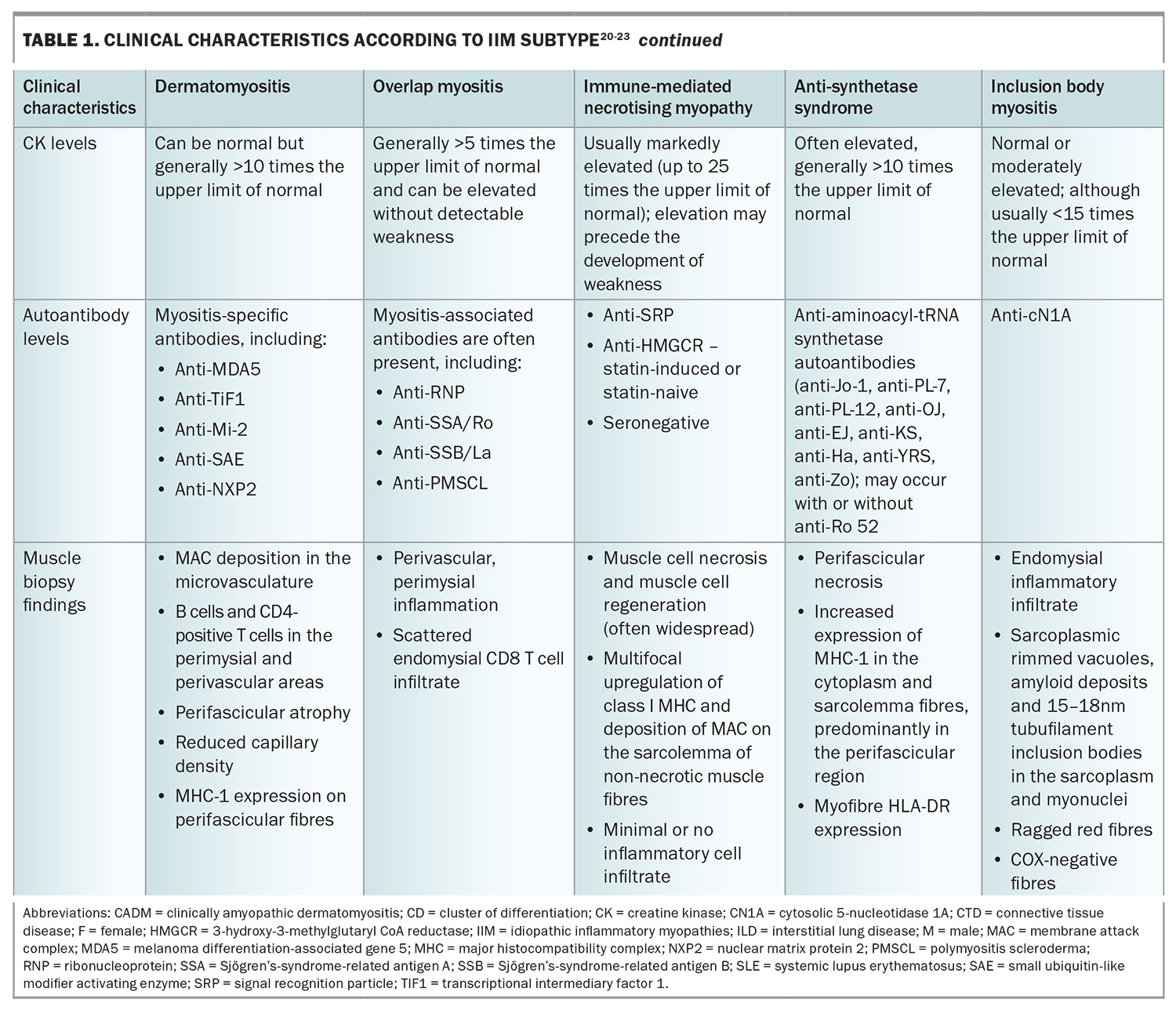
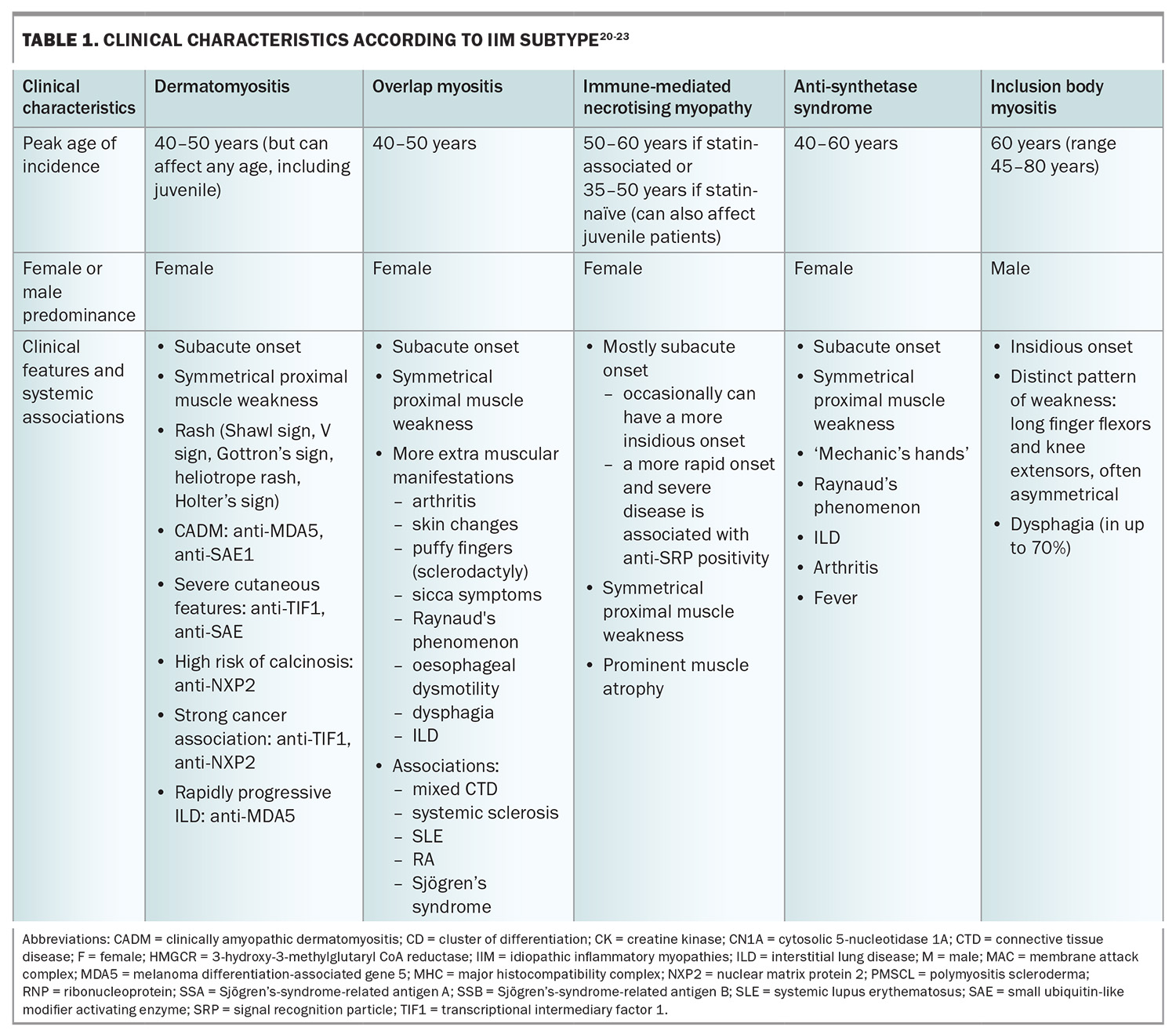
The clinical manifestations of IIM are variable; however, the majority present with progressive muscle weakness with or without other systemic features (Table 1 and Table 1 cont.), and are often found to have histopathological findings of inflammatory muscle infiltrates. The variations in clinical manifestations, serological differences and specific histopathological findings help distinguish the IIM phenotypes from one another.
The overall prevalence of IIM appears to be between 8.7 to 25 people per 100,000 population. In general, most subtypes have a female predominance, with the exception of IBM, which is more common in men. The prevalence and incidence of IIM subtypes are variable, and are influenced by factors such as sex, age, ethnicity, geographical location and ultraviolet light exposure.1-5
This article provides an overview of IIM, including its pathogenesis, clinical presentation and associations, and a suggested approach to its diagnosis and management.
Classification of IIM
The IIM classification has evolved over the years, with the identification of myositis-specific autoantibodies, as well as the recognition of newer disease subtypes. The European Alliance of Associations for Rheumatology (EULAR) and American College of Rheumatology (ACR) classification criteria for IIM were published in 2017 and incorporate 16 weighted variables – including clinical, laboratory and muscle biopsy features – to help identify IIM and accurately categorise the subtype of research participants.
One drawback of the EULAR/ACR criteria, owing to the methodology employed and the relative rarity of certain autoantibodies, was the grouping of IMNM and ASyS under the PM subheading, despite the differences in immunopathogenesis, organ involvement and response to therapies between each of these subtypes. Additionally, of all myositis-specific antibodies, only anti-Jo-1 antibodies were included in the criteria. Although these criteria are reasonably sensitive and specific for the diagnosis of IIM and the distinction from its mimics, they are not sufficiently specific for all currently recognised myositis subtypes or to use as diagnostic criteria in clinical practice.
Pathogenesis
Like many other autoimmune diseases, the pathogenesis of IIM is thought to be the result of environmental trigger(s) in genetically susceptible individuals, leading to loss of self-tolerance (the immune system’s ability to recognise self-produced antigens as non-threats while appropriately responding to foreign substances), causing chronic self-directed inflammation.
The major genetic risk factors for autoimmune myopathies relate to polymorphisms in the human leukocyte antigen (HLA) region of the major histocompatibility complex. Dysregulated presentations of self-antigens by HLA molecules may result in loss of self-tolerance and development of autoimmune responses.6 Key HLA associations include the 8.1 ancestral haplotype HLA-A1, B8; DRB3*0101 for DM, PM and IBM; and HLA-DRB1*11:01 in anti-3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR) IMNM in the adult European and UK population.7,8,9
Cigarette smoking and ultraviolet light exposure are recognised environmental triggers. Epidemiological studies have indicated an increased incidence of DM in regions with greater ultraviolet light exposure, particularly for patients with DM with anti-Mi-2 and anti-TIF1-gamma antibodies.10-12 A positive association between cigarette smoking and anti-Jo-1 autoantibody frequency was reported in European IIM patients with HLA-DRB1*03.13
Infection may also trigger disease. Suggested mechanisms include loss of self-tolerance, for example via molecular mimicry or inflammatory signalling. Observational data from various centres have revealed a rise in new DM cases coinciding with the surge in COVID-19 infections.14,15
Exposure to certain medications can be associated with the development of myositis. Statins predominantly potentiate the development of anti-HMGCR antibody-associated IMNM, which usually occurs a few years after statin exposure.16 However, anti-HMGCR antibody-associated IMNM can also occur in statin-naïve individuals. Other implicated drugs include immune checkpoint inhibitors, anti-TNF agents and D-penicillamine.17,18
Clinical presentation
Apart from those with IBM, patients with IIM most commonly present with subacute, progressive symmetrical weakness. Common symptoms include difficulty raising the arms above the head, trouble climbing stairs or standing from sitting on a low chair. There may be associated myalgia and muscle tenderness, although prominent muscle pain is unusual. Involvement of the pharyngeal muscles can lead to dysphagia, and respiratory muscle involvement can result in nocturnal hypoventilation and associated sleep disruption.19 On examination, there is typically symmetrical proximal weakness. Neck flexor weakness may be an indication of bulbar involvement and is important not to miss. Muscle atrophy may be seen, particularly in IMNM, even at presentation. Muscle swelling (oedema) with tenderness can also be present, particularly in DM.
IBM, in contrast to the other forms of IIM, typically presents with slowly progressive asymmetrical muscle weakness, with prominent involvement of deep finger flexors and quadriceps muscles. Dysphagia is frequently present. IBM is associated with marked morbidity, with a significant proportion of affected patients becoming wheelchair dependent within 10 to 20 years from disease onset. Unlike other forms of IIM, there is no extramuscular involvement.
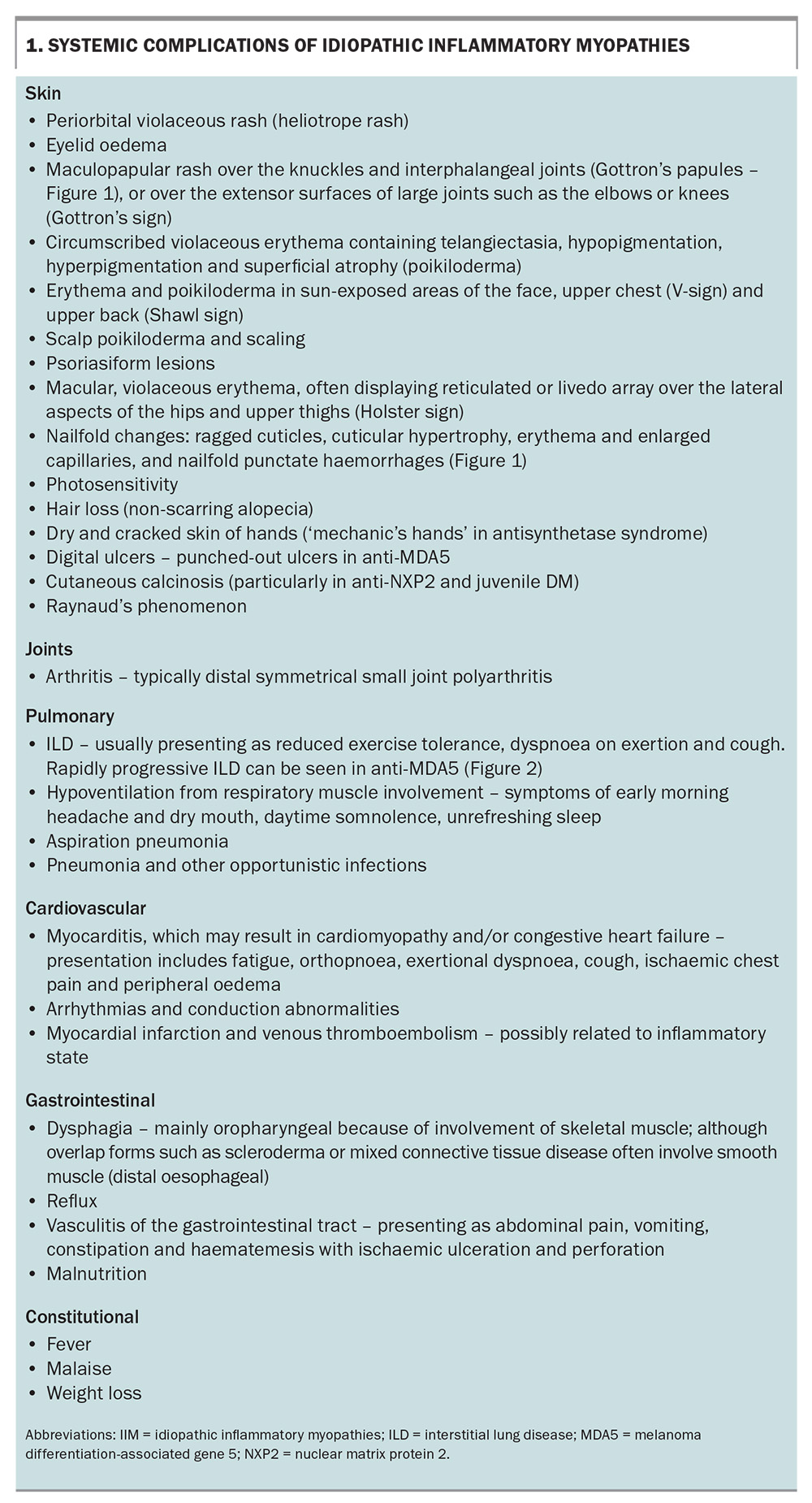
A thorough systemic enquiry must be performed to detect both muscular and extramuscular manifestations of IIM. Box 1 lists the potential systemic complications of IIM and the associated symptoms to screen for, both for diagnosis as well as monitoring for disease relapse.
Malignancy and IIM
An important consideration is the association between IIM and malignancy. This association is the highest within three years of IIM diagnosis, with the highest risk within the first year.24 A systematic review and meta-analysis found that, compared with the general population, patients with DM and PM had overall relative risks of malignancy of 4.66 and 1.75, respectively. The risk remains increased but gradually falls off over the 10 years after IIM diagnosis.25 All patients with a new diagnosis of IIM should undergo age- and sex-appropriate screening as per local guidelines. Depending on risk stratification, patients with certain clinical risk factors may require a more in-depth malignancy screen. One widely recognised and used guideline is the International Guideline for Idiopathic Inflammatory Myopathy-Associated Cancer Screening: An International Myositis Assessment and Clinical Studies Group (IMACS) Initiative.26 It provides recommendations addressing IIM-associated cancer risk stratification, cancer screening modalities and screening frequency.
Risk factors and protective factors
The risk of cancer varies in accordance with certain clinical, laboratory and serological features. Of the IIM subtypes, DM carries the highest risk of malignancy. Identified clinical risk factors are older age at IIM onset, male sex, cutaneous ulceration, presence of dysphagia and severe or refractory disease. Interestingly, laboratory risk factors include lower levels of creatine kinase (CK) and serum lactate dehydrogenase. In terms of serological risk factors, the highest association with cancer was found in patients with anti-TIF1-gamma antibody positivity, followed by patients with anti-NXP2 positivity. Antibodies with moderate risk include anti-SAE1, anti-HMGCR, anti-Mi-2 and anti-MDA5.27 Protective clinical factors are interstitial lung disease, Raynaud’s phenomenon and arthritis.
Types of cancers associated with IIM
The types of cancer that patients with IIM may be at higher risk of differ by IIM subtype and the geographical region. Cancers associated with DM that have been reported in studies in Scandinavian countries have included ovarian, lung, pancreatic, stomach and colorectal cancers, as well as non-Hodgkin lymphoma.28 An analysis of two large cohorts of 400 patients from the USA found that the most frequently reported malignancies in patients with DM were breast (24.5%), hematological (17.0%), colorectal (9.4%) and prostate (9.4%).29
Investigations
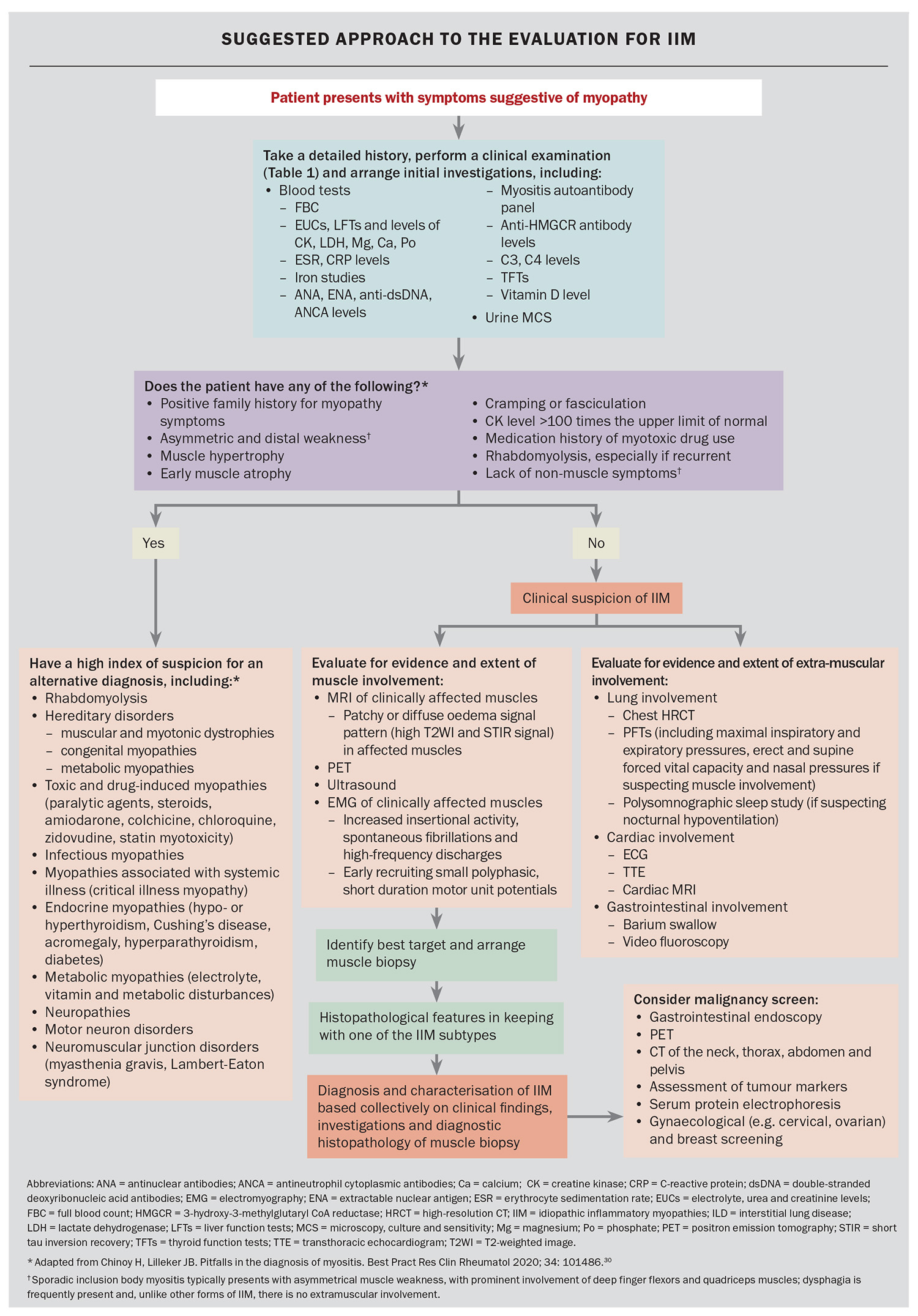
Following a comprehensive history and examination, the workup for possible IIM consists largely of blood tests, imaging and muscle biopsy. As part of the initial workup, clinicians should also consider investigations to rule out alternative forms of muscle disease, such as abnormal thyroid function or electrolyte disturbances. Nerve conduction studies and electromyography can also be used in some cases to distinguish an inflammatory myopathy from a noninflammatory myopathy, and to exclude an underlying neurogenic or neuromuscular junction disorder. The Flowchart provides a suggested algorithm that can be used in the workup of patients with IIM.
It is worth noting that CK levels vary across different subtypes of IIM and can be normal in some forms of amyopathic myositis, or in IBM, independent of muscle weakness or disease severity. In a Japanese cohort of IIM patients with anti-MDA5 positivity, only 20% were found to have elevation of CK.31-33 However, when CK levels are significantly elevated, they serve as useful markers for the monitoring of treatment response and relapse. Along with CK levels, aspartate aminotransferase and alanine aminotransferase levels are also elevated, which can sometimes be erroneously interpreted as indicating liver disease.
More specific blood tests consist of immunological testing – looking for the presence of myositis-associated and myositis-specific antibodies. The recognition of these autoantibodies has helped with the characterisation of myositis as distinct subtypes, as highlighted in Table 1 and Table 1 cont. They have important diagnostic and prognostic value because of their distinct disease manifestations and associations. The choice and intensity of treatment, and modalities for malignancy screening, are increasingly informed by the identification of myositis-specific antibodies.
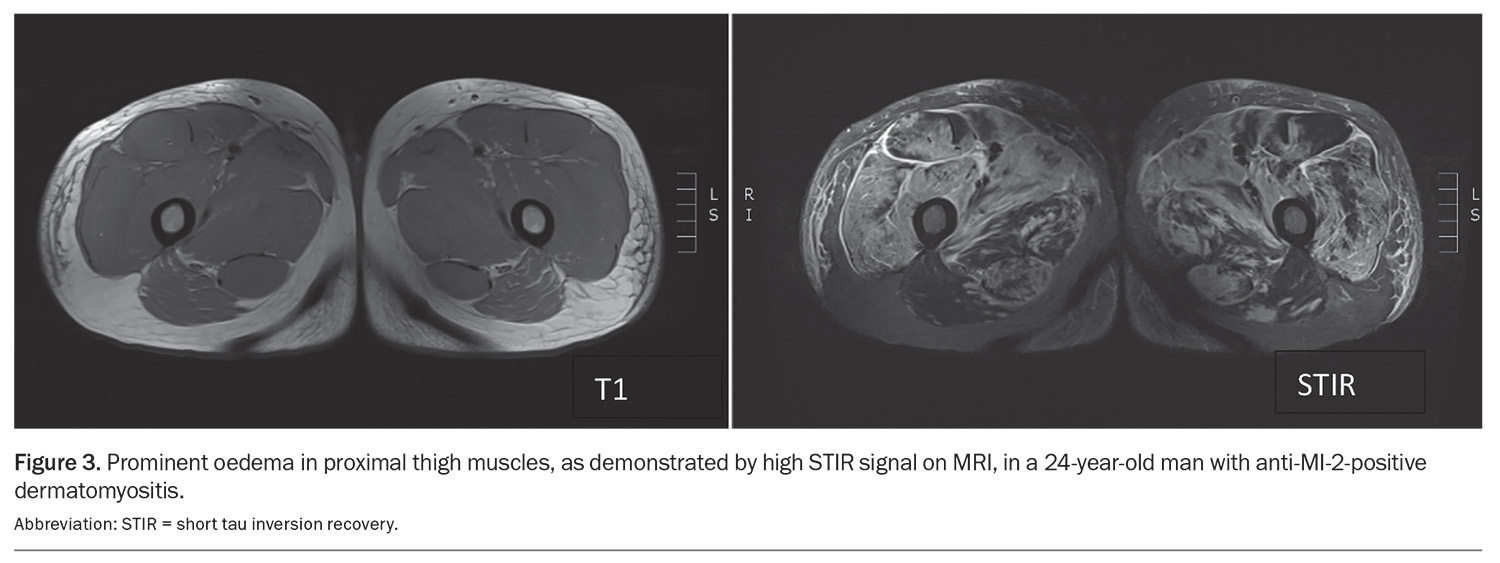
In terms of muscle imaging, MRI is an important imaging modality that can be used to evaluate the extent and pattern of muscle involvement, identify an appropriate site for muscle biopsy and monitor response to treatment (Figure 3). The pattern of muscle involvement may help differentiate between the IIM subtypes. For example, pelvic and adductor muscles are commonly involved in IMNM but are relatively spared in DM and PM patients.34 Conversely, muscle inflammation in IBM is frequently found in the anterior muscles of the thigh (quadriceps) and forearm (flexor digitorum profundus), and the distal part of thigh muscles, with relative sparing of the rectus femoris muscles.35
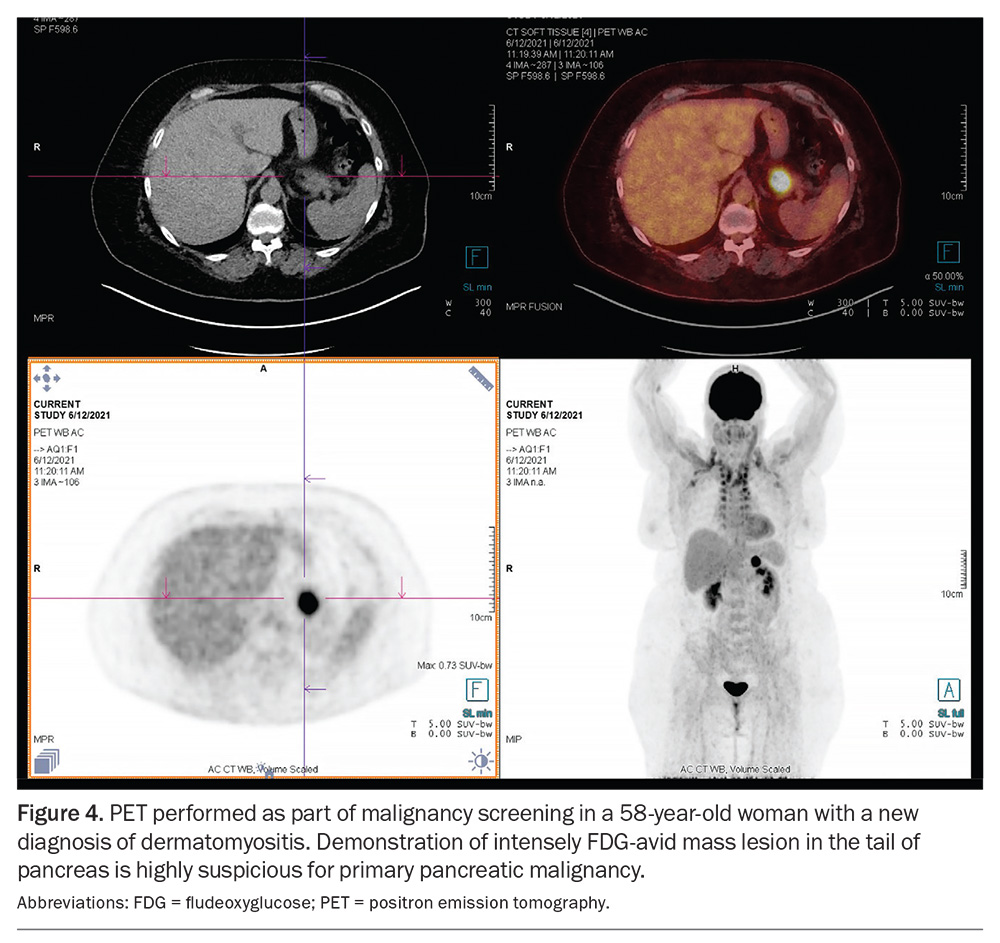
Positron emission tomography-computed tomography (PET-CT) is an increasingly used imaging modality in the workup of IIM (Figure 4). It has proven to be useful in the screening for occult cancers in patients with IIM at a higher risk of underlying malignancies, such as those with DM with anti-TIF1-gamma antibodies. PET-CT imaging can evaluate the extent and activity of muscle inflammation, although its sensitivity is poorer than MRI. Lastly, it can help detect other organ involvement in IIM, such as lung uptake in ILD.36
Specialist referral
Once a diagnosis of myositis is suspected, prompt referral to a specialist with expertise and experience in managing myositis should be done as soon as possible, so as not to delay the diagnosis and commencement of treatment. A delay in treatment might result in irreversible damage to muscles and other organs such as the lungs, as in the case of rapidly progressive ILD.
It is helpful for GPs to perform initial blood tests to help rule out differential diagnoses and narrow down the diagnosis, and to consider other basic investigations to evaluate for the evidence of extra-muscular involvement. Referral, however, should not be delayed while awaiting test results. Timely diagnosis and early specialist referral are crucial – all patients with new-onset or active myositis are considered category 1 (appointment within 30 calendar days). If patients are systemically unwell with potential complications of either the disease or therapy, requiring emergent review, they should be referred to the emergency department (via ambulance if necessary) or as per local emergency care protocols. Red flags that should prompt consideration for a referral to the emergency department include:
- rapidly progressive disease (over days to weeks)
- severe weakness (including dysphagia)
- respiratory failure (in the setting of respiratory muscle weakness or ILD)
- heart failure
- severe cutaneous lesions.
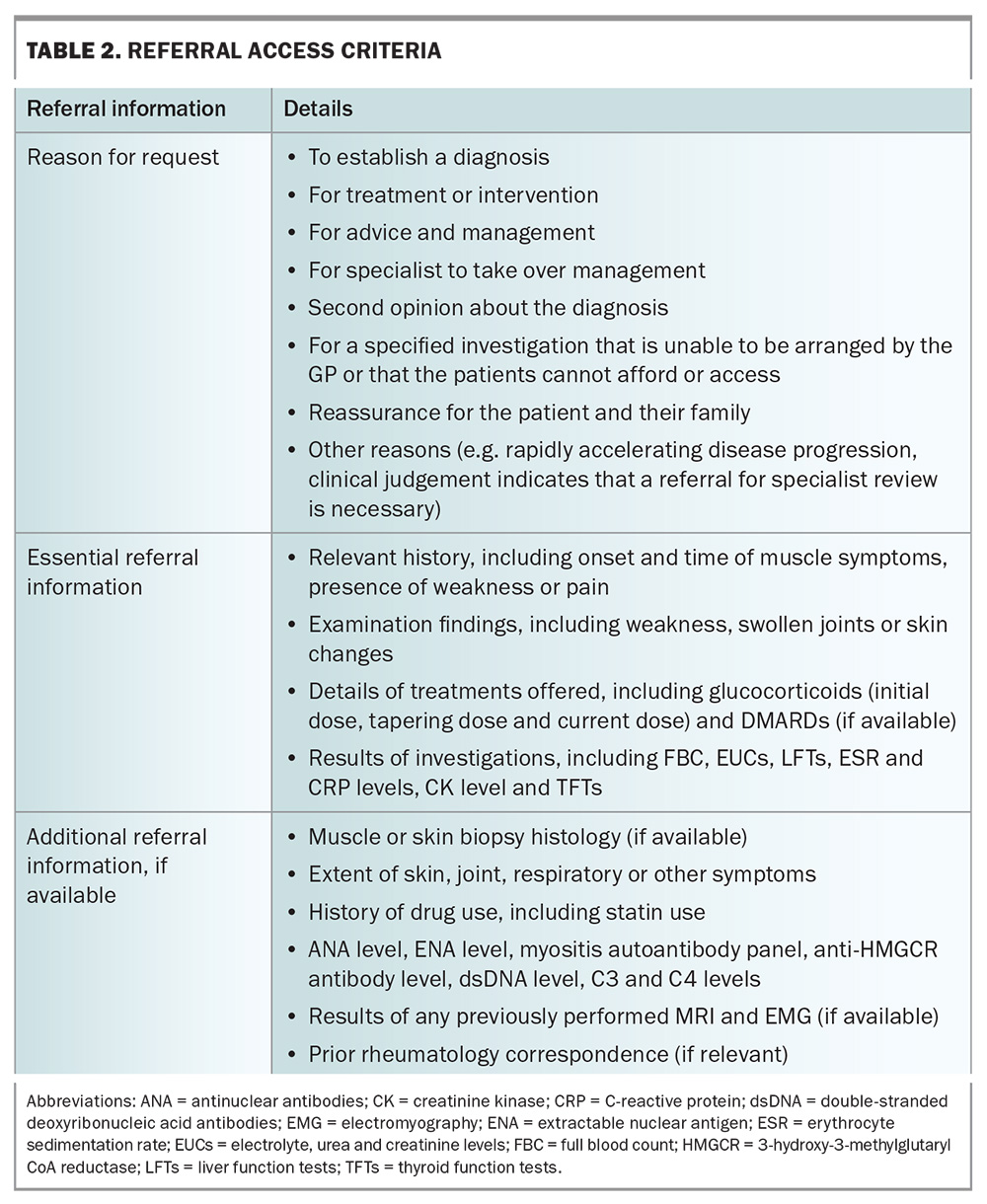
A list of helpful details to include in a referral to a rheumatology service for a patient with suspected IIM is demonstrated in Table 2 (adapted from the Department of Health Myositis Referral Access Criteria). Box 2 lists some websites that contain relevant information about the referral process for patients with suspected myositis in different states and territories.
Management
The focus of management in patients with IIM is on restoring muscle strength, preserving functionality and quality of life, and preventing other organ damage. This usually requires a multidisciplinary approach, including primary care with input from allied health and various specialties, depending on the disease manifestations and systemic involvement. Care should be holistic, addressing not just the physical and psychological aspects of disease, but also its social implications.
Patients should be primarily under the care of a specialist with expertise and experience in managing myositis, with periodic reviews for as long as their disease is active. GPs also have an important role to play in the care of patients with IIM, including working closely with specialists to support medication adherence, as well as appropriate monitoring for medication side effects. Recognising disease relapse is critical, and, as primary care physicians are often the first point of contact for patients, they play a key role in flagging patients with potential disease flares to their specialists. Other important roles include ongoing management of key comorbidities such as cardiovascular risk factors, bone health, mood and sleep disturbances, and screening for malignancy (if not already conducted). Monitoring for infection and ensuring vaccinations are up to date are also important given the risks associated with immunosuppressive treatments.
The mainstay of treatment for most IIM (apart from IBM) is immunosuppression combined with a personalised exercise program. Immunosuppressant choice, primarily dictated by the treating specialist, is based on the disease severity and rate of progression, and individualised, based on a patient’s comorbidities and access to treatment.
Glucocorticoids
Corticosteroids are the first-line treatment for most patients with IIM. Prednisolone is usually initiated at a dose of 1 mg/kg, then slowly tapered to the lowest possible dose to keep the disease controlled. In severe cases, or where there is significant end-organ damage, intravenous methylprednisolone induction therapy may be required and is usually administered at doses of 500 to 1000 mg daily for three days. The duration and dosing of ongoing oral steroid therapy is highly individualised. In most cases, additional steroid-sparing therapies are initiated very early in the disease course or at the same time as initiation of steroids to reduce overall steroid requirements. Given the well-established side effects of prolonged steroid use, it is vital that patients undergo regular monitoring of their blood pressure, blood sugar levels, bone health and mental health (screening for mood disturbances), with the assistance of the patient’s primary care team.
Nonbiologic DMARDs
The usual choices for a second-line agent are methotrexate, azathioprine, and mycophenolate mofetil. There are no head-to-head trials comparing these drugs, although all have shown efficacy in the treatment of IIM. Treatment choice is clinician-dependent and based on patient characteristics.
Methotrexate is an antifolate that inhibits lymphocyte proliferation. Typical doses used are between 10 and 20 mg weekly, with the addition of folic acid to reduce some of the side effects.
Azathioprine is a purine antimetabolite that interferes with T and B cell proliferation. The initial dose is 25 to 50 mg daily, with increments of 25 mg per week to target dose of 2 to 3 mg/kg of the patient’s ideal body weight.
Mycophenolate is another anti-metabolite that blocks the proliferation of T and B lymphocytes by inhibiting guanosine synthesis. Typical starting doses are 500 mg daily or twice daily and increased by 500 mg weekly to a target dose of 1000 to 1500 mg twice daily.
Another class of medications is the calcineurin inhibitors: cyclosporin and tacrolimus. They work by impairing the transcription of interleukin-2 and other cytokines in T lymphocytes, and hence interfere with T cell activation, proliferation, and differentiation. Combination therapy with another DMARD may be required for patients with severe or refractory disease.
Cyclophosphamide is a nitrogen mustard-alkylating agent that blocks both T and B cell proliferation. It is used infrequently in IIM and usually reserved for cases where multiple other lines of therapy have failed or in patients with highly aggressive and organ-threatening disease manifestations. It can be given as a weekly intravenous infusion of 0.6 to 1 g/m2 for up to six to 12 months.
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) has considerable value in the treatment of patients with IIM, particularly in cases where immunosuppressants are contraindicated, such as in the setting of fulminant infections or pregnancy. A trial conducted in the Netherlands demonstrated that IVIG monotherapy used as first-line treatment in patients with IIM led to at least moderate improvement in nearly half of patients, with a fast clinical response in the majority of responders.37 IVIG has a complex immunomodulatory mechanism of action, including reduction of complement activation and membrane attack complex deposition in muscle fibres and muscle capillaries; reduced and altered profile of cytokines and adhesion molecules; reduced T cell activation and migration.38 It is usually administered at doses of 1 g/kg monthly (maintenance dose), but the dose or interval between infusions can be altered based on disease severity and treatment responsiveness.
Biologic DMARDS
Rituximab is a monoclonal humanised chimeric antibody to CD20 that acts through the depletion of B cells in the circulation. It is usually given as two doses of 1000 mg, two weeks apart. This course can be repeated in six to 12 months, often guided by reconstitution of B cells. Several small, open-label studies have reported benefit in patients with severe and refractory IIM.39-41
Tocilizumab, an antagonist of the interleukin-6 receptor, showed efficacy in an open-label pilot study involving patients with refractory IIM.42 However, in a multicentre, randomised, double-blinded, placebo-controlled trial in adult patients with refractory DM and PM, it unfortunately did not meet the primary or secondary efficacy outcomes.43
Abatacept is a cytotoxic T lymphocyte- associated protein 4 (CTLA-4) analogue that inhibits costimulatory signalling between antigen-presenting cells and T cells. Case reports and small open-label studies have reported benefit in patients with DM and IMNM. There is an ongoing trial to evaluate the efficacy and safety of abatacept in combination with standard therapy, compared with standard therapy alone in improving disease activity in adults with active IIM (ClinicalTrials.gov Identifier NCT02971683).
Janus kinase (JAK) inhibitors suppress JAK, which are cytoplasmic protein tyrosine kinases that mediate key nuclear signal transduction and downstream activation of inflammatory pathways. JAK inhibitors that have been studied in patients with IIM include tofacitinib, ruxolitinib and baricitinib. There have been several case series showing the effectiveness of JAK inhibitors in severe DM, including anti-MDA5 myositis and severe refractory calcinosis.44 Currently, a phase 2a trial with baricitinib (JAK 1/2 inhibitor) is ongoing (ClinicalTrials.gov Identifier: NCT04208464).
Emerging therapies
There are currently multiple trials ongoing for several agents including lenabasum (a cannabinoid receptor type-2 agonist), sifalimumab (anti-interferon-α monoclonal antibody), ustekinumab (interleukin-12/interleukin-23 inhibitor), efgargitimod (neonatal FcFn inhibitor), ravulizumab and PF-06823859 (selective, humanised immunoglobulin 1-neutralising monoclonal antibody directed against human interferon-β).
Management of IBM
The focus of management in IBM is largely nonpharmacological, as there are no effective treatments currently available. Unlike the other subtypes of myositis, IBM is typically refractory to standard immunosuppressive therapy.45 Some studies have found some short-term improvement in dysphagia with IVIG therapy, and therefore IVIG may be trialled for dysphagia.46 There have been some recent studies looking at the use of arimoclomol and bimagrumab, although they did not meet their primary endpoints. There are trials of a humanised anti-KLRG1 antibody and sirolimus ongoing at the time of writing.
Conclusion
The IIM are heterogeneous disorders that affect muscle, often along with extra-muscular manifestations, which result in significant disability and impact on patients’ quality of life. The diagnosis is based on features from clinical assessment, serological testing and imaging, with or without muscle biopsy, and often requires the expertise from several specialities, including rheumatology, immunology, neurology, dermatology and respiratory medicine. Early diagnosis and effective therapy remain key for optimal prognosis. MT
COMPETING INTERESTS: None.
References
1. Barnabe C, Joseph L, Belisle P, et al. Prevalence of systemic lupus erythematosus and systemic sclerosis in the First Nations population of Alberta, Canada. Arthritis Care Res (Hoboken) 2012; 64: 138-143.
2. Dobloug C, Garen T, Bitter H, et al. Prevalence and clinical characteristics of adult polymyositis and dermatomyositis; data from a large and unselected Norwegian cohort. Ann Rheum Dis. 2015; 74: 1551-1556.
3. Bernatsky S, Joseph L, Pineau CA, et al. Estimating the prevalence of polymyositis and dermatomyositis from administrative data: age, sex and regional differences. Ann Rheum Dis 2009; 68: 1192-1196.
4. Svensson J, Arkema EV, Lundberg IE, Holmqvist M. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology (Oxford) 2017; 56: 802-810.
5. Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017; 76: 1955-1964.
6. Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: the immune system in health and disease. 5th ed. New York: Garland Science; 2001. Autoimmune responses are directed against self antigens. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK27155/ (accessed October 2024).
7. McHugh NJ, Tansley SL. Autoantibodies in myositis. Nat Rev Rheumatol 2018; 14: 290-302.
8. Candore G, Lio D, Colonna Romano G, Caruso C. Pathogenesis of autoimmune diseases associated with 8.1 ancestral haplotype: effect of multiple gene interactions. Autoimmun Rev 2002; 1: 29-35.
9. Miller FW, Chen W, O’Hanlon TP, et al. Genome-wide association study identifies HLA 8.1 ancestral haplotype alleles as major genetic risk factors for myositis phenotypes. Genes Immun 2015; 16: 470-480.
10. Hengstman GJ, van Venrooij WJ, Vencovsky J, Moutsopoulos HM, van Engelen BG. The relative prevalence of dermatomyositis and polymyositis in Europe exhibits a latitudinal gradient. Ann Rheum Dis 2000; 59: 141-142.
11. Shah M, Targoff IN, Rice MM, Miller FW, Rider LG; Childhood Myositis Heterogeneity Collaborative Study Group. Brief report: ultraviolet radiation exposure is associated with clinical and autoantibody phenotypes in juvenile myositis. Arthritis Rheum 2013; 65: 1934-1941.
12. Parkes JE, Rothwell S, Oldroyd A, Chinoy H, Lamb JA; Myositis Genetics Consortium (MYOGEN). Genetic background may contribute to the latitude-dependent prevalence of dermatomyositis and anti-TIF1-γ autoantibodies in adult patients with myositis. Arthritis Res Ther 2018; 20: 117.
13. Chinoy H, Adimulam S, Marriage F, et al. Interaction of HLA-DRB1*03 and smoking for the development of anti-Jo-1 antibodies in adult idiopathic inflammatory myopathies: a European-wide case study. Ann Rheum Dis 2012; 71: 961-965.
14. Gokhale Y, Patankar A, Holla U, et al. Dermatomyositis during COVID-19 pandemic (a case series): is there a cause effect relationship? J Assoc Physicians India 2020; 68: 20-24.
15. Tonutti A, Motta F, Ceribelli A, Isailovic N, Selmi C, De Santis M. Anti-MDA5 antibody linking COVID-19, type i interferon, and autoimmunity: a case report and systematic literature review. Front Immunol 2022; 13: 937667.
16. Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol 2018; 14: 215-224.
17. Liu SW, Velez NF, Lam C, et al. Dermatomyositis induced by anti-tumor necrosis factor in a patient with juvenile idiopathic arthritis. JAMA Dermatol 2013; 149: 1204-1208.
18. Carroll GJ, Will RK, Peter JB, Garlepp MJ, Dawkins RL. Penicillamine induced polymyositis and dermatomyositis. J Rheumatol 1987; 14: 995-1001.
19. Bourke SC. Respiratory involvement in neuromuscular disease. Clin Med (Lond) 2014; 14: 72-75.
20. Ashton C, Paramalingam S, Stevenson B, Brusch A, Needham M. Idiopathic inflammatory myopathies: a review. Intern Med J 2021; 51: 845-852.
21. Naddaf E. Inclusion body myositis: Update on the diagnostic and therapeutic landscape. Front Neurol 2022; 13: 1020113.
22. Yongchairat K, Tanboon J, Waisayarat J. Clinical spectrums and outcomes of necrotizing autoimmune myopathy versus other idiopathic inflammatory myopathies: a multicenter case-control study. Clin Rheumatol 2019; 38: 3459-3469.
23. Noguchi E, Uruha A, Suzuki S, et al. Skeletal muscle involvement in antisynthetase syndrome. JAMA Neurol 2017; 74: 992-999.
24. Qiang JK, Kim WB, Baibergenova A, Alhusayen R. Risk of malignancy in dermatomyositis and polymyositis. J Cutan Med Surg 2017; 21: 131-136.
25. Dani L, Ian Che W, Lundberg IE, Hellgren K, Holmqvist M. Overall and site-specific cancer before and after diagnosis of idiopathic inflammatory myopathies: A nationwide study 2002-2016. Semin Arthritis Rheum 2021; 51: 331-337.
26. Oldroyd AGS, Callen JP, Chinoy H, et al. International guideline for idiopathic inflammatory myopathy-associated cancer screening: an International Myositis Assessment and Clinical Studies Group (IMACS) initiative. Nat Rev Rheumatol 2023; 19: 805-817.
27. Marzęcka M, Niemczyk A, Rudnicka L. Autoantibody markers of increased risk of malignancy in patients with dermatomyositis. Clin Rev Allergy Immunol 2022; 63: 289-296.
28. Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 2001; 357: 96-100.
29. Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore) 2018; 97: e9639.
30. Chinoy H, Lilleker JB. Pitfalls in the diagnosis of myositis. Best Pract Res Clin Rheumatol 2020; 34: 101486.
31. Sizemore TC. In the idiopathic inflammatory myopathies, how significant is creatine kinase levels in diagnosis and prognosis? A case study and review of the literature. J Rheum Dis Treat 2015: 1: ISSN: 2469-5726.
32. Benveniste O, Guiguet M, Freebody J, et al. Long-term observational study of sporadic inclusion body myositis. Brain 2011; 134: 3176-3184.
33. Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T. Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology (Oxford) 2010; 49: 1726-1733.
34. Day JA, Bajic N, Gentili S, Patel S, Limaye V. Radiographic patterns of muscle involvement in the idiopathic inflammatory myopathies. Muscle Nerve 2019; 60: 549-557.
35. Dion E, Cherin P, Payan C, et al. Magnetic resonance imaging criteria for distinguishing between inclusion body myositis and polymyositis. J Rheumatol 2002; 29: 1897-1906.
36. Selva-O’Callaghan A, Gil-Vila A, Simó-Perdigó M, Trallero-Araguás E, Alvarado-Cárdenas M, Pinal-Fernandez I. PET scan: nuclear medicine imaging in myositis. Curr Rheumatol Rep 2019; 21: 64.
37. Lim J, Eftimov F, Verhamme C, et al. Intravenous immunoglobulins as first-line treatment in idiopathic inflammatory myopathies: a pilot study. Rheumatology (Oxford) 2021; 60: 1784-1792.
38. Wang DX, Shu XM, Tian XL, et al. Intravenous immunoglobulin therapy in adult patients with polymyositis/dermatomyositis: a systematic literature review. Clin Rheumatol 2012; 31: 801-806.
39. Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum 2005; 52: 601-607.
40. Brulhart L, Waldburger JM, Gabay C. Rituximab in the treatment of antisynthetase syndrome. Ann Rheum Dis 2006; 65: 974-975.
41. Noss EH, Hausner-Sypek DL, Weinblatt ME. Rituximab as therapy for refractory polymyositis and dermatomyositis. J Rheumatol 2006; 33: 1021-1026.
42. Li S, Li W, Jiang W, He L, Peng Q, Wang G, Lu X. The efficacy of tocilizumab in the treatment of patients with refractory immune-mediated necrotizing myopathies: an open-label pilot study. Front Pharmacol 2021; 12: 635654.
43. Oddis CV, Rockette HE, Zhu L, et al. Randomized trial of tocilizumab in the treatment of refractory adult polymyositis and dermatomyositis. ACR Open Rheumatol 2022; 4: 983-990.
44. Zeng R, Glaubitz S, Schmidt J. Antibody therapies in autoimmune inflammatory myopathies: promising treatment options. Neurotherapeutics 2022; 19: 911-921.
45. Schmidt K, Schmidt J. Inclusion body myositis: advancements in diagnosis, pathomechanisms, and treatment. Curr Opin Rheumatol 2017; 29: 632-638.
46. Mohannak N, Pattison G, Hird K, Needham M. Dysphagia in patients with sporadic inclusion body myositis: management challenges. Int J Gen Med 2019; 12: 465-474.