Keratinocyte skin cancers: updates on diagnosis and management
Keratinocyte cancers, formerly known as non-melanoma skin cancers, comprise basal cell carcinoma and cutaneous squamous cell carcinoma. GPs are at the forefront of the detection and management of keratinocyte cancers, which are an important cause of morbidity and mortality in Australia. This updated summary for GPs incorporates the latest advances in management.
- Australia has one of the highest rates of skin cancer in the world, with keratinocyte cancers being the most common skin cancers encountered in general practice.
- The Cancer Council Australia Clinical practice guidelines for keratinocyte cancer are regularly updated and are a useful tool for GPs to provide evidence-based patient care.
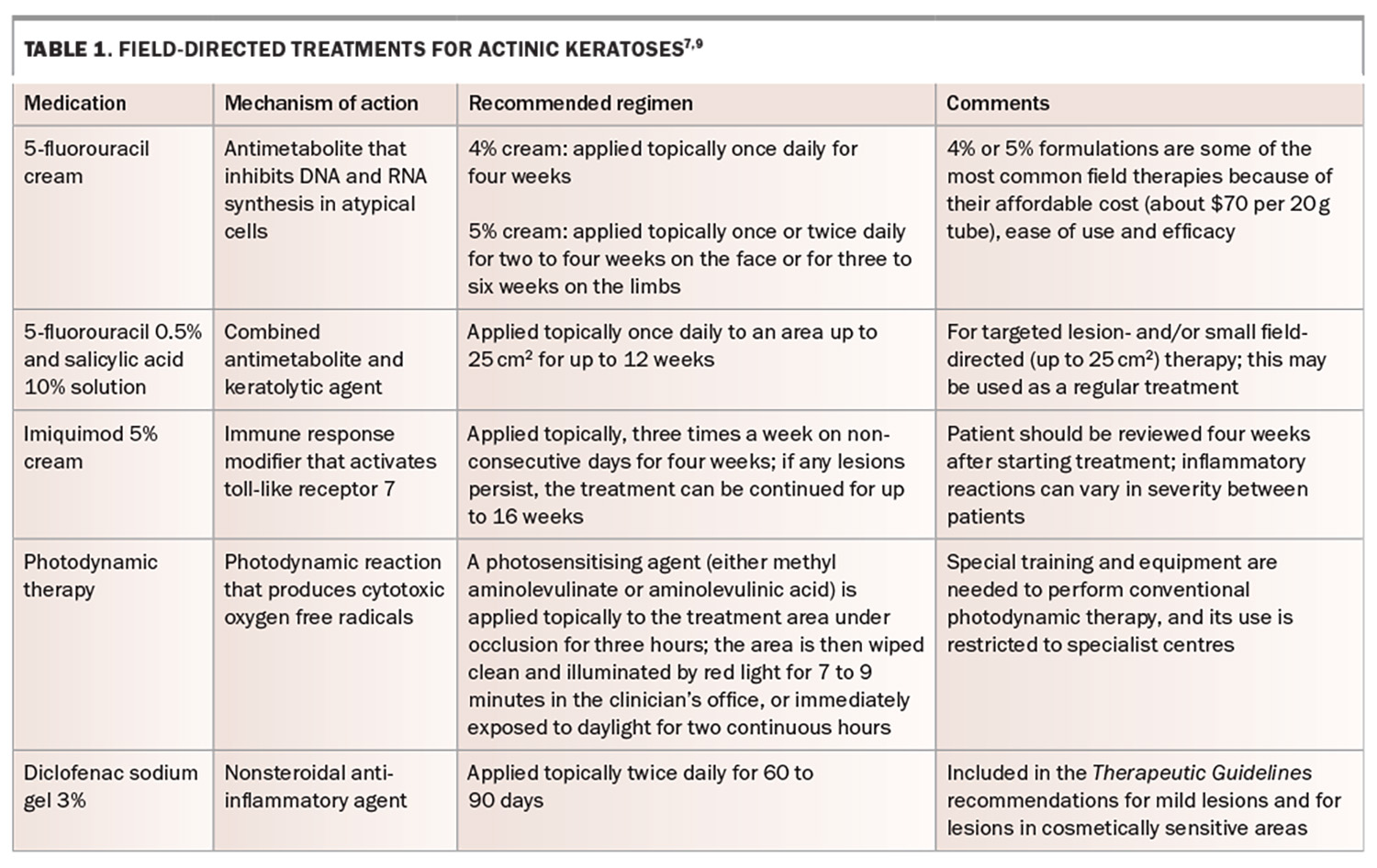
- The 5-fluorouracil 0.5% and salicylic acid 10% solution is a new combined antimetabolite and keratolytic agent for targeted lesion-directed and/or small field-directed (up to 25 cm2) therapy for actinic keratoses.
- Brachytherapy with rhenium-188 is now TGA-listed for the treatment of thin keratinocyte cancers.
- PBS-funded systemic therapies are now available for patients with locally advanced or metastatic keratinocyte cancers who are not suitable candidates for surgery or radiotherapy. These therapies include monoclonal antibodies (cetuximab and cemiplimab) for cutaneous squamous cell carcinoma, and hedgehog pathway inhibitors (sonidegib and vismodegib) for basal cell carcinoma.
- Patients with locoregional advanced keratinocyte cancers should be managed by a multidisciplinary team, comprising dermatologists, surgeons and radiation and medical oncologists.
Australia has one of the highest rates of skin cancer in the world, with keratinocyte cancers being the most common type. Keratinocyte cancers, formerly known as non-melanoma skin cancers, comprise basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC) (cSCC). Actinic keratosis (AK) is a potentially premalignant cutaneous lesion that may transform into cSCC if left untreated, although this is rare.
In 2021, keratinocyte cancers caused 760 deaths in Australia and was the most common cancer recorded as the principal diagnosis for hospitalisation.1 An estimated 69% of Australians will undergo at least one excision for keratinocyte cancer in their lifetime, and this is likely to increase as the population ages.2 In 2021 alone, the total cost to the Australian Government for new patients with keratinocyte cancers was $426.2 million.3 However, statistics on the morbidity and mortality related to keratinocyte cancers are likely underestimated as they are not notifiable diseases in any state or territory except Tasmania.1
GPs have an important role in the prevention, early detection and management of keratinocyte cancers in Australia. Skin consultations account for about 17% of GP consultations, and skin cancers are the second most common reason for specialist referral.4 Australia’s high survival rate for skin cancers reflects the success of primary care services for the early detection and evidence-based treatment of skin cancers. GPs are also well positioned to educate patients on the importance of sun-safe behaviours and to detect skin cancers at the earliest opportunity through routine skin checks and opportunistic skin screening.
This update incorporates the latest guidelines and published literature on the diagnosis and management of keratinocyte cancers, to aid GPs with evidence-based treatment plans.
Actinic keratoses
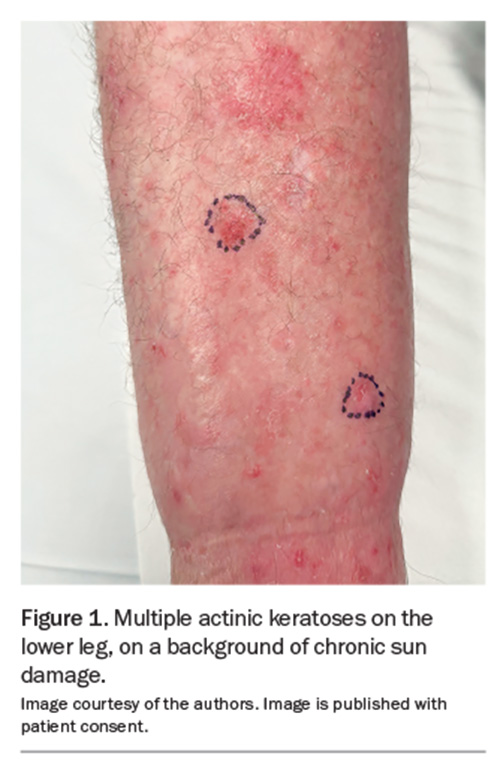
AKs, also known as solar keratoses, are potentially premalignant lesions caused by damage to keratinocytes as a result of cumulative sun exposure. They are found on sites of chronic ultraviolet (UV) radiation exposure, most commonly on the face, scalp, ears and dorsal aspects of the limbs (Figure 1). The lesions progress to SCC at the rate of 0.075% to 0.1% per lesion per year, sometimes extrapolated to up to 10% over 10 years.5 The spontaneous regression rate of AKs is also highly variable and has been reported to be about 15% to 63% per year.1 Unfortunately, it is not possible to target higher-risk AKs as there are no clinically defining features that determine which AKs will progress to become cSCCs.
Associated factors
AKs are clinically significant, not only because they may progress to cSCC, but also because the presence of multiple AKs indicates significant, chronic UV radiation exposure. It is recommended that patients with multiple AKs undergo regular full skin examinations with their GP to assist in the early detection and management of skin cancers.
Presentation and diagnosis
AKs have varied clinical presentations. They are classically described as gritty macules, papules or plaques on an erythematous base, often with rough yellow or white scale. They can also present as hyperkeratotic, pigmented or atrophic lesions. The lesions are typically asymptomatic but may sting or itch.
The diagnosis of an AK is predominantly clinical. Biopsy should be considered if there is concern that the lesion may be an early cSCC or other keratinocyte cancer. Hallmark signs raising concern include tenderness, bleeding, inflammation and growth in height or thickness.
On biopsy, the distinction between an AK and a cSCC is the extent of keratinocyte atypia. Keratinocyte atypia is confined to the lower portion of the epidermis in an AK. In contrast, cSCC comprises keratinocyte atypia that occupies the entire epidermis and may infiltrate deeper into the dermis.
Treatment of actinic keratosis
Evidence-based treatment for AK is informed by the Cancer Council Australia’s Clinical practice guidelines for keratinocyte cancer and the American Academy of Dermatology’s Guidelines of care for the management of actinic keratosis.6,7
Lesion-directed treatment
The first-line modality for localised treatment of AK is liquid nitrogen cryotherapy. The duration of therapy varies depending on the lesion size and location, but a freeze of about 3 to 5 seconds as part of a single or double freeze–thaw cycle is generally recommended.8 This usually causes erythema, oedema and, sometimes, a blister that heals over 7 to 10 days. Cryotherapy should be avoided if the diagnosis is uncertain, and prolonged cryotherapy should be avoided on the lower legs, where healing is poor.9
The 5-fluorouracil 0.5% and salicylic acid 10% solution has also been approved for use on targeted individual AKs (see Product Information for full details).
Curettage and cautery or electrodesiccation, and shave treatments may also help treat AK. However, these treatments are often reserved for larger, thicker lesions or if the diagnosis is uncertain.
Field-directed treatment
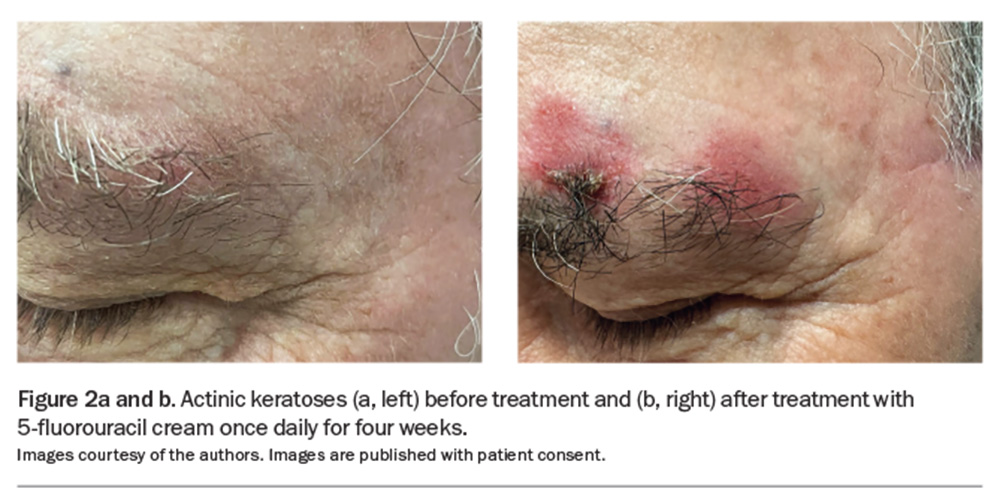
Field-directed treatment should be considered for areas that contain multiple AKs or lesions without distinct borders that allow for targeted localised treatment (Table 1).7,9 Patient education about these therapies is paramount, as the therapies act, in part, by inducing an inflammatory reaction, including redness, soreness and crusting. Patients are best counselled about this before therapy, including being shown photographs of expected reactions (Figure 2a and 2b).
Squamous cell carcinoma
cSCC is the second most common skin cancer in Australia. The age-standardised incidence rate of cSCC is about 387 per 100,000 people aged 14 years and older.10 The incidence increases steeply with age from mid-adulthood and appears higher in men than in women across all age groups. However, the current available evidence on national incidence rates is out of date and likely inaccurate because of a lack of statutory reporting of keratinocyte cancers.2 The primary concern with cSCC is its ability to metastasise, with several large studies demonstrating a mortality rate of more than 70%.11
The most common sites of cSCC are the head and neck areas in men, and the upper limbs followed by the head and neck areas in women. After accounting for body surface area, the highest incidence of cSCC is on the face, particularly the lips, ears, nose, cheek and eyelids.12
Risk factors
cSCC can arise de novo with no risk factors or triggers. However, most cSCCs arise in individuals with significant risk factors, including:13
- Fitzpatrick skin types I to III
- cumulative exposure to UV radiation
- male sex (male to female ratio of 3:1)
- increased age (average age of onset in the mid-60s)
- chronic inflammation or sites of previous trauma or scarring, especially in darker-skinned individuals
- immunosuppression, most significantly in patients with solid organ or stem cell transplant, with chronic leukaemia or on immunosuppressive agents
- infection with oncogenic human papillomavirus, particularly periungual and anogenital SCC
- rare hereditary cancer syndromes
- environmental exposures (e.g. arsenic, nitrosamines, alkylating agents)
- exposure to ionising radiation.
Presentation and diagnosis
cSCC has a spectrum of presentations. Bowen’s disease, also known as cSCC in situ, typically presents as an erythematous patch or plaque with scale and, in rare cases, pigmentation. Without treatment, 2 to 5% of cases of Bowen’s disease progress to involve the dermis, defined as invasive cSCC.14 Invasive cSCC typically presents as an erythematous keratotic papule or nodule, which may be tender on palpation. On dermoscopy, invasive cSCC tends to have looped or hairpin and serpentine vessels. Regional lymph nodes should be examined, and suspected metastases should be confirmed via fine needle aspiration.
The diagnosis of cSCC is established via biopsy. On histopathological examination, Bowen’s disease shows keratinocyte atypia involving the full thickness of the epidermis. If invasive cSCC is suspected, the biopsy should be deep enough to determine the extent of dermal involvement. The pathology report should include a synoptic checklist of information useful for prognosis, including the tumour subtype, degree of differentiation, tumour thickness and presence or absence of perineural, vascular or lymphatic spread.6 Key factors indicative of higher risk and poorer prognosis for cSCC are summarised in Box 1.15,16
Treatment of invasive cSCC
Surgery
Surgical treatment options for cSCC include local excision and Mohs micrographic surgery. Low-risk lesions can be treated with surgical excision, curettage and cautery, or punch excision. Lateral and deep margins must be adequate to ensure complete excision. Adequate deep margins are particularly important, as inadequate deep margins significantly increase the risk of recurrence.17 For the best cosmetic result, tumours should be excised along relaxed skin tension lines, but along a line that avoids distortion.
For patients with cSCC with features of poor prognosis, referral to a multidisciplinary team or to a specialist for assessment and treatment should be considered (Box 1).18
Radiotherapy
Radiotherapy for cSCC has improved significantly in the past few decades. Modified fractionation schedules and more precise fractionation techniques allow for an improved balance between targeting tumour cells and minimising effects on normal tissue. Radiotherapy for cSCC may be considered for patients who are not appropriate surgical candidates, such as in cases of frailty, comorbidities or high surgical or bleeding risk. Referral to a radiation oncologist as part of multidisciplinary care should be considered for patients with stage T3 or T4 primary tumours, persistent or recurrent cSCC, and following incomplete surgical excision as an alternative to re-excision.
Systemic treatment
Locoregional advanced cSCC represents an advanced stage of disease that may present de novo or after previous surgery and radiotherapy.19 The goal of treatment is to clear local disease and prevent further recurrence or regional metastasis. Surgery, radiotherapy, chemotherapy or a combination of treatments may be needed. Patients should be assessed on a case-by-case basis in a multidisciplinary setting.6
Systemic drug treatments should be considered for patients with locoregionally advanced or metastatic SCC who are not suitable candidates for surgery or radiotherapy.20 Both cetuximab, an epidermal growth factor receptor inhibitor, and cemiplimab, a programmed cell death protein-1 inhibitor, are PBS listed for the treatment of metastatic or locally advanced cSCC in patients who are unsuitable for curative surgical resection or radiotherapy, and who have a WHO performance status of 0 (fully active) or 1 (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature).
Chemoprophylaxis
Chemoprophylaxis agents, including acitretin, capecitabine and vitamin B3 (nicotinamide), have been used in practice to reduce the risk of keratinocyte cancers in solid organ transplant recipients who develop multiple or high-risk keratinocyte cancers.6 A 2022 Australian study involving 22 solid organ transplant recipients showed that acitretin, a synthetic retinoid, appears to be well tolerated and effective in reducing keratinocyte cancers for at least five years.21 However, these therapies are not without adverse effects and decisions should be made by dermatologists experienced in their use for this purpose.22
Basal cell carcinoma
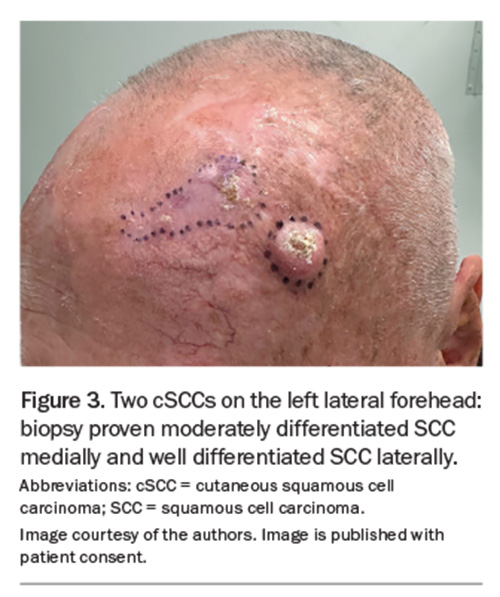
BCC has the highest incidence of all cancers in Australia and accounts for 70% of all keratinocyte cancers.1 The most recent (2011–14) reports of BCC incidence in Australia estimate an annual incidence of 770 affected people per 100,000.23 However, when the incidence of lesions is considered, the rates are considerably higher. BCC incidence rates are highest on the face, followed by the upper limbs, trunk and lower limbs. Among the facial sites, BCCs most commonly occur on the nose, followed by the forehead and temple, then the cheeks and perioral region and then the ears (Figure 3).24
Risk factors
As with cSCC, exposure to UV radiation is the greatest risk factor for BCC. However, unlike SCC, the risk is associated with intense episodes of burning rather than cumulative exposure.25 Other risk factors include:6
- exposure to artificial UV radiation, including psoralen and UVA radiation
- exposure to ionising radiation
- exposure to arsenic
- presence of rare hereditary syndromes (e.g. naevoid BCC syndrome [Gorlin’s syndrome], Bazex–Dupré–Christol syndrome, Rombo syndrome).
Presentation and diagnosis
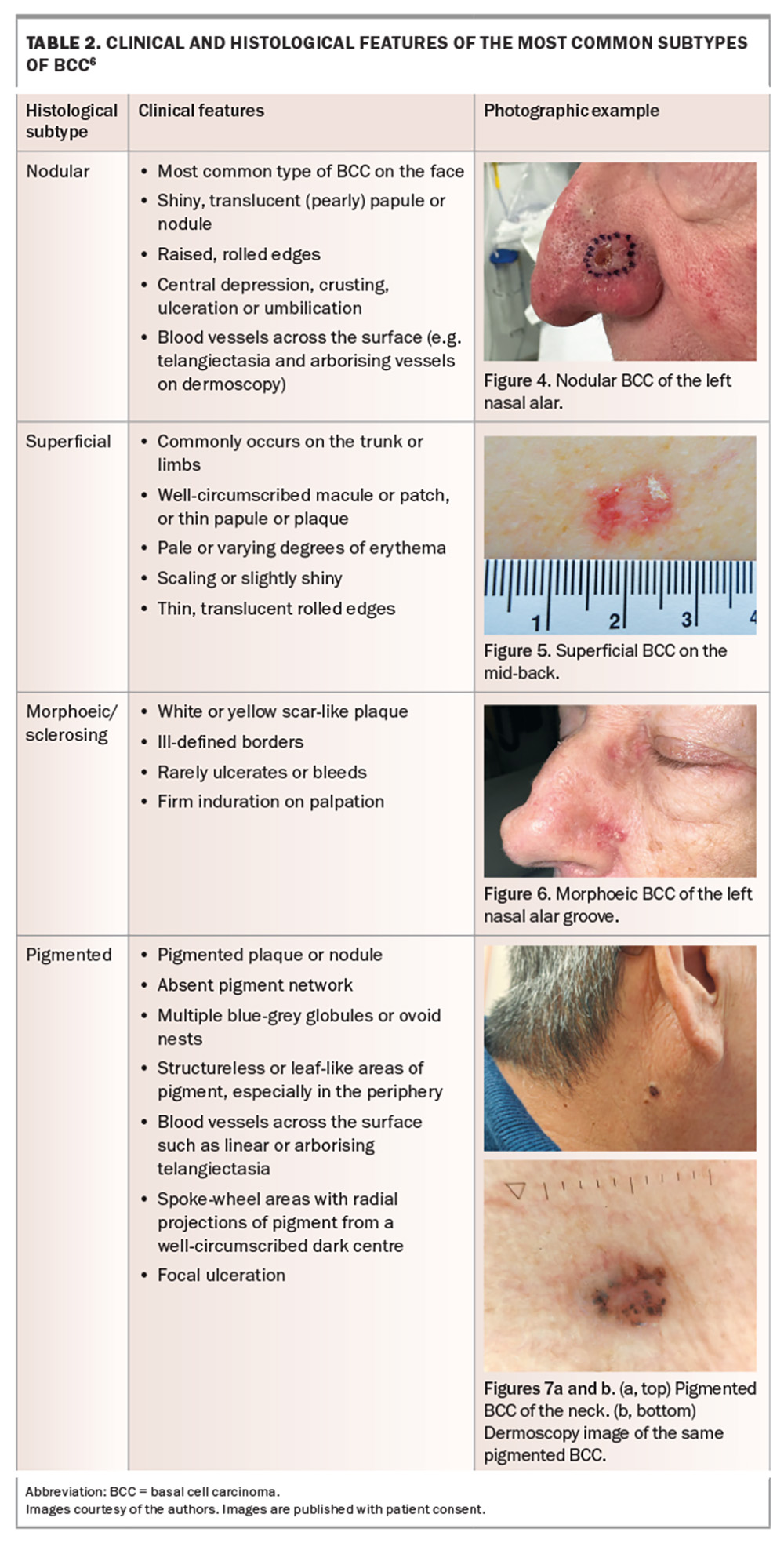
Evaluation of a suspected BCC entails a comprehensive clinical examination supplemented with dermoscopy. BCCs are usually diagnosed via biopsy. The clinical features and histopathology for the different subtypes of BCC are outlined in Table 2.6 Not all clinical and histological features may be present for a specific lesion.
BCCs rarely metastasise, and the main clinical concern is local destruction. If allowed to progress, BCCs can cause significant morbidity and represent a significant burden on healthcare services. Key factors indicative of higher risk and poorer prognosis for BCC are summarised in Box 1.15,16
Treatment of BCC
Treatment options for low-risk BCC
Small, low-risk, superficial BCC may be treated with cryotherapy, imiquimod cream or photodynamic therapy. Cryotherapy is performed with a double freeze–thaw cycle of 20 to 30 seconds. This causes a significant blister that heals in about three to four weeks. It can lead to hypopigmentation; therefore, it is important to counsel patients about this. Long-term follow up is essential, as late recurrences may occur.6
Similarly, topical treatment with imiquimod 5% cream is more intensive than for AKs, with therapy applied by the patient once daily, on five consecutive nights per week for six weeks.26 The degree of inflammation is variable and depends partly on the specific skin lesion and genetic factors. If severe inflammation occurs, patients may need to take a break from therapy for a week or two to allow time for the reaction to settle before continuing treatment. Skin biopsy is required for PBS-reimbursed prescription of imiquimod 5% cream. There is usually a good cosmetic outcome with little scarring after imiquimod treatment. Several studies and clinical trials have shown that imiquimod is superior to 5-fluorouracil in treating superficial BCCs.26,27
Photodynamic therapy may be considered for superficial and thin nodular BCCs. Two treatment sessions, one week apart, are usually required, with the lesions first descaled or debulked before applying the photosensitising agent.
Brachytherapy utilising the beta emitter radioisotope rhenium-188 is now TGA-listed (but not PBS-funded) for the treatment of thin BCC and cSCCs (<3 mm thick) in a single treatment session.28 The area requiring treatment is covered with a protective foil before the rhenium-188 compound is applied on top using a special applicator and left in place for 30 to 180 minutes. Although studies have shown that healing and the rates of remission are favourable, the caveat is that long-term data, while being accrued, are lacking.28,29
Surgical treatment options
Surgery is usually the first-line therapy for nonsuperficial BCCs (Box 1). It usually involves elliptical excision of the lesion and repair of the defect by side-to-side (primary) closure. Very large lesions or lesions in anatomically difficult areas may require flap or skin graft repair. Curettage and electrodessication may be considered as an option for some lesions.
Referral to a specialist is recommended for the consideration of other treatment options, such as Mohs micrographic surgery and postsurgical adjuvant treatments, for lesions with features of poor prognosis in certain cases (Box 1).6
Mohs micrographic surgery is a highly specialised and meticulous surgical technique. It involves histopathological examination of frozen sections of almost the entire peripheral and deep margins of the excised tissue, in contrast with standard specimen processing, where only 0.1 to 1% of the surgical margin is examined. Mohs micrographic surgery may be considered an alternative to surgical excision for the following types of BCCs:6
- aggressive histological subtypes (e.g. infiltrating, micronodular, sclerosing)
- residual or recurrence following previous treatment
- poorly defined clinical border
- located in an anatomically difficult area or an area of large size, especially on the face.
Radiotherapy
Radiotherapy using curative doses is an alternative treatment for BCC if the patient declines surgery or surgery is inappropriate because of patient factors including frailty, tumour-related factors (e.g. if tissue conservation or cosmesis is a high priority, such as in BCC of the eyelid) or treatment-related factors (e.g. concurrent anticoagulant therapy). For patients with a stage T3 or T4 primary BCC or persistent or recurrent BCC, referral to a radiation oncologist for an opinion regarding radiation therapy should be considered.
Systemic therapy
Metastatic BCC is rare. If suspected, confirmation is required via biopsy. Patients with complex locally advanced disease are best treated by a multidisciplinary team that includes surgeons, dermatologists, radiation oncologists and medical oncologists. For patients who are not suitable candidates for surgery or radiotherapy, oral therapy with a hedgehog pathway inhibitor (sonidegib or vismodegib) should be considered.30 Both agents have similar efficacy and are listed on the PBS for the treatment of metastatic or locally advanced BCC, for which neither surgery nor curative radiotherapy is appropriate.
When should a GP refer?
GPs are well positioned to educate patients on the importance of sun-safe behaviours, to detect skin cancers at the earliest opportunity and to provide initial management. Recommendations on when to consider referring patients for specialist or multidisciplinary care are outlined in Box 2.
Patients requiring specialised management, including photodynamic therapy, Mohs micrographic surgery and brachytherapy, may be referred to a dermatologist. Referrals to plastic surgery and surgical oncology specialists are appropriate for lesions that require complex surgical management or have higher-risk features. For patients who are unable to undergo surgery because of lesion features or comorbidities, referral for a radiation oncology opinion is appropriate. For complex tumours and metastatic tumours, referral to a multidisciplinary team that includes dermatologists, surgeons and medical and radiation oncologists is recommended for planning and management.
Conclusion
Keratinocyte cancers are the most common cancers encountered in Australia, and GPs play a crucial role in their detection and early management. Full skin examination is recommended for patients at risk of skin cancers, as many areas of the skin cannot be adequately monitored by patients themselves. Prevention through sun-safe education, early detection and appropriate treatment can help reduce the impact these cancers have on patients’ lives and the healthcare system. MT
COMPETING INTERESTS: Dr Miao and Dr Phan: None. Associate Professor Shumack receives advisory board payments from Oncobeta.
References
1. Australian Institute of Health and Welfare (AIHW). Cancer in Australia. Canberra: AIHW; 2021. Available online at: https://www.aihw.gov.au/getmedia/0ea708eb-dd6e-4499-9080-1cc7b5990e64/aihw-can-144.pdf (accessed July 2024).
2. Olsen CM, Pandeya N, Green AC, Silva Ragaini B, Venn A, Whiteman DC. Keratinocyte cancer incidence in Australia: a review of population-based incidence trends and estimates of lifetime risk. Public Health Res Pract 2022; 32: 3212203.
3. Gordon LG, Leung W, Johns R, et al. Estimated healthcare costs of melanoma and keratinocyte skin cancers in Australia and Aotearoa New Zealand in 2021. Int J Environ Res Public Health 2022; 19: 3178.
4. Royal Australian College of General Practitioners (RACGP). 2022 RACGP curriculum and syllabus for Australian general practice. Melbourne: RACGP; 2022. Available online at: https://www.racgp.org.au/education/education-providers/curriculum/curriculum-and-syllabus/units/dermatological-presentations#ref-num-1 (accessed July 2024).
5. Dodds A, Chia A, Shumack S. Actinic keratosis: rationale and management. Dermatol Ther 2014; 4: 11-31.
6. Cancer Council Australia. Clinical practice guidelines for keratinocyte cancer v1.4. Sydney: Cancer Council Australia; 2024. Available online at: https://www.cancer.org.au/clinical-guidelines/skin-cancer/keratinocyte-cancer (accessed July 2024).
7. Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol 2021; 85: e209-e233.
8. Arisi M, Guasco Pisani E, Calzavara-Pinton P, Zane C. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol 2022; 15: 357-365.
9. Dermatology Expert Group. Solar damage and skin cancer. In: eTG complete (Internet). Melbourne: Therapeutic Guidelines Limited, 2022.
10. National Cancer Control Initiative (NCCI). National Cancer Control Initiative 1997-2002 report. Melbourne: NCCI; 2003. Available online at: https://www.canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/national-cancer-control-initiative-1997-2002-report (accessed July 2024).
11. Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol 2016; 17: 491-508.
12. Buettner PG, Raasch BA. Incidence rates of skin cancer in Townsville, Australia. Int J Cancer 1998; 78: 587-593.
13. Que SK, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 2018; 78: 237-247.
14. Drake AL, Walling HW. Variations in presentation of squamous cell carcinoma in situ (Bowen’s disease) in immunocompromised patients. J Am Acad Dermatol 2008; 59: 68-71.
15. Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol 2016; 152: 419-428.
16. Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med 2018; 379: 363-374.
17. Sepehripour S, Dawood O, Hatter S, et al. An assessment of histological margins and recurrence of completely excised cutaneous SCC. J Plast Reconstr Aesthet Surg 2020; 73: 899-903.
18. Stanganelli I, Spagnolo F, Argenziano G, et al. The multidisciplinary management of cutaneous squamous cell carcinoma: a comprehensive review and clinical recommendations by a panel of experts. Cancers 2022; 14: 377.
19. Venables ZC, Autier P, Nijsten T, et al. Nationwide incidence of metastatic cutaneous squamous cell carcinoma in England. JAMA Dermatol 2019; 155: 298-306.
20. Schmults CD, Blitzblau R, Aasi SZ, et al. NCCN Guidelines® insights: squamous cell skin cancer, version 1.2022. J Natl Compr Canc Netw 2021; 19: 1382-1394.
21. Allnutt KJ, Vogrin S, Li J, et al. A long-term cohort study of acitretin for prevention of keratinocyte carcinoma in solid organ transplant recipients. Australas J Dermatol 2022; 63: e121-e126.
22. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol 2006; 155: 916-925.
23. Pandeya N, Olsen CM, Whiteman DC. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust 2017; 207: 339-343.
24. Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol 2009; 129: 323-328.
25. Green A, Battistutta D, Hart V, Leslie D, Weedon D. Skin cancer in a subtropical Australian population: incidence and lack of association with occupation. The Nambour Study Group. Am J Epidemiol 1996; 144: 1034-1040.
26. Marks R, Gebauer K, Shumack S, et al. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: results of a multicenter 6-week dose-response trial. J Am Acad Dermatol 2001; 44: 807-813.
27. Jansen MH, Mosterd K, Arits AH, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol 2018; 138: 527-533.
28. Cipriani C, Desantis M, Dahlhoff G, et al. Personalized irradiation therapy for NMSC by rhenium-188 skin cancer therapy: a long-term retrospective study. J Dermatolog Treat 2022; 33: 969-975.
29. Castellucci P, Savoia F, Farina A, et al. High dose brachytherapy with non sealed 188 Re (rhenium) resin in patients with non-melanoma skin cancers (NMSCs): single center preliminary results. Eur J Nucl Med Mol Imaging 2021; 48: 1511-1521.
30. Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol 2020; 100: adv00140.