‘I plucked a hair out of my mole’ – an enlarging ulcerated nodule
A 75-year-old woman presents with an ulcerated lesion on her neck. What is the diagnosis?
Case presentation
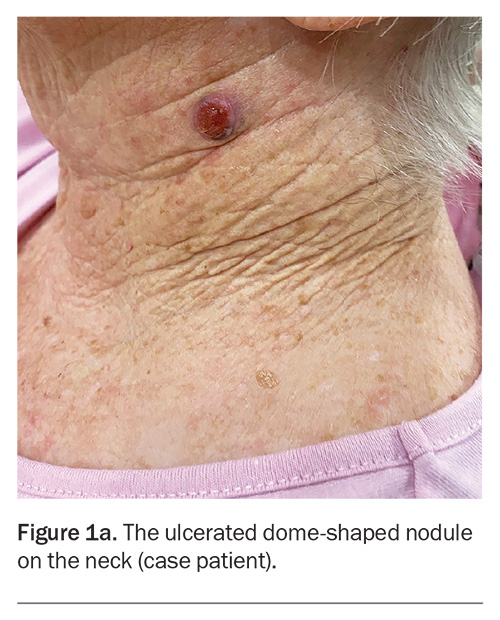
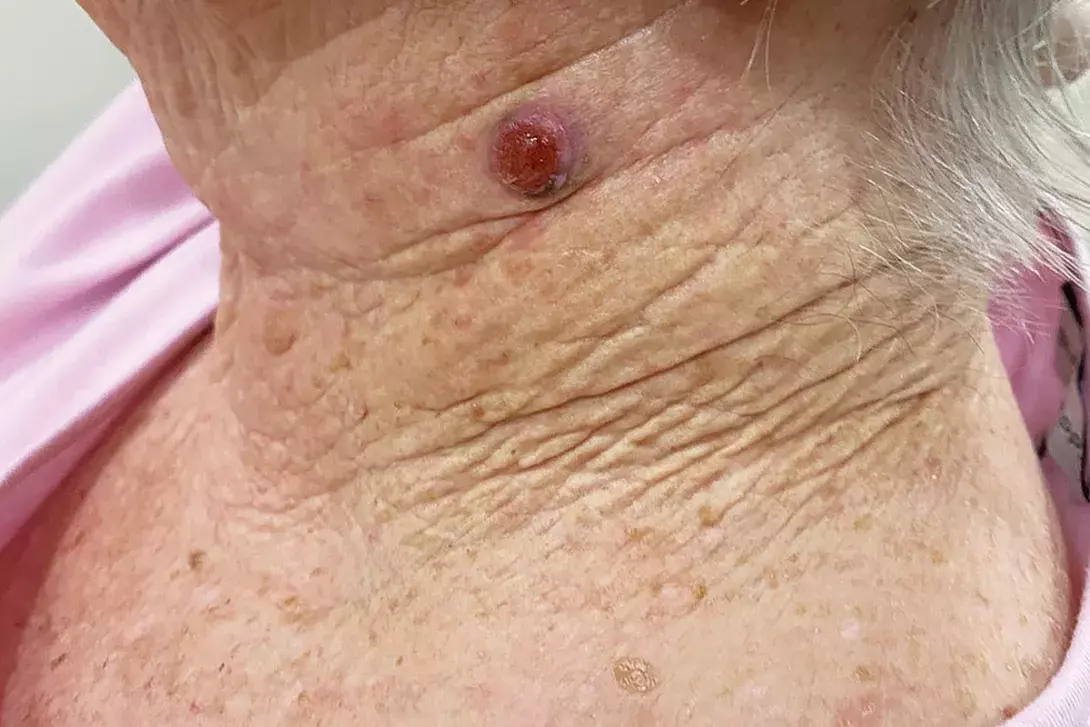
A 75-year-old woman presents after noticing change in a lesion on her neck. She says it was present and unchanged for many years, until three weeks ago when she plucked a hair from the ‘mole’ and noticed some mild yellowish discharge. She is concerned that it could be infected because it is enlarging.
The patient has a medical history of hypertension, ischaemic heart disease and temporal lobe epilepsy. Thirty years ago, she had a melanoma on her shoulder, which was excised.
On examination, an ulcerated, red, dome-shaped nodule is evident on the left side of the patient’s neck (Figure 1a). The lesion is firm and slightly tender. There is no surrounding erythema.
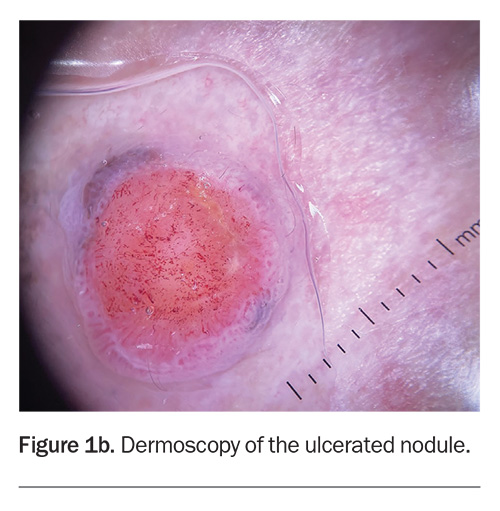
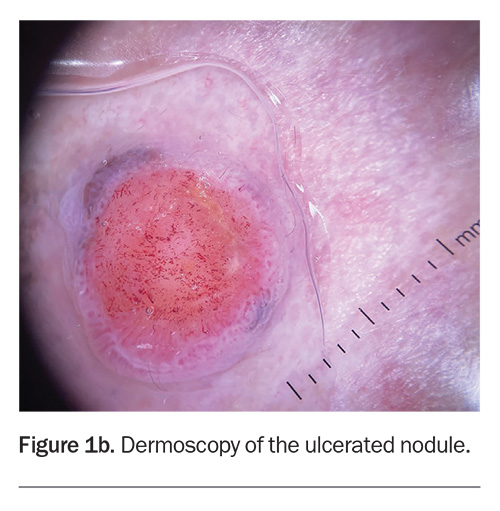
Dermoscopy shows a lesion with a well-defined border (Figure 1b). There is a large central circular area of ulceration, 9 mm in diameter, with a hint of grey-brown pigment at the periphery along the pink border of the lesion. The vessel pattern in the ulcerated area is polymorphous.
The patient is afebrile and examination of her neck, chin and supraclavicular areas reveals no palpable lymph nodes.
Differential diagnoses
Conditions to consider among the differential diagnoses for a patient presenting with an enlarging ulcerated nodule include the following.
Pyogenic granuloma
A pyogenic granuloma is a benign proliferation of the capillary blood vessels of the skin.1 It tends to occur in children and young adults, with minor trauma being a common trigger. Other associated factors include pregnancy and medications.2 Clinically, a pyogenic granuloma presents as a painless fleshy nodule that grows rapidly over a few weeks. It can be smooth or ulcerated and, given its vascular nature, can bleed easily.3
For the case patient, the history of minor trauma (hair removal) could be suggestive for a pyogenic granuloma, although these nodules tend to be more common in younger individuals. This was not the correct diagnosis.
Carbuncle
A carbuncle is a spreading infection of a hair follicle, commonly caused by Staphylococcus aureus. It presents as a tender, inflamed and fluctuant nodule, often with multiple pustules or discharging tracts.4 Carbuncles can be associated with systemic symptoms such as fever and lymph node enlargement. Predisposing factors include diabetes mellitus and immunodeficiency.5
The case patient’s nodule was not fluctuant on examination and she had no systemic symptoms or lymph node enlargement. The history of a discharge from her lesion when she removed the hair was suggestive of an infectious process, but its clinical appearance (no pustules or sinuses or surrounding erythema) was not typical for a carbuncle. This was not the correct diagnosis.
Basal cell carcinoma
The most common type of skin cancer, basal cell carcinomas are tumours arising from keratinocytes in the skin. The nodular subtype presents as a slowly enlarging nodule that can bleed and ulcerate, either spontaneously or with minor trauma.6 Clinically, it classically has a rolled edge on its border. Dermoscopy is useful in the diagnosis of nodular basal cell carcinoma and most commonly reveals a translucent pink stroma with branching ‘arborising’ thin vessels that are in sharp focus unless ulceration occurs.7 Diagnosis usually requires a biopsy for histopathological assessment.
For the case patient, the clinical history and dermoscopic features of the lesion (e.g. lack of typical vessel patterns) were not consistent with a basal cell carcinoma. This was not the correct diagnosis.
Squamous cell carcinoma
Cutaneous squamous cell carcinomas account for 20% of all skin cancers.8 Poorly differentiated subtypes can present as red, ulcerated, rapidly growing nodules.9 Risk factors include chronic exposure to ultraviolet radiation and immunosuppression. Squamous cell carcinomas are more common in patients who are taking immunosuppressive therapy after an organ transplant.8 A biopsy is usually required for histological confirmation. This was not the correct diagnosis for the case patient.
Keratoacanthoma
Keratoacanthomas are skin tumours that present as rapidly growing nodules that form dome- or crater-like lesions, often with a central keratin plug. There are multiple variants, with solitary keratoacanthoma being the most common subtype. After growing rapidly and reaching a stable size, it characteristically undergoes spontaneous resolution over a period of months. A biopsy is usually required for diagnosis. Differentiating a keratoacanthoma from a well differentiated squamous cell carcinoma can be difficult for clinicians and histopathologists alike and, thus, such lesions are managed as a variant of cutaneous squamous cell carcinoma.10 This was not the correct diagnosis for the case patient.
Nodular melanoma
This is the correct diagnosis. Nodular melanoma, which accounts for 15% of all melanomas, is a subtype that tends to grow vertically and to present as a rapidly enlarging nodule (over weeks to months).11 Most patients are over 50 years of age at diagnosis and have risk factors for melanoma, such as previous melanoma, multiple atypical naevi and fair skin.
About one-third of nodular melanomas are not pigmented (amelanotic) and the lesions can be symmetrical with regular borders, which makes diagnosis more challenging.12,13 However, on dermoscopy, some pigment clues can be evident in lesions that are hypomelanotic (have scant pigment) – for example, Figure 1b shows a greyish-brown crescent of pigment at the 11 o’clock and 4 o’clock positions on the border, which represents melanin within the malignant melanocytes. The other diagnoses considered above would not be expected to display this pigment clue (with the exception of basal cell carcinomas, which can demonstrate blue-grey ovoid nests and/or a very specific ‘spoke-wheel’ pigmentation).
Nodular melanomas can be challenging to diagnose and tend to be thicker than other melanoma subtypes at diagnosis due to vertical growth, which can contribute negatively to prognosis.14 The EFG acronym (elevated, firm and growing) was developed to help in the identification of skin changes in a lesion that may be suggestive for nodular melanoma. Any EFG nodule should prompt consideration of an excisional biopsy to rule out a nodular melanoma.15
Management of a confirmed melanoma involves definitive surgery, with the extent depending on several factors, such as the thickness of the lesion and its location. Referral to a multidisciplinary team should be considered for discussion of treatment options with systemic therapies, which include targeted therapies (BRAF and MEK inhibitors) and immunotherapies.16 The latest melanoma guidelines can be found on the Cancer Council Australia website (https://www.cancer.org.au/clinical-guidelines/skin-cancer/melanoma).
Outcome
For the case patient, an excisional biopsy in the form of an elliptical excision with a 2 mm margin around the lesion was performed. The diagnosis of a nodular melanoma (Breslow thickness 3.0 mm and Clark level 4) was confirmed histologically, with no precursor lesion identified. We postulate that our patient mistook her lesion as a change occurring in a mole and that the lesion was actually new. It is much less likely for a nodular melanoma than for a superficial spreading melanoma to be associated with a pre-existing naevus.17
The patient was referred to a multidisciplinary team for further management. A head and neck surgeon performed a wide excision, along with a sentinel lymph node biopsy. Results of her lymph node biopsy returned only fibrofatty tissue, with no lymphoid tissue identified. Given the propensity for nodular melanoma to metastasise, compassionate access to pembrolizumab was arranged, which has been shown to increase chances of survival.18
A full body skin check was performed postoperatively, which identified a melanoma in situ on the patient’s thigh and a squamous cell carcinoma in situ on her cheek. Both lesions were subsequently managed with successful outcomes. MT
COMPETING INTERESTS: None.
References
1. Plachouri K-M, Georgiou S. Therapeutic approaches to pyogenic granuloma: an updated review. Int J Dermatol 2019; 58: 642-648.
2. Park SH, Lee JH, Tak MS, Lee HJ, Choi HJ. A research of pyogenic granuloma genesis factor with immunohistochemical analysis. J Craniofac Surg 2017; 28: 2068-2072.
3. Wollina U, Langner D, França K, Gianfaldoni S, Lotti T, Tchernev G. Pyogenic granuloma – a common benign vascular tumor with variable clinical presentation: new findings and treatment options. Open Access Maced J Med Sci 2017; 5: 423-426.
4. Troxell T, Hall CA. Carbuncle. StatPearls. Treasure Island (FL): StatPearls Publishing [internet]; 2023.
5. Stulberg DL, Penrod MA, Blatny RA. Common bacterial skin infections. Am Fam Physician 2002; 66: 119-124.
6. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med 2015; 88: 167-179.
7. Reiter O, Mimouni I, Dusza S, Halpern AC, Leshem YA, Marghoob AA. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol 2021; 85: 653-664.
8. de Jong E, Lammerts MUPA, Genders RE, Bouwes Bavinck JN. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol 2022; 36 (Suppl 1): 6-10.
9. Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol 2017; 31: 1828-1833.
10. Tisack A, Fotouhi A, Fidai C, Friedman BJ, Ozog D, Veenstra J. A clinical and biological review of keratoacanthoma. Br J Dermatol 2021; 185: 487-498.
11. Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell Melanoma Res 2011; 24: 879-897.
12. Dessinioti C, Geller AC, Whiteman DC, et al. Not all melanomas are created equal: a review and call for more research into nodular melanoma. Br J Dermatol 2021; 185: 700-710.
13. Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res 2019; 29: 221-230.
14. Mar V, Roberts H, Wolfe R, English DR, Kelly JW. Nodular melanoma: a distinct clinical entity and the largest contributor to melanoma deaths in Victoria, Australia. J Am Acad Dermatol 2013; 68: 568-575.
15. Kelly JW. Nodular melanoma: how current approaches to early detection are failing. J Drugs Dermatol 2005; 4: 790-793.
16. Cancer Council Australia. Clinical practice guidelines for the diagnosis and management of melanoma. Available online at: https://www.cancer.org.au/clinical-guidelines/skin-cancer/melanoma (accessed April 2024).
17. Pan Y, Adler NR, Wolfe R, McLean CA, Kelly JW. Nodular melanoma is less likely than superficial spreading melanoma to be histologically associated with a naevus. Med J Aust 2017; 207: 333-338.
18. Patel SP, Othus M, Chen Y, et al. Neoadjuvant-Adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med 2023; 388: 813-823.