A new dawn for polymyalgia rheumatica: from diagnosis to emerging treatments

There has been little change in diagnostic and management approaches for polymyalgia rheumatica (PMR) since the 1950s. In the past decade, major advances have occurred in our understanding of the condition’s unique pathology and the potential role for imaging in confirming its diagnosis. With recent US FDA approval of the first biologic agent for relapsing disease, modern medicine may soon become a reality for patients with PMR.
- Polymyalgia rheumatica (PMR) is characterised by sudden, profound pain and stiffness at the shoulders and hips, leading to significant disability and psychological distress among affected individuals.
- About 20% of PMR cases overlap with giant cell arteritis, emphasising the need for vigilant assessment of cranial and large-vessel symptoms, including visual change, which necessitates immediate referral for ophthalmic assessment.
- Although no specific autoantibody exists for PMR diagnosis, the diagnostic approach should exclude relevant differential diagnoses, such as rheumatoid arthritis, and may be complemented by modern imaging techniques such as ultrasound, MRI and 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
- Prednisolone remains the primary treatment, but recent studies have challenged the utility of traditional tapering strategies because of the development of persistent symptoms in many patients and long-term steroid-related risks.
- Steroid-sparing agents, including the biologic agents sarilumab, tocilizumab and rituximab, have shown promise in recent randomised clinical trials, potentially transforming the future of PMR management.
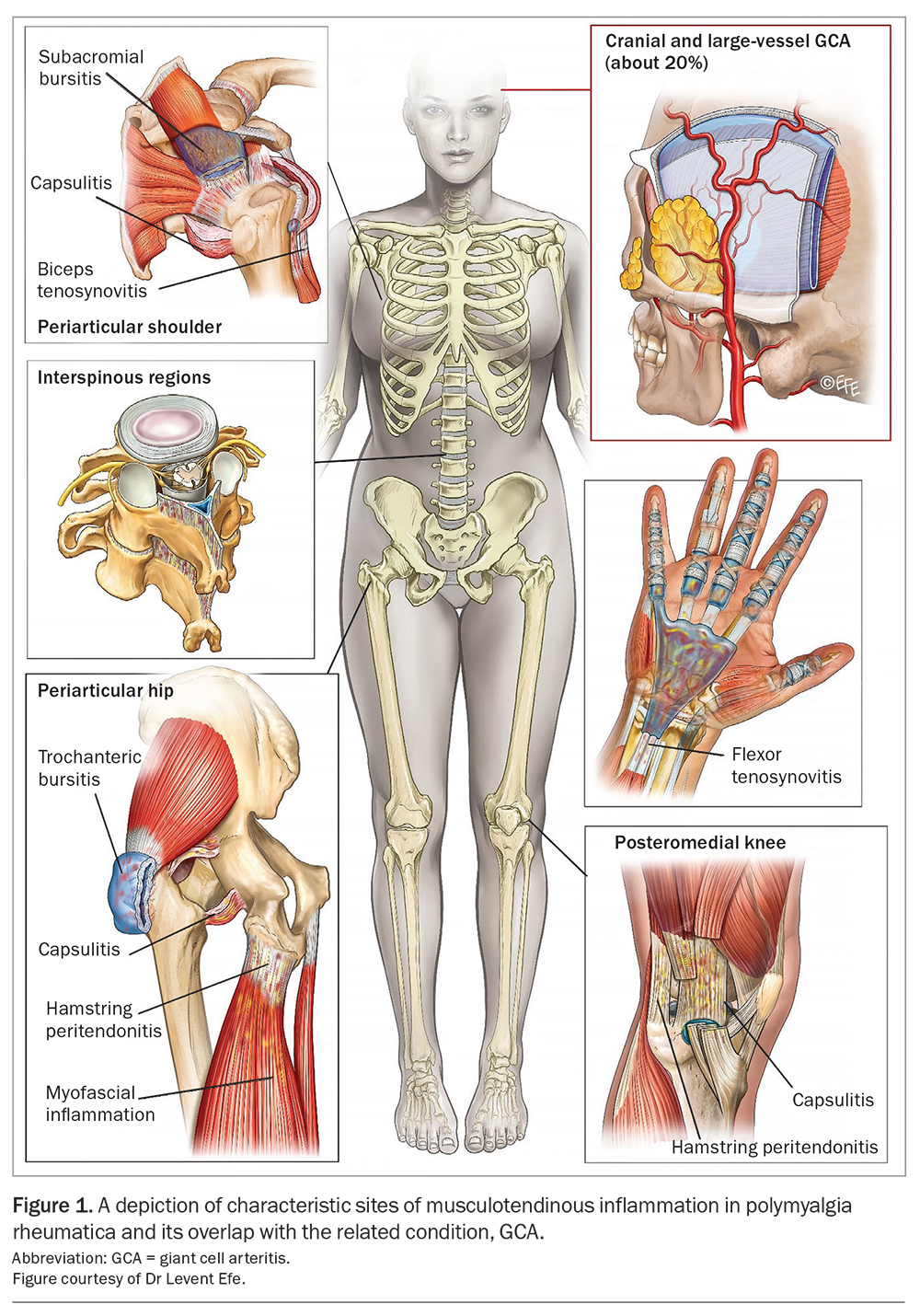
Polymyalgia rheumatica (PMR) is the most common inflammatory rheumatic disease among patients 50 years of age and older, with a peak incidence in the eighth decade and lifetime prevalence of 2.43% in women and 1.66% in men.1,2 Until recently, the exact cause of the profound shoulder and hip pain and stiffness associated with PMR was elusive. With the aid of modern imaging techniques, PMR is now recognised by its distinctive pattern of inflammation at key musculotendinous structures (Figure 1).3 Ultrasound findings such as subacromial and trochanteric bursitis and biceps tenosynovitis may be used to compliment the traditional clinical approach to diagnosis.4 Furthermore, although prednisolone remains the mainstay of PMR treatment, increased recognition of a relapsing disease phenotype and greater appreciation of the risks associated with long-term low-dose glucocorticoid therapy have prompted a rethink of the current management paradigm to one that incorporates steroid-sparing agents and disease-modifying antirheumatic drugs (DMARDs). This article discusses the diagnostic work-up for patients with suspected PMR, as well as the management approaches for newly diagnosed and relapsing disease.
Clinical presentation and diagnostic work-up for polymyalgia rheumatica
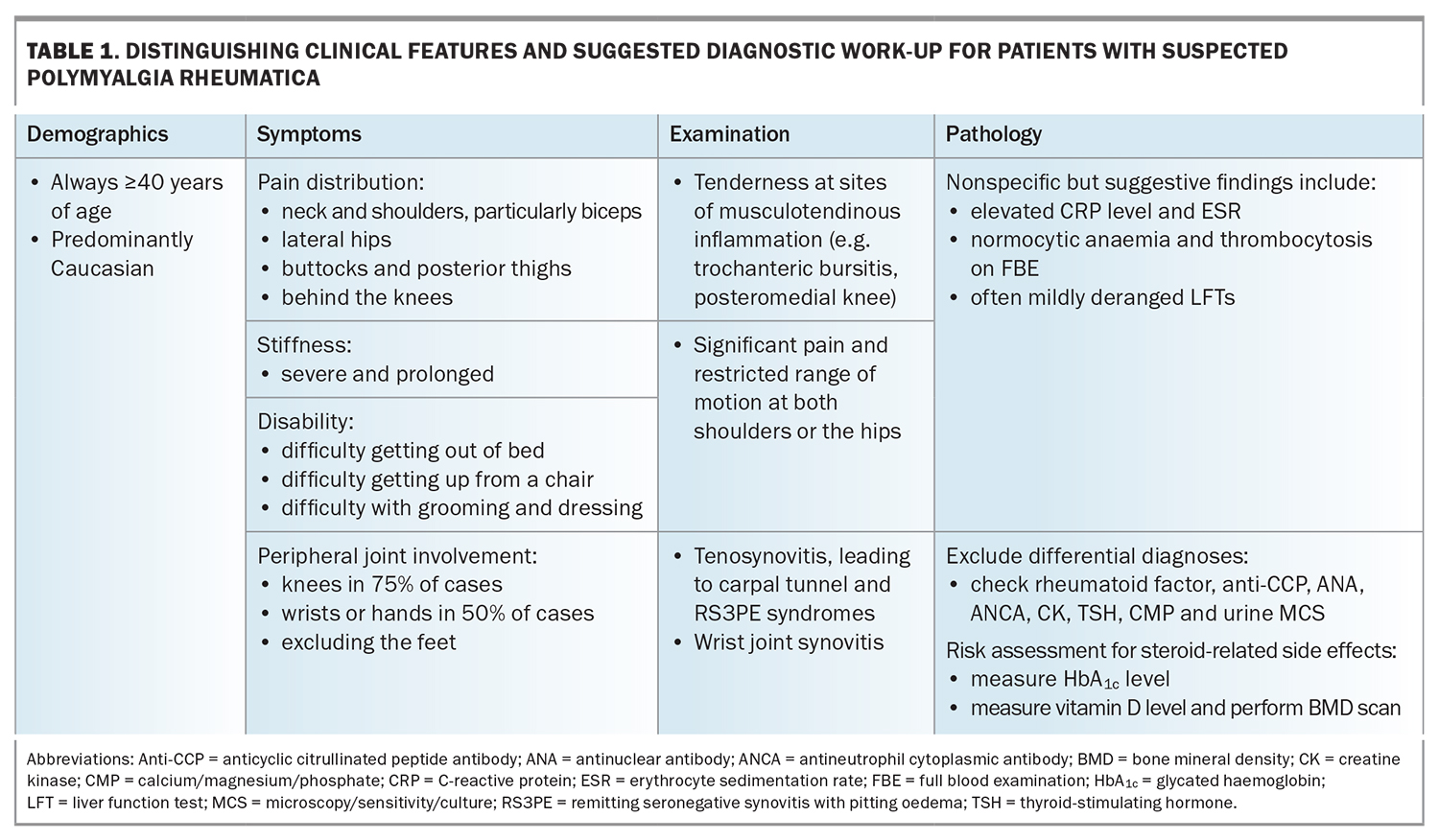
Patients with PMR typically describe a sudden onset of pain and stiffness at both of their shoulder and hip joints (Table 1). These symptoms result in profound disability and, consequently, significant psychological distress, with the acuity and severity often leading affected individuals to believe they have developed a life-threatening illness.5 The degree and duration of stiffness are similarly a key point of clinical differentiation from other forms of inflammatory arthritis, such as rheumatoid arthritis (RA) or psoriatic arthritis; the stiffness predominates in the early hours of the morning, but can persist to a lesser degree all day and tends to worsen following periods of inactivity. Eliciting a history of pain in the buttocks, posterior thighs and behind the knees can be particularly helpful owing to recognised involvement of the hamstring tendons at their anatomical origin (the ischial tuberosities) and insertion (at the medial tibial condyle).6
An overlap between PMR and its related condition giant cell arteritis (GCA) occurs in about 20% of PMR cases, although up to 50% of patients diagnosed with GCA experience musculoskeletal symptoms consistent with PMR.7 It is therefore imperative that clinical features suggestive of both cranial and large-vessel (involving the aorta and its main branches) GCA are sought in individuals suspected of having PMR, including a persistent headache, scalp or temporal artery tenderness, jaw claudication or constitutional symptoms (fatigue, fevers or night sweats and unintentional loss of weight). Any change in vision may indicate acute ischaemic optic neuropathy, which can result in irreversible blindness and should prompt immediate referral for ophthalmological assessment and consideration of intravenous pulse methylprednisolone therapy. Similarly, in recognition of this risk, empiric high-dose prednisolone (50 mg/day) must be commenced in patients with other suggestive GCA symptoms who are awaiting appropriate diagnostic tests (temporal artery biopsy for cranial disease, and advanced imaging modalities including positron emission tomography/computed tomography [PET/CT] or magnetic resonance angiography to assess for large vessel involvement).
Pain and swelling of the hand and wrist joints can suggest an alternative inflammatory arthritis diagnosis; however, this manifestation is recognised to occur in about half of all patients with PMR.8 Although tenosynovitis occurs frequently in PMR, little difference has been found in the rates of synovitis and bone erosions compared with healthy controls.9 This predilection for tendon involvement can lead to the development of carpal tunnel syndrome (due to local compression of the median nerve) and, when particularly severe, remitting seronegative synovitis with peripheral oedema syndrome, characterised by diffuse, glove-like swelling of the hands. Generally, such manifestations are profoundly steroid-responsive and hence may also emerge for the first time when prednisolone is weaned.
There is no recognised pathogenic autoantibody associated with PMR. Initial investigations therefore aim to exclude relevant differential diagnoses including:
- RA (rheumatoid factor and anticyclic citrullinated peptide antibodies [anti-CCP])
- connective tissue diseases (antinuclear antibody [ANA])
- small-vessel vasculitis (antineutrophil cytoplasmic antibodies [ANCA])
- myositis (creatinine kinase [CK])
- hypothyroidism (thyroid-stimulating hormone [TSH])
- infection (urine microscopy, culture and sensitivity [MCS])
- malignancy (calcium).
Nonspecific findings that can be supportive of a PMR diagnosis include normocytic anaemia and thrombocytosis on full blood count and mildly deranged liver function test results (typically an obstructive or mixed pattern). These abnormalities are reflective of high levels of interleukin-6 (IL-6) in the peripheral circulation, which is known to be the predominant cytokine driving PMR.10,11 The systemic markers of inflammation, C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR), are usually elevated two- to tenfold their normal ranges; however, a subset of patients with PMR may present with normal levels (7 to 20%).7,12 Finally, it is worthwhile assessing each patient’s risk of developing steroid-related side effects, including diabetes mellitus and osteoporosis, from the outset and re-evaluating these at regular intervals thereafter (every three to six months for diabetes and annually for osteoporosis).
Imaging
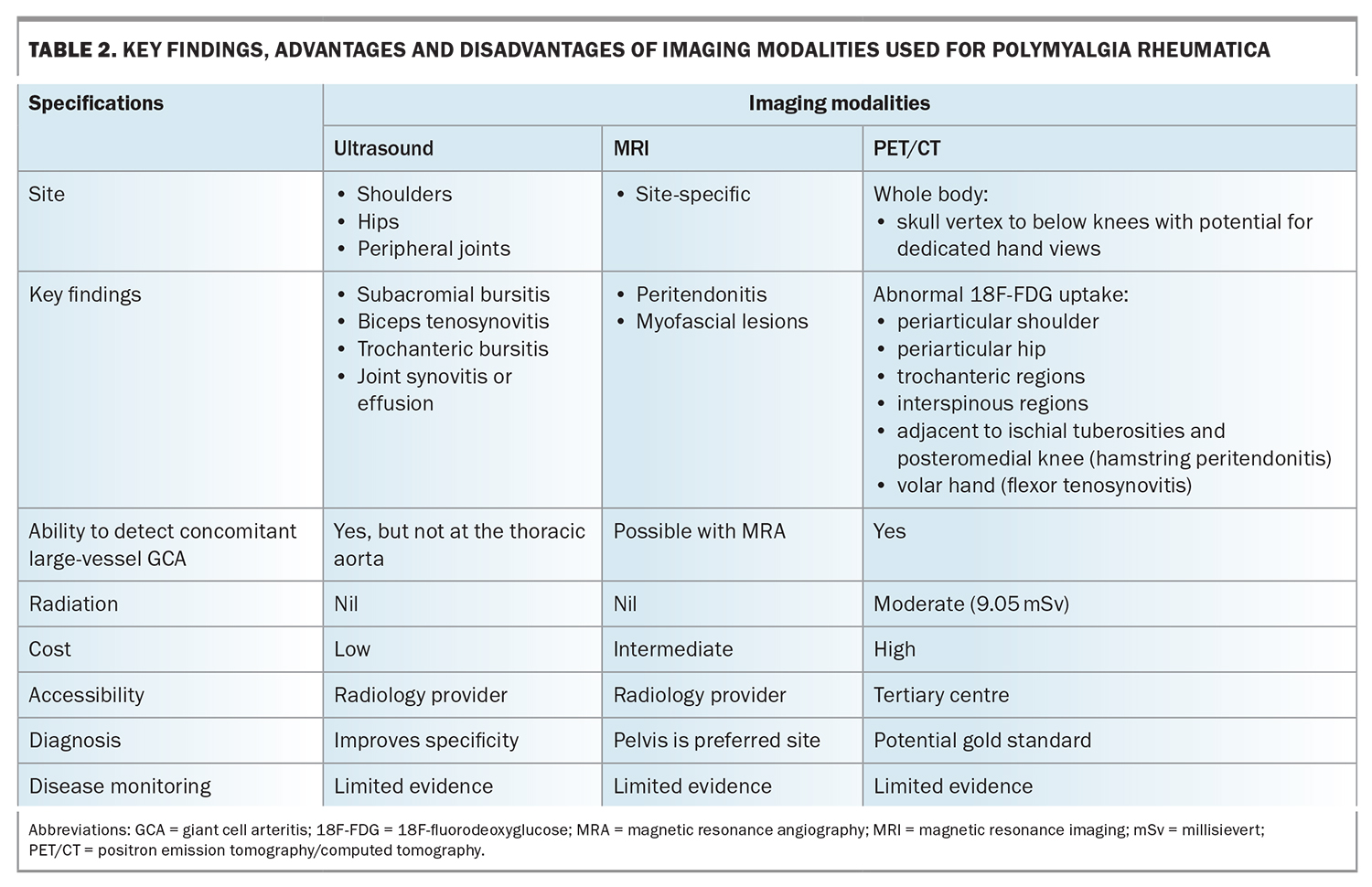
Although imaging is not needed to diagnose PMR, it can be a helpful addition in certain cases, including in patients presenting with atypical clinical features (e.g. normal CRP level and ESR) or an unacceptably high risk of glucocorticoid-induced adverse events (e.g. poorly controlled diabetes, established osteoporosis), or if there is concern about concomitant large-vessel GCA (e.g. profound constitutional symptoms). Imaging may reduce the likelihood of misclassification, with 11% of patients eventually being given an alternative diagnosis in a 10-year follow-up study.13 In Table 2, the features of PMR detectable using modern imaging techniques are outlined, alongside the relevant advantages and disadvantages of each modality.
Ultrasound
On ultrasound, bilateral subacromial-subdeltoid bursitis represents the imaging hallmark of PMR (sensitivity, 66%; specificity, 89%).14,15 Additional suggestive features include biceps tenosynovitis and bilateral trochanteric bursitis; however, synovitis at the glenohumeral or hip joints are less discriminatory from a diagnostic standpoint.15 Again, this reflects the key difference in PMR’s pathology compared with analogous rheumatic diseases – its predominance for periarticular musculotendinous structures. Although individual abnormalities may be seen, for example, in a patient with rotator cuff disease, it is the combination of these findings at both shoulders or one shoulder and one hip that increases the likelihood of a PMR diagnosis.4 Accordingly, this is reflected in the 2012 European League Against Rheumatism/American College of Rheumatology Classification Criteria with the inclusion of an optional ultrasound scoring component. There is presently insufficient evidence however to advocate for routinely scanning the temporal arteries or great vessels in patients with suspected PMR if symptoms suggestive of concomitant GCA are not present.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) can detect the same inflammatory changes as those detected using ultrasound, but with superior sensitivity and specificity.15 Additionally, MRI can identify newly recognised imaging features of PMR, including peritendonitis and myofascial inflammatory lesions.16,17 Peritendonitis is known to correlate anatomically with areas of periarticular inflammation routinely visualised at the shoulder and hip joints in patients with PMR. On the other hand, myofascial inflammatory lesions, defined by high T2-short tau inversion recovery (STIR) signal within the affected muscle or the formation of a line around it, are less frequently observed. The finding is unique to PMR and likely reflects inflammation from the affected peritendon extending across the musculotendinous junction into the adjacent perimysium. Using magnetic resonance angiography, it is additionally possible to detect concentric wall thickening and mural contrast enhancement consistent with large vessel vasculitis in GCA.
Positron emission tomography/computed tomography
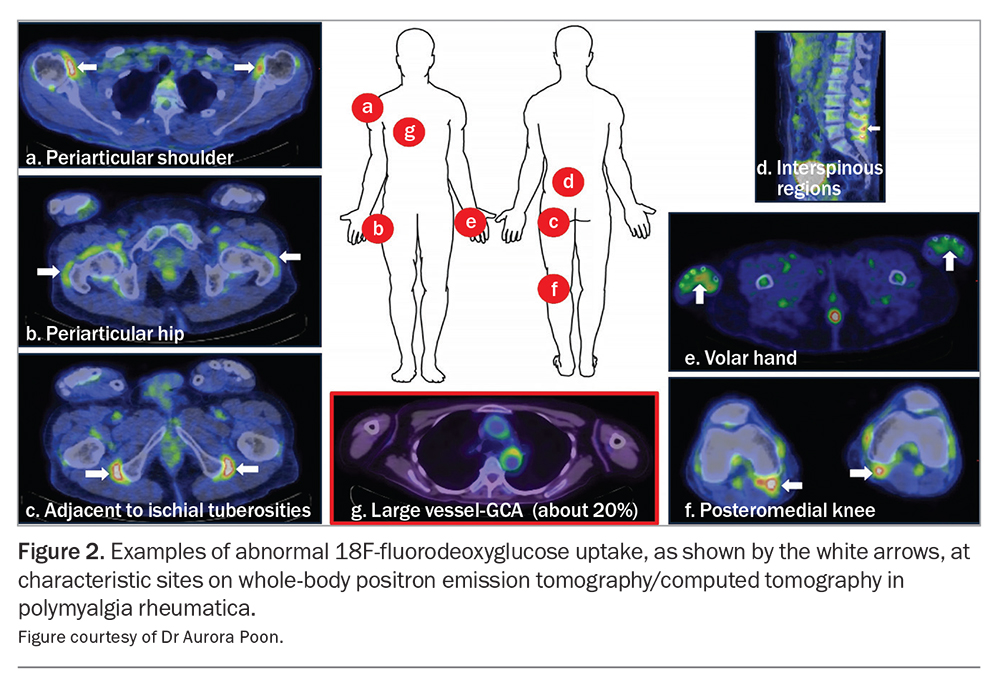
The most significant breakthrough for PMR in recent years involves the recognition of 18F-fluorodeoxyglucose (18F-FDG) PET/CT as a ‘one-stop shop’ for diagnosis given its capacity to document distinctive pathology throughout the whole body, assess for concomitant large-vessel GCA and exclude relevant differentials such as infection and malignancy.18 As with other imaging modalities, individual musculoskeletal findings are insufficient for diagnostic purposes; however, the presence of abnormalities at three sites has been shown to achieve sensitivity (90.9%) and specificity (92.4%) values far exceeding those achieved by clinical classification criteria. Examples of characteristic PMR findings on whole-body PET/CT are demonstrated in Figure 2. Availability, cost and radiation exposure represent drawbacks to the routine use of this technology as part of the diagnostic work-up for suspected PMR.
Initial treatment and disease activity assessment
Prednisolone remains the initial treatment of choice for PMR, with a recommended dose of 12.5 to 25 mg daily. Evidence-based guidelines suggest then gradual tapering over 12 to 18 months until complete cessation.19,20 Unfortunately, this approach appears inconsistent with the natural history of the disease experienced by most patients with PMR. A recent study found 50% of patients still required glucocorticoid therapy beyond two years, and another study documented 40.1% of participants taking prednisolone at a median 5.16 years follow up.2,21 Increasingly, the adverse impacts of long-term, low-dose steroids are also being recognised. Twice as many patients with PMR experience glucocorticoid-induced side effects than those with RA (due to earlier DMARD introduction), and a large primary care cohort study also showed that patients taking prednisolone at a dose less than 5 mg daily had a 40% increased risk of both infection and major adverse cardiovascular events.22-24
Assessing PMR disease activity can be difficult owing to the absence of a validated biomarker. Relapse is arbitrarily defined as the return of symptoms in combination with elevated markers of systemic inflammation. However, a normal CRP level and ESR have been documented in up to 25% of patients with PMR experiencing disease relapse.25 Although the use of imaging for disease activity assessment is in its infancy, improvements following treatment have been consistently observed on ultrasound, MRI and PET/CT compared with findings at diagnosis.26-29 As complete resolution of abnormalities occurs infrequently, the significance of persistent imaging features is unknown and is an area of ongoing research. Further evaluation for concomitant GCA should be considered in patients with persistently elevated CRP levels or ESR despite clinically quiescent PMR and in those with intractable symptomatology; the latter may be due to large vessel involvement, as shown in more than 60% of cases in a recent PET/CT study.30
Relapsing disease
Current guidelines advocate for specialist referral with a view to consider a steroid-sparing agent in patients with PMR who have experienced recurrent relapse, along with those who exhibit an inadequate response to initial prednisolone dosing or who have an unacceptably high risk of glucocorticoid-induced adverse events.20 Methotrexate is considered the first-line DMARD, although evidence to support its efficacy is mixed.31-33 The use of biologic medications represents an exciting new frontier in PMR research, with the potential for application in future clinical practice. In the past two years, three randomised controlled trials (PMR-SPARE, SEMAPHORE and BRIDGE-PMR) showed higher rates of glucocorticoid-free remission and lower cumulative prednisolone doses in patients with PMR treated with the IL-6 blocker, tocilizumab, or the B-cell-depleting drug, rituximab.34-36 Results of the pivotal SAPHYR study, which evaluated sarilumab (a monoclonal antibody that targets IL-6 receptors), have subsequently led to the US FDA approving this medication as the first biologic for relapsing PMR.37 In Australia, none of these options are available yet on the PBS for this indication; however, the future is nonetheless looking brighter for patients with PMR courtesy of a shift away from steroid monotherapy as an acceptable long-term management strategy.
Conclusion
PMR is a common inflammatory condition that disproportionately affects older adults and women. Its unique pathology at musculotendinous structures has become apparent in recent years and facilitated major advances in the approach to its diagnosis. Moving forward, biologic medications will most likely be adopted to manage relapsing disease, thereby propelling PMR into the modern medicine era. MT
COMPETING INTERESTS: Dr Harris: None. Dr Owen has received consulting fees from Abbvie; received honoraria from Abbvie, Janssen, Novartis, Roche and Rheumatology Republic; and participated on the advisory board for Abbvie.
ACKNOWLEDGEMENTS: The authors wish to thank Dr Levent Efe and Dr Aurora Poon for their assistance with preparing the figures.
References
1. Crowson CS, Matteson EL, Myasoedova E, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum 2011; 63: 633-639.
2. Partington RJ, Muller S, Helliwell T, Mallen CD, Sultan AA. Incidence, prevalence and treatment burden of polymyalgia rheumatica in the UK over two decades: a population-based study. Ann Rheum Dis 2018; 77: 1750-1756.
3. Owen CE, Liew DFL, Buchanan RRC. Musculotendinous inflammation: the defining pathology of polymyalgia rheumatica? J Rheumatol 2019; 46: 1552-1555.
4. Dasgupta B, Cimmino MA, Maradit-Kremers H, et al. 2012 Provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2012; 64: 943-954.
5. Twohig H, Mitchell C, Mallen C, Adebajo A, Mathers N. "I suddenly felt I’d aged": a qualitative study of patient experiences of polymyalgia rheumatica (PMR). Patient Educ Couns 2015; 98: 645-650.
6. Owen CE, Poon AMT, Lee ST, et al. Fusion of positron emission tomography/computed tomography with magnetic resonance imaging reveals hamstring peritendonitis in polymyalgia rheumatica. Rheumatology 2018; 57: 345-353.
7. Salvarani C, Pipitone N, Versari A, Hunder GG. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol 2012; 8: 509-521.
8. Salvarani C, Cantini F, Macchioni P, et al. Distal musculoskeletal manifestations in polymyalgia rheumatica: a prospective followup study. Arthritis Rheum 1998; 41: 1221-1226.
9. Cimmino MA, Parodi M, Zampogna G, Barbieri F, Garlaschi G. Polymyalgia rheumatica is associated with extensor tendon tenosynovitis but not with synovitis of the hands: a magnetic resonance imaging study. Rheumatology 2011; 50: 494-499.
10. Camellino D, Soldano S, Cutolo M, Cimmino MA. Dissecting the inflammatory response in polymyalgia rheumatica: the relative role of IL-6 and its inhibition. Rheumatol Int 2018; 38: 1699-1704.
11. Alvarez-Rodriguez L, Lopez-Hoyos M, Mata C, et al. Circulating cytokines in active polymyalgia rheumatica. Ann Rheum Dis 2010; 69: 263-269.
12. Cantini F, Salvarani C, Olivieri I, et al. Erythrocyte sedimentation rate and C-reactive protein in the evaluation of disease activity and severity in polymyalgia rheumatica: a prospective follow-up study. Semin Arthritis Rheum 2000; 30: 17-24.
13. Gonzalez-Gay MAG, García-Porrúa C, Vázquez-Caruncho M, Dababneh A, Hajeer A, Ollier WE. The spectrum of polymyalgia rheumatica in Northwestern Spain: incidence and analysis of variables associated with relapse in a 10-year study. J Rheumatol 1999; 26: 1326-1332.
14. Cantini F, Salvarani C, Olivieri I, et al. Shoulder ultrasonography in the diagnosis of polymyalgia rheumatica: a case-control study. J Rheumatol 2001; 28: 1049-1055.
15. Mackie SL, Koduri G, Hill CL, et al. Accuracy of musculoskeletal imaging for the diagnosis of polymyalgia rheumatica: systematic review. RMD Open 2015; 1: e000100.
16. Fruth M, Buehring B, Baraliakos X, Braun J. Use of contrast-enhanced magnetic resonance imaging of the pelvis to describe changes at different anatomic sites which are potentially specific for polymyalgia rheumatica. Clin Exp Rheumatol 2018; 114: 86-95.
17. Laporte J-P, Garrigues F, Huwart A, et al. Localized myofascial inflammation revealed by magnetic resonance imaging in recent-onset polymyalgia rheumatica and effect of tocilizumab therapy. J Rheumatol 2019; 46: 1619-1626.
18. Hofman MS. Fluorodeoxyglucose positron emission tomography/computed tomography: a "one stop shop" for diagnosing polymyalgia rheumatica. BMJ 2014; 348: f7705.
19. Dasgupta B, Borg FA, Hassan N, et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology 2010; 49: 1594-1597.
20. Dejaco C, Singh YP, Perel P, et al. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74: 1799-1807.
21. Muller S, Hider SL, Sokhal BS, Lawton SA, Helliwell T, Mallen CD. Long-term use of glucocorticoids for polymyalgia rheumatica: follow-up of the PMR Cohort Study. Rheumatol Adv Pract 2022; 6: rkac034.
22. Wu J, Keeley A, Mallen C, Morgan AW, Pujades-Rodriguez M. Incidence of infections associated with oral glucocorticoid dose in people diagnosed with polymyalgia rheumatica or giant cell arteritis: a cohort study in England. CMAJ 2019; 191: E680-E688.
23. Pujades-Rodriguez M, Morgan AW, Cubbon RM, Wu J. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PloS Med 2020; 17: e1003432.
24. Hoes JN, Jacobs JWG, Verstappen SMM, Bijlsma JWJ, van der Heijden GJMG. Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: a meta-analysis. Ann Rheum Dis 2009; 68: 1833-1838.
25. Dejaco C, Singh YP, Perel P, et al. Current evidence for therapeutic interventions and prognostic factors in polymyalgia rheumatica: a systematic literature review informing the 2015 European League Against Rheumatism/American College of Rheumatology recommendations for the management of polymyalgia rheumatica. Ann Rheum Dis 2015; 74: 1808-1817.
26. Jiménez-Palop M, Naredo E, Humbrado L, et al. Ultrasonographic monitoring of response to therapy in polymyalgia rheumatica. Ann Rheum Dis 2010; 69: 879-882.
27. Macchioni P, Catanoso MG, Pipitone N, Boiardi L, Salvarai C. Longitudinal examination with shoulder ultrasound of patients with polymyalgia rheumatica. Rheumatology 2009; 48: 1566-1569.
28. Huwart A, Garrigues F, Jousse-Joulin S, et al. Ultrasonography and magnetic resonance imaging changes in patients with polymyalgia rheumatica treated by tocilizumab. Arthritis Res Ther 2018; 20: 11.
29. Palard-Novello X, Querellou S, Gouillou M, et al. Value of (18)F-FDG PET/CT for therapeutic assessment of patients with polymyalgia rheumatica receiving tocilizumab as first-line treatment. Eur J Nucl Med Mol Imaging 2016; 43: 773-779.
30. Prieto-Peña D, Martínez-Rodríguez I, Loricera J, et al. Predictors of positive (18)F-FDG PET/CT-scan for large vessel vasculitis in patients with persistent polymyalgia rheumatica. Semin Arthritis Rheum 2019; 48: 720-727.
31. Caporali R, Cimmino MA, Ferraccioli G, et al; Systemic Vasculitis Study Group of the Italian Society for Rheumatology. Prednisone plus methotrexate for polymyalgia rheumatica: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2004; 141: 493-500.
32. Ferraccioli G, Salaffi F, de Vita S, Casatta L, Bartoli E. Methotrexate in polymyalgia rheumatica: preliminary results of an open, randomized study. J Rheumatol 1996; 23: 624-628.
33. van der Veen MJ, Dinant HJ, van Booma-Krankfort C, van Albada-Kuipers GA, Bijlsma JW. Can methotrexate be used as a steroid sparing agent in the treatment of polymyalgia rheumatica and giant cell arteritis? [Erratum appears in Ann Rheum Dis 1996; 55: 563]. Ann Rheum Dis 1996; 55: 218-223.
34. Bonelli M, Radner H, Kerschbaumer A, et al. Tocilizumab in patients with new onset polymyalgia rheumatica (PMR-SPARE): a phase 2/3 randomised controlled trial. Ann Rheum Dis 2022; 81: 838-844.
35. Devauchelle-Pensec V, Carvajal-Alegria G, Dernis E, et al. Effect of tocilizumab on disease activity in patients with active polymyalgia rheumatica receiving glucocorticoid therapy: a randomized clinical trial. JAMA 2022; 328: 1053-1062.
36. Marsman DE, den Broeder N, van den Hoogen FHJ, den Broeder AA, van der Maas A. Efficacy of rituximab in patients with polymyalgia rheumatica: a double-blind, randomised, placebo-controlled, proof-of-concept trial. Lancet Rheumatol 2021; 3: e758-e766.
37. Spiera RF, Unizony S, Warrington KJ, et al. Sarilumab for relapse of polymyalgia rheumatica during glucocorticoid taper. N Engl J Med 2023; 389: 1263-1272.