Lung cancer screening: an update for primary care
With data from international programs backing the efficacy of lung cancer screening, the Australian National Lung Cancer Screening Program is due to launch in 2025. It will be available to current or ex-smokers aged 50 to 70 years who meet eligibility criteria. The program is anticipated to prevent lung cancer mortality through early detection.
- The Australian National Lung Cancer Screening Program will commence in 2025.
- Lung cancer is the most common cause of cancer-related deaths in Australia. The proposed screening program will aim to reduce lung cancer mortality through early detection in high-risk individuals.
- The Medical Services Advisory Committee has recommended that lung cancer screening be limited to current- or ex-smoking individuals who are aged between 50 and 70 years, have a smoking history of more than 30 pack-years and have quit smoking less than 10 years ago.
- Eligible applicants will be offered a low-dose CT scan of the chest every two years. Patients with pulmonary nodules that are deemed high risk will be recommended for referral for specialist review.
- It is anticipated that within the first 10 years of the lung cancer screening program, 12,000 lung cancer deaths will be prevented and more than 50,000 quality-adjusted life-years will be gained.
The National Lung Cancer Screening Program (NLCSP) is due to commence in July 2025. The program aims to detect lung cancers at an early stage by screening high-risk individuals with low-dose CT (LDCT). This article provides an overview of key concepts in lung cancer screening.
Health impact of lung cancer in Australia
Lung cancer is currently the leading cause of cancer-related deaths in Australia in both men and women. In 2023, there were more than 14,000 new cases of lung cancer and more than 8000 lung cancer-related deaths.1,2 Lung cancer has a poor prognosis, with a five-year survival rate of 25.7% compared with an average survival rate of 72.5% from all other cancers in Australia.3
Rationale for lung cancer screening
Lung cancer is typically detected at a late stage, with more than 40% of cases Stage IV at the time of diagnosis.4 At this late stage, the lung cancer has spread to other organs and treatment options are limited.5 The prognosis of lung cancer varies significantly based on stage, with the five-year survival rate reducing from 67.7% for Stage I lung cancer to 3.8% for Stage IV lung cancer.4
Lung cancer screening aims to facilitate early detection and improve survival. National cancer screening programs currently exist for breast cancer, bowel cancer and cervical cancer.6 Data from existing screening programs in the USA indicate that this approach is viable, with the five-year survival rate of lung cancers detected in a screening program much higher than that detected outside of a screening program (64% vs 19%, respectively).7 In comparison, the advent of immunotherapy using checkpoint inhibitors improved five-year mortality of lung cancer by 14% to 23.7% across all stages.8 Therefore, the anticipated benefit from the lung cancer screening program in reducing cancer-specific mortality is at least comparable to recent advances in systemic therapies.
Evidence of lung cancer screening programs
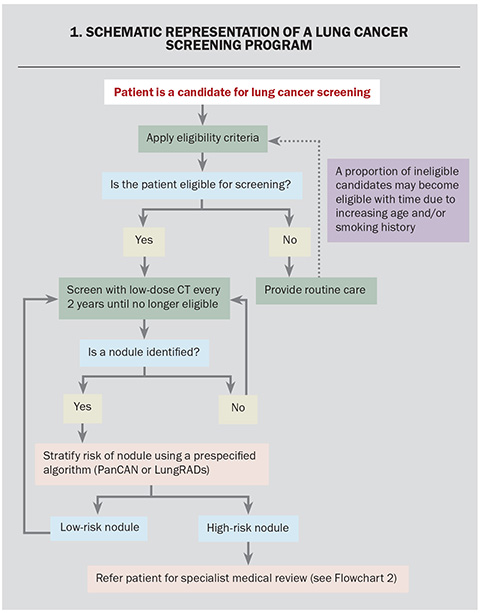
The efficacy of lung cancer screening using LDCT in reducing cancer-specific mortality has been demonstrated in randomised control trials. Findings from four landmark trials in lung cancer screening are presented below. Three of these trials (NLST, NELSON, ILST) investigated lung cancer screening in populations at risk from smoking exposure. The fourth trial (TALENT) investigated the efficacy of lung cancer screening in candidates with nonsmoking-related risk factors such as family history. A schematic representation of a program is presented in Flowchart 1.
The National Lung Screening Trial (NLST)
The National Lung Screening Trial (NLST) compared the efficacy of LDCT with that of chest x-rays (CXR) for lung cancer screening in high-risk smoking candidates.9 The study found that LDCT was more effective at detecting cancers than CXR. The cohort who underwent screening using LDCT also had a 20% reduction in lung cancer-specific mortality. These results suggest that LDCT is a more effective screening modality than CXR.
The Dutch-Belgian randomised lung cancer screening trial (NELSON)
The NELSON study established the effectiveness of LDCT in lung cancer screening in high-risk smoking candidates.10 The NELSON study compared outcomes of two cohorts: those who underwent screening using LDCT (intervention arm) and those who underwent no screening (control arm). The study found that LDCT was effective in detecting early-stage cancers, with 46.8% of cancers identified in the intervention arm diagnosed at Stage I compared with only 6.9% diagnosed in the control arm. This was associated with a reduction in cancer-related mortality of 24% in the intervention arm compared with the control arm. The result of this study supports the use of LDCT in lung cancer screening.
The International Lung Screening Trial (ILST)
The International Lung Screening Trial (ILST) is the first screening trial to include an Australian population.11 The study compared the effectiveness of different screening eligibility criteria and nodule management algorithms in lung cancer screening. Preliminary results indicate that risk-prediction models are superior to categorical criteria for selecting candidates for lung cancer screening.12 The results from remaining outcomes are awaiting publication.
The Taiwan Lung Cancer Screening in Never-Smoker Trial (TALENT)
The TALENT study is a prospective cohort study assessing the efficacy of lung cancer screening in nonsmoking candidates with at least one risk factor for lung cancer.13 The risk factors assessed included family history of lung cancer, passive smoking, environmental exposures, COPD and prior history of tuberculosis, which are not included in most current lung cancer screening eligibility criteria. The study found that participants with a family history of lung cancer had a significantly higher cancer detection rate of 2.6% compared with a detection rate of 1.2% in those without risk factors. This result supports the need to consider broadening lung cancer screening criteria to include high-risk nonsmokers such as candidates with a positive family history.
Existing lung cancer screening programs
The effectiveness of lung cancer screening has been evaluated in multiple randomised control studies, with the landmark NLST and NELSON study demonstrating a mortality benefit.9,10,14-19 Given this large body of evidence, lung cancer screening programs have been established in the USA, UK, Canada, Poland, China, Taiwan and South Korea.20
The Australian Lung Cancer Screening Program
The Australian NLCSP is currently under development and is due to commence by July 2025. The Medical Services Advisory Committee (MSAC) has published its recommendations for the proposed program.21,22 Some key features of the proposed lung cancer screening program are presented below.
Eligibility criteria for lung cancer screening
The MSAC recommends that lung cancer screening be offered to current- or ex-smoking individuals who meet eligibility criteria.21,22 Their proposed eligibility criteria require applicants to be aged 50 to 70 years, have at least a 30 pack-year smoking history, either currently smoking or have quit smoking within the past 10 years.23 The proposed MSAC selection criteria are comparable to those used by the United States Preventative Services Task Force (USPSTF), which uses categorical factors, age and smoking history to select applicants for lung cancer screening.24 Other existing screening programs utilise additional risk factors to select candidates for lung cancer screening. The Canadian Ontario Lung Screening Program and UK-based Targeted Lung Health Check Program both use risk prediction models which incorporate additional risk factors, including ethnicity, education level, personal history of lung cancer and family history of lung cancer, to select screening applicants.25 The Taiwan-based screening program is the first to include nonsmoking participants, provided they have a family history of lung cancer.26 The USPSTF criteria have also evolved over time to address screening inequities, and are now enrolling participants with a broader age range and less intense smoking history, based on local data.24 The Australian lung cancer screening eligibility criteria may also evolve with improved understanding of local lung cancer epidemiology.
Screening intervals
MSAC recommends that eligible applicants undergo LDCT every two years for lung cancer screening.21 This recommendation was based on results from the NELSON and Multicentric Italian Lung Detection (MILD) trials.10,27 The NELSON study found that a screening period of two years did not lead to a significant increase in the proportion of advanced cancers identified.10 Similarly, the MILD study found that two-year screening intervals reduced the total number of scans performed without significantly increasing lung cancer mortality or the proportion of advanced cancers identified on screening.27 Based on these results, a screening interval of two years may reduce health expenditure and radiation exposure without significantly impacting the efficacy of the screening program.
Risk stratification of pulmonary nodules
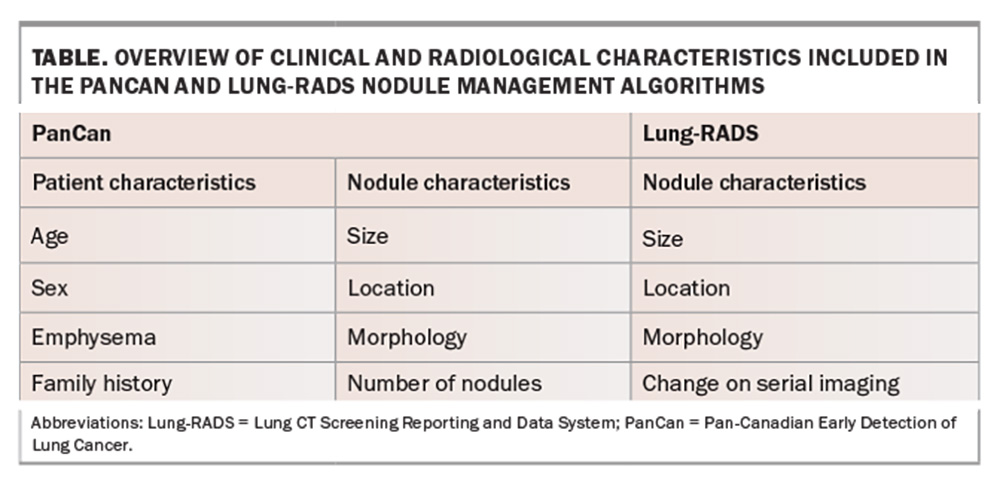
All lung nodules identified during the screening process will need to undergo risk assessment to determine further action. Nodule stratification algorithms, such as Pan-Canadian Early Detection of Lung Cancer (PanCan) and Lung CT Screening Reporting and Data System (Lung-RADS) (Table), stratify nodules based on the risk of malignancy.28 MSAC currently recommends using the PanCan algorithm for nodules detected on baseline screening scans and Lung-RADs algorithm for nodules detected on subsequent screening scans.28,29 The PanCan algorithm uses patient (age, sex, family history) and nodule characteristics (nodule size, nodule location, nodule type, morphology) to predict the risk of malignancy.28 The Lung-RADs algorithm uses radiological features of identified pulmonary nodules and the change on serial imaging for risk stratification.29 Depending on the risk profile of the patient and the radiological features of nodules identified, the patient may be discharged, remain within the screening program or referred for specialist respiratory review.
Management of pulmonary nodules identified on screening
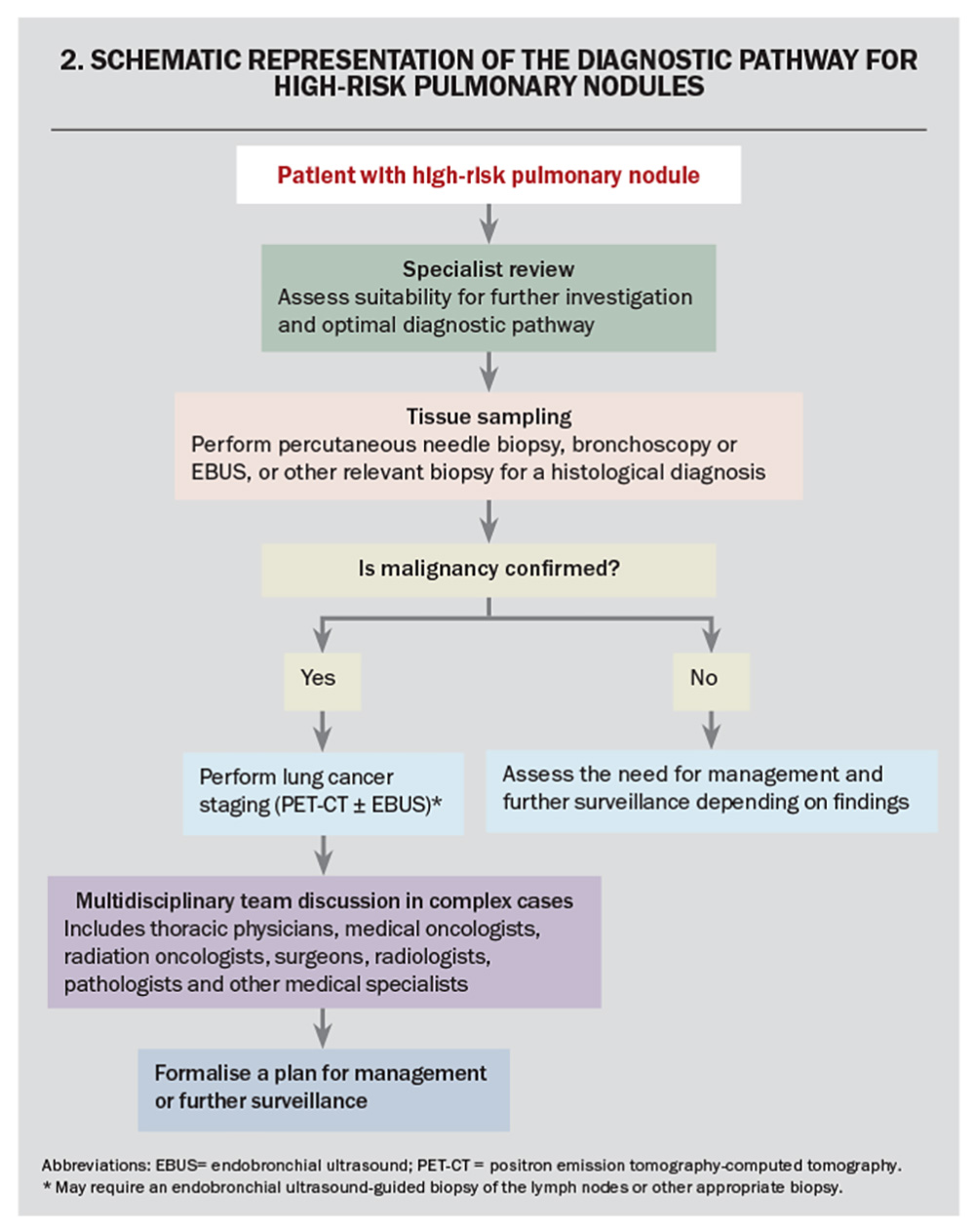
Pulmonary nodules have a broad differential that includes benign, infective, immunological and malignant pathology. Therefore, the diagnosis of lung cancer requires histopathological confirmation using tissue obtained via image-guided biopsy, surgery or bronchoscopy.30 Once the lung cancer diagnosis has been confirmed, the patient requires staging to determine the extent of spread, which may require PET-CT, endobronchial ultrasound-guided sampling of lymph nodes and/or biopsy of suspected distal metastases.30 Complex cases require management in a multidisciplinary team that includes thoracic physicians, oncologists, surgeons, radiologists, pathologists and other medical and allied specialties.31 A schematic representation for the diagnostic pathway for the management of patients with high-risk pulmonary nodules is presented in Flowchart 2.
Projected impact of lung cancer screening
The national Report on the Lung Cancer Screening Enquiry estimates that within the first 10 years of the lung cancer screening program more than 70% of lung cancers will be diagnosed at an early stage, 12,000 lung cancer deaths will be avoided, and more than 50,000 quality-adjusted life-years will be gained.4
Benefits beyond early detection of lung cancer
Opportunities for smoking cessation
Tobacco smoking is the most important modifiable risk factor for lung cancer. The proposed targeted lung cancer screening program offers opportunities to incorporate interventions for smoking cessation. Studies have shown that participation in a screening program increases readiness to quit smoking, increases cessation attempts and improves the chances of success.32,33 Therefore, there is a strong incentive to integrate smoking cessation into the lung cancer screening program.
Incidental findings on LDCT
Incidental findings will be identified on LDCT as a part of the screening program. A retrospective analysis of NLST data identified incidental findings in 33.8% of LDCT images, and 89.1% of these findings were deemed reportable.34 The most common findings were emphysema, coronary artery calcification and nonpulmonary mass lesions.34 Therefore, the use of LDCT may also identify other pathologies and allow early intervention.
Potential harms of lung cancer screening
Radiation exposure
CT uses ionising radiation to produce images and cumulative exposure carries the risk of inducing cancers.35 The LDCT used in the screening program reduces the ionising radiation from 7 mSV (used in conventional CT) to 1.5 mSV.4 Based on a single centre study, the number of induced cancers after 10 years of screening with LDCT was estimated to be one cancer per 108 lung cancers identified.35 In the context of a high-risk population, the benefit from a reduction in lung cancer mortality is generally thought to outweigh the risk from radiation exposure.4
False positives
False positives refer to the detection of noncancerous nodules during lung cancer screening. Potential harms from false positives include psychological distress and unnecessary investigations or procedures. Lung cancer screening trials found false-positive rates between 9.6% to 28.9%.36 Data from US screening programs indicate that 0.09% to 0.56% of participants underwent a biopsy and 0.5% to 1.3% underwent a surgical procedure for a false-positive result.36
Psychological burden
Data from existing studies indicate that lung cancer screening is associated with significant psychological burden. Analyses from the NELSON study demonstrated that indeterminant screening results were associated with an increased level of distress over the short term based on the Impact of Effects Scale (IES).37 Data from the NLST study showed that true positives were also associated with negative impacts on quality of life based on 36-Item Short Form Survey (SF-36) scores.38 Given the potential impacts of screening on quality of life, adequate support services will need to be implemented alongside the NLCSP.
Overdiagnosis
Overdiagnosis refers to the detection of cancers that would not have been clinically significant, and is a concept applied to populations rather than individuals.39 The rates of overdiagnosis in screening trials vary widely between 6.3% to 67.2%.21,39 Potential strategies to reduce overdiagnosis include limiting screening to high-risk individuals and effective risk stratification of identified nodules.39
Future directions
Computer-aided detection and radiomics
The Australian NLCSP will generate a large volume of LDCT images. Computer-aided detection (CAD) uses software that aids the radiologist in detecting nodules by removing unwanted background in LDCT images and highlighting findings suspected to be pulmonary nodules.40 In doing so, CAD may shorten image reporting times and reduce the risk of missing nodules.
Radiomics software predicts whether an identified nodule is likely to be cancerous. This is achieved by analysing nodule features (size and morphology) to predict the likelihood that a pulmonary nodule is cancerous.41 Given the lung cancer screening program is likely to generate large numbers of scans, radiomics may become a valuable adjunct in risk stratification of identified nodules.
Optimising eligibility criteria for lung cancer screening
The proposed lung cancer screening program will target current- and ex-smoking individuals. However, it is known that 10% to 25% of lung cancers occur in people without a significant smoking history and this approach may therefore lead to the exclusion of some high-risk individuals.41 Epidemiological studies suggest that women and those with a family history of lung cancer are at higher risk of lung cancer, even without a significant smoking history.42,43 Most recently, the TALENT study demonstrated that individuals with a family history of lung cancer had a significantly higher cancer detection rate.13 The lung cancer detection rate in individuals with a positive family history was 2.7%, which is higher than the overall lung cancer detection rate in the NLST study (1.1%) and NELSON study (0.9%) despite no significant smoking history.14 Given comparable lung cancer detection rates, the TALENT study supports the need to consider broadening selection criteria for lung cancer screening to include high-risk nonsmokers with a family history. The selection criteria for lung cancer screening may evolve with improved understanding of lung cancer risk factors and epidemiology. The mortality benefit of LDCT lung cancer screening in nonsmoking participants without additional risk factors has yet to be determined.
The role of GPs in lung cancer screening
The Australian NLCSP is under development and the role of GPs has not been formally announced. Based on experience from the US screening program, the roles that GPs could play in the lung cancer screening program include:44
- identifying suitable candidates
- shared decision making with patients and multidisciplinary teams
- follow up in patients with abnormal and incidental findings
- maintaining engagement or adherence to the screening program.
Population engagement will be key to the success of the NLCSP. Other Australian cancer screening programs have achieved varying degrees of engagement, with 41%, 50% and 68% of eligible individuals participating in colorectal, breast and cervical cancer screening, respectively.45-47 The uptake of lung cancer screening programs varies widely across the world. Estimates from existing screening or pilot programs in the USA, South Korea, China, the UK and Japan show varying uptake rates of up to 5.8%, 23%, 31%, 52.6% and 53.4% of eligible candidates, respectively.48,49 Some contributing factors to low uptake for lung cancer screening programs include lack of awareness, underestimation of cancer risk, fatalistic view of lung cancer and smoking-related stigma.50 Poor uptake will reduce both the number of individuals screened and cancer deaths prevented by the NLCSP.
Australian GPs will likely provide pivotal support for the program. About 82% of the people in Australia have attended a GP practice in the past 12 months, which provides GPs with the greatest coverage of the Australian population among healthcare practitioners.51 Furthermore, studies have shown that endorsement from a primary care physician is an important motivating factor and increases the readiness of patients to engage in cancer screening programs.52 The efforts of Australian GPs to support the NLCSP will be vital to its success.
Conclusion
The Australian NLCSP has the potential to improve patient outcomes through early detection and integration of preventative measures, such as smoking cessation. The success of the screening program will depend on effective implementation and adequate uptake. Although the role of Australian GPs has not been announced, their support will be key to the program’s success. MT
COMPETING INTERESTS: Dr Hu: None. Professor Stone has received past speaker honoraria from Astra Zeneca, Merck Sharp & Dohme and The Limbic; previous support for conference attendance from Astra Zeneca; and has been on the Advisory Board for Bristol Myers Squibb. She is currently Deputy Board Chair for the Thoracic Oncology Group Australasia and was appointed Editor-in-Chief, Journal of Thoracic Oncology Clinical Research Reports in 2023.
References
1. Australian Institute of Health and Welfare (AIHW). Cancer mortality by age visualisation. AIHW; Canberra, 2023. Available online at: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-mortality-by-age-visualisation (accessed July 2024).
2. Australian Institute of Health and Welfare (AIHW). Cancer incidence by age visualisation. AIHW; Canberra, 2023 Available online at: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-incidence-by-age-visualisation (accessed July 2024).
3. Australian Institute of Health and Welfare (AIHW). Cancer survival by age visualisation. AIHW; Canberra, 2023. Available online at: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-survival-by-age-visualisation (accessed July 2024).
4. Cancer Australia. Report on the Lung Cancer Screening Enquiry. Cancer Australia; NSW, 2020. Available online at: https://www.canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/report-lung-cancer-screening-enquiry (accessed July 2024).
5. Lababede O, Meziane MA. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist 2018; 23: 844-848.
6. Australian Government Department of Health and Aged Care. Screening for Cancer. Updated November, 2023. Available online at: https://www.health.gov.au/topics/cancer/screening-for-cancer (accessed July 2024).
7. Young RP, Hopkins RJ. Measures of outcome in lung cancer screening: maximising the benefits. J Thorac Dis 2016; 8: E1317-E1320.
8. Ferreira M, Reckamp KL. Editorial: impact of immunotherapy in lung cancer. Front Oncol. 2022; 12: 1083524.
9. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: 395-409.
10. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020; 382: 503-513.
11. Lim KP, Marshall H, Tammemägi M, et al; ILST (International Lung Screening Trial) Investigator Consortium. Protocol and rationale for the International Lung Screening Trial. Ann Am Thorac Soc 2020; 17: 503-512.
12. Tammemägi MC, Ruparel M, Tremblay A, et al. USPSTF2013 versus PLCOm2012 lung cancer screening eligibility criteria (International Lung Screening Trial): interim analysis of a prospective cohort study. Lancet Oncol 2022; 23: 138-148.
13. Chang GC, Chiu CH, Yu CJ, et al. Low-dose CT screening among never-smokers with or without a family history of lung cancer in Taiwan: a prospective cohort study. Lancet Respir Med 2024; 12: 141-152.
14. Yang W, Qian F, Teng J, et al. Community-based lung cancer screening with low-dose CT in China: Results of the baseline screening. Lung Cancer 2018; 117: 20-26.
15. Infante M, Cavuto S, Lutman FR, et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med 2015; 191: 1166-1175.
16. Paci E, Puliti D, Lopes Pegna A, et al; the ITALUNG Working Group. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017; 72: 825-831.
17. Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening - results from the randomized German LUSI trial. Int J Cancer 2020; 146: 1503-1513.
18. Field JK, Vulkan D, Davies MPA, et al; Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg Health Eur 2021; 10: 100179.
19. Pastorino U, Silva M, Sestini S, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol 2019; 30: 1162-1169.
20.Poon C, Wilsdon T, Sarwar I, Roediger A, Yuan M. Why is the screening rate in lung cancer still low? A seven-country analysis of the factors affecting adoption. Front Public Health 2023; 11: 1264342.
21. Medical Services Advisory Committee (MSAC). Public Summary Document: Application No. 1699 – National Lung Cancer Screening Program. Australian Government; Canberra, 2022. Available online at: https://www1.health.gov.au/internet/msac/publishing.nsf/Content/C77B956C49CD6841CA25876D000392
DF/$File/1699%20-%20Final%20PSD_Mar-Apr2022_v2redacted.pdf (accessed July 2024).
22. Medical Services Advisory Committee (MSAC). Attachments to the Public Summary Document: Application No. 1699 – National Lung Cancer Screening Program. Australian Government; Canberra, 2022. Available online at: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1699-public (accessed July 2024).
23. US Preventive Services Task Force; Krist AH, Davidson KW, Mangione CM, et al. Screening for lung cancer: US preventive Services Task Force Recommendation Statement. JAMA 2021; 325: 962-970.
24. Cancer Council Australia. Policy context and impact. Lung Cancer Screening Prevention Policy. Cancer Council, 2023. Available online at: https://www.cancer.org.au/about-us/policy-and-advocacy/early-detection/lung-cancer/policy-context-and-impact (accessed July 2024).
25. Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013; 368: 728-736.
26. Lung Cancer Policy Network. Learning from Taiwan: implementing a national lung cancer screening programme. August, 2023. Available online at: https://www.lungcancerpolicynetwork.com/learning-from-taiwan-implementing-a-national-lung-cancer-screening-programme/ (accessed July 2024).
27. Pastorino U, Sverzellati N, Sestini S, et al. Ten-year results of the Multicentric Italian Lung Detection trial demonstrate the safety and efficacy of biennial lung cancer screening. Eur J Cancer 2019; 118: 142-148.
28. van Riel SJ, Ciompi F, Jacobs C, etb al. Malignancy risk estimation of screen-detected nodules at baseline CT: comparison of the PanCan model, Lung-RADS and NCCN guidelines. Eur Radiol 2017; 27: 4019-4029.
29. McKee BJ, Regis SM, McKee AB, Flacke S, Wald C. Performance of ACR Lung-RADS in a clinical CT lung screening program. J Am Coll Radiol 2016; 13 (2 Suppl): R25-R29.
30. Rami-Porta R, Call S, Dooms C, et al. Lung cancer staging: a concise update. Eur Respir J 2018; 5: 1800190.
31. Hardavella G, Frille A, Theochari C, et al. Multidisciplinary care models for patients with lung cancer. Breathe (Sheff) 2020; 16: 200076.
32. Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung cancer (Amsterdam, Netherlands) 2007; 56: 125-134.
33. Williams PJ, Philip KEJ, Buttery SC, et al. Immediate smoking cessation support during lung cancer screening: long-term outcomes from two randomised controlled trials. Thorax 2024; 79: 269-273.
34. Gareen IF, Gutman R, Sicks J, et al. Significant incidental findings in the National Lung Screening Trial. JAMA Intern Med 2023; 183: 677-684.
35. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017; 356: j347.
36. Jonas DE, Reuland DS, Reddy SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021; 325: 971-987.
37. van den Bergh KA, Essink-Bot ML, Borsboom GJ,et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010; 102: 27-34.
38. Gareen IF, Duan F, Greco EM, et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer 2014; 120: 3401-3409.
39. Brodersen J, Voss T, Martiny F, Siersma V, Barratt A, Heleno B. Overdiagnosis of lung cancer with low-dose computed tomography screening: meta-analysis of the randomised clinical trials. Breathe (Sheff) 2020; 16: 200013.
40. Binczyk F, Prazuch W, Bozek P, Polanska J. Radiomics and artificial intelligence in lung cancer screening. Transl Lung Cancer Res 2021; 10: 1186-1199.
41. Couraud S, Zalcman G, Milleron B, Morin F, Souquet PJ. Lung cancer in never smokers--a review. Eur J Cancer 2012; 48: 1299-1311.
42. Siegel DA, Fedewa SA, Henley SJ, Pollack LA, Jemal A. Proportion of never smokers among men and women with lung cancer in 7 US States. JAMA Oncology 2021; 7: 302-304.
43. Coté ML, Liu M, Bonassi S, et al. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. Eur J Cancer 2012; 48: 1957-1968.
44. Mańczuk M, Przepiórka I, Cedzyńska M, et al. Actual and potential role of primary care physicians in cancer prevention. Cancers (Basel) 2023; 15: 427.
45. Australian Institute of Health and Welfare (AIHW). National Cervical Screening Program. AIHW; Canberra, July 2023. Available online at: https://www.aihw.gov.au/reports/cancer-screening/national-cancer-screening-programs-participation/contents/national-cervical-screening-program (accessed July 2024).
46. Australian Institute of Health and Welfare (AIHW). Breast cancer screening participation. AIHW; Canberra, July 2023. Available online at: https://www.aihw.gov.au/reports/cancer-screening/national-cancer-screening-programs-participation/contents/breastscreen-australia/participation (accessed July 2024).
47. Australian Institute of Health and Welfare (AIHW). Bowel cancer screening participation. AIHW; Canberra, July 2023. Available online at: https://www.aihw.gov.au/reports/cancer-screening/national-cancer-screening-programs-participation/contents/national-bowel-cancer-screening-program/participation (accessed July 2024).
48. Poon C, Wilsdon T, Sarwar I, Roediger A, Yuan M. Why is the screening rate in lung cancer still low? A seven-country analysis of the factors affecting adoption. Front Public Health 2023; 11: 1264342.
49. Dickson JL, Hall H, Horst C,et al. Uptake of invitations to a lung health check offering low-dose CT lung cancer screening among an ethnically and socioeconomically diverse population at risk of lung cancer in the UK (SUMMIT): a prospective, longitudinal cohort study. Lancet Public health 2023; 8: e130-e140.
50. Cavers D, Nelson M, Rostron J, et al. Understanding patient barriers and facilitators to uptake of lung screening using low dose computed tomography: a mixed methods scoping review of the current literature. Respir Res 2022; 23: 374.
51. Australian Bureau of Statistics (ABS). Patient experiences. 2022-23 financial year. ABS; Canberra, 2023. Available online at: https://www.abs.gov.au/statistics/health/health-services/patient-experiences/latest-release (accessed July 2024).
52. Zajac IT, Whibley AH, Cole SR, et al. Endorsement by the primary care practitioner consistently improves participation in screening for colorectal cancer: a longitudinal analysis. J Med Screen 2010; 17: 19-24.