Clascoterone: a new topical acne medication
Acne vulgaris is a common condition, and most patients with mild-to-moderate acne can be treated with topical therapy. Clascoterone is a new topical cream with a novel mode of action that has just been released in Australia. It is the first topical prescription product that acts specifically on the sebaceous gland and functions as an antiandrogen and anti-inflammatory agent. Currently, there are few real-world data on its use in Australia and North America.
- Clascoterone is a new topical cream approved for use in patients aged 12 years or over with acne vulgaris. It is available on private prescription.
- It is the first topical prescription product that acts specifically on the sebaceous gland and functions as an antiandrogen and anti-inflammatory agent.
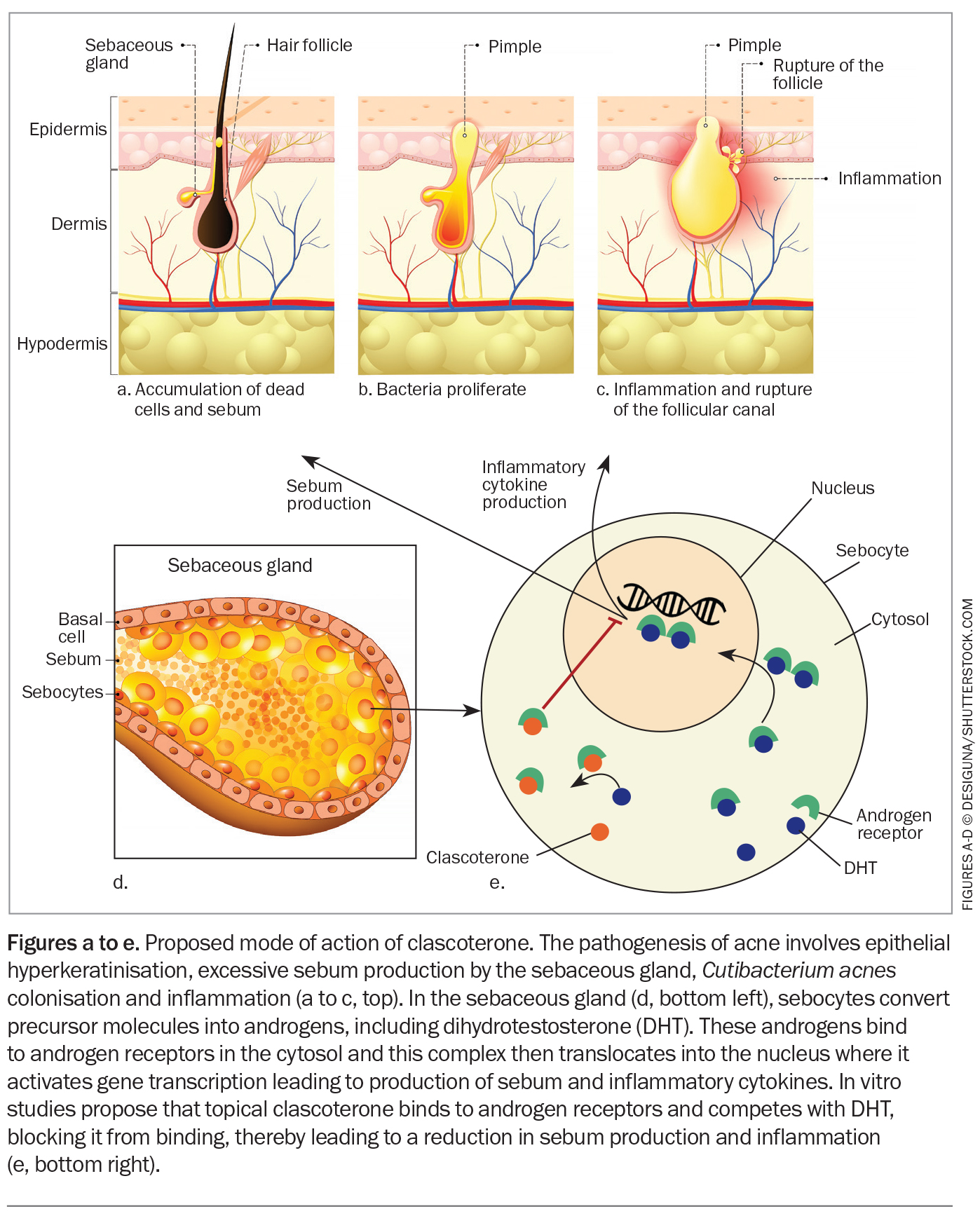
- Although its precise mechanism of action is yet to be established, clascoterone is thought to compete with androgens for receptors in the sebocytes, thereby preventing androgens from activating the transcription of genes involved in acne pathogenesis.
- Because of its tolerability profile observed in clinical trials, clascoterone may be a good treatment option for those with sensitive skin.
- Given cost constraints, it is likely that clascoterone will be used primarily for facial acne.
Clascoterone (cortexolone 17 alpha-propionate) is an androgen-receptor inhibitor that is thought to work in acne by decreasing sebum production and inflammation. It is formulated as a topical 1% cream and was approved in March 2024 by the TGA for the treatment of facial and truncal acne vulgaris in patients aged 12 years and over.1 It is available on private prescription in a 60 g tube and is applied twice daily on the face and trunk after cleansing.1 A thin even layer of clascoterone should be applied over the entire acne-prone area (not spot treating) in the morning and evening, avoiding the eyes, mouth and mucosal areas. Although clascoterone is effective for acne on both the face and trunk, it is likely that it will be used primarily for facial acne due to cost constraints.
Clascoterone is characterised by a fused four-ring backbone that is structurally identical to dihydrotestosterone and spironolactone.2 It is rapidly metabolised to its probable primary metabolite, cortexolone.3 In phase 2a pharmacokinetic studies, the plasma concentrations of cortexolone were generally below or near the lower limit of quantification (0.5 ng/mL) in patients with acne treated twice daily with clascoterone for 14 days.3
How does it work?
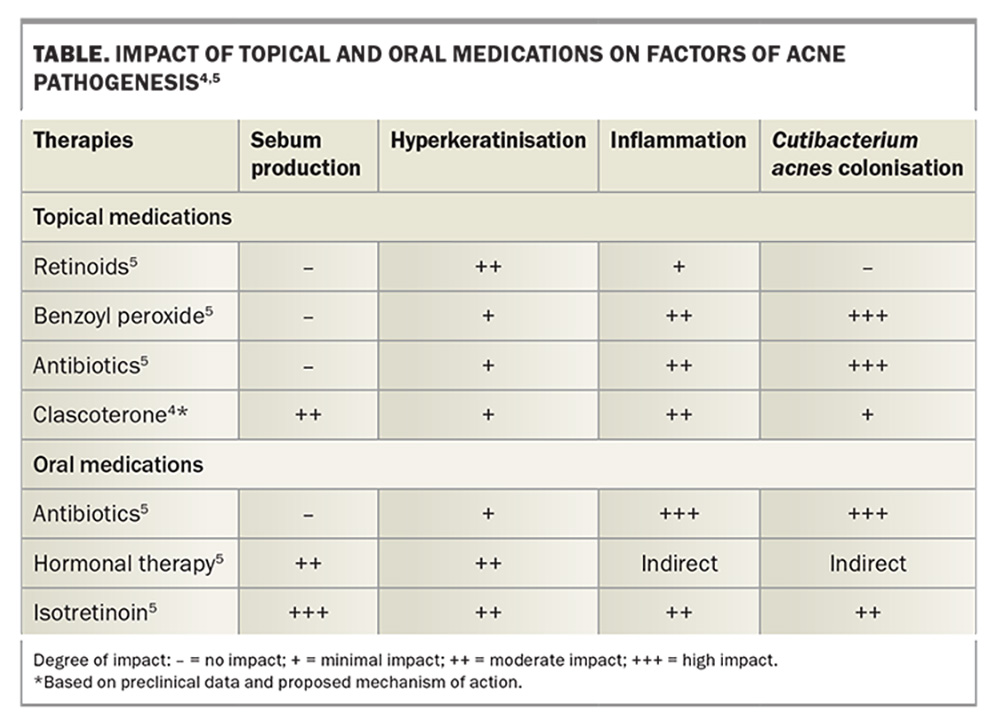
Androgens have a key role in acne development. Clascoterone is thought to compete with androgens for receptors, thereby preventing androgens from activating the transcription of genes involved in acne pathogenesis. The precise mechanism of action is yet to be established; however, in vitro evidence suggests that clascoterone competes with dihydrotestosterone and binds to the androgen receptor in sebocytes with high affinity. Clascoterone is therefore thought to act by inhibiting downstream signalling pathways involved in sebum secretion and inflammation (Figure).4 This is innovative, as the only other agents that affect sebum production are oral antiandrogens, such as spironolactone (Table).4,5
When and in whom is clascoterone used?
Clascoterone has been approved for the topical treatment of acne vulgaris in both male and female patients aged 12 years and older.1 Safety and efficacy data have shown it to be effective in facial and truncal acne for up to 12 months.6-8 It has been used in the USA since its launch there in 2021.
Clascoterone differs significantly from other commonly used topical acne prescription medications in that is does not contain an antibiotic (therefore, minimising antibiotic resistance), a topical retinoid or benzoyl peroxide (thereby minimising epidermal barrier damage and skin irritation). It may prove to be a well-tolerated topical acne product for patients who are concerned about side effects like burning, stinging, redness and itch.
Safety and efficacy
Two identical multicentre, randomised, double-blind, parallel-group, vehicle-controlled, phase 3 studies found no serious treatment-emergent adverse effects and no systemic adverse reactions with clascoterone treatment. The most common treatment-emergent adverse events were nasopharyngitis, headache and oropharyngeal pain, which occurred with the same frequency in the vehicle-control group.6,7 One percent or fewer patients treated with clascoterone or the vehicle cream complained of erythema, dryness and itch.6,7
In the extension trial of 9 to 12 months’ usage, the side-effect profile of clascoterone remained the same; however, the success rate significantly improved with time. About half of all patients had clear or almost clear facial acne at the end of the study and over 50% with truncal acne achieved clear or almost clear skin.8
Important precautions and interactions
Local skin reactions or irritation may occur with clascoterone therapy, so concurrent use of other potentially irritant products, such as facial scrubs, should be avoided.1 However, overseas reports suggest that clascoterone is extremely well tolerated with a low potential for skin barrier damage, unlike many of the currently available topical products that contain a retinoid or benzoyl peroxide.6,9
Clascoterone is not indicated for use in pregnancy and lactation as no data exist for safety in these groups.1
Clascoterone is indicated for use in patients 12 years of age and older, as younger patients have a theoretical risk of developing hyperkalaemia. This was seen in a maximal usage study where younger patients applied more product than older patients. The risk is due to the chemical composition similarity with spironolactone.1,10 Of note, the TGA has approved the use of clascoterone only in patients aged 12 years and older in whom there is minimal risk of hyperkalaemia.
Another theoretical risk of clascoterone use is hypothalamic pituitary axis (HPA) suppression due to the largely inactive metabolite, cortexolone, which has weak glucocorticoid properties. In a pharmacokinetic study involving maximum use conducted in 42 patients, suppression of the HPA axis was observed after 14 days in 5% of adults (one in 20) who applied 6 g clascoterone twice daily and 9% of adolescents (two in 22) who applied 4 g clascoterone twice daily. HPA axis function returned to normal in all patients during follow up, which took place four weeks after discontinuing clascoterone treatment.3 However, because of the small sample size of patients included in the maximal use trial, it has been deemed that there is no clear association between HPA axis suppression and clascoterone systemic exposure.11 If HPA axis suppression develops with clascoterone use, then stopping its application is recommended. This is unlikely to occur if the cream is applied to the face only.
Recent studies suggest that HPA axis suppression occurred when the dose was two- to six-times higher than recommended.12 An ongoing Canadian study has reported similar interim data [unpublished data]. Although clascoterone is effective for truncal use, its cost may limit this usage and it may be used more often for facial acne.
Clascoterone is not known to interact with any other topical or oral acne medications. Because of this potential lack of interaction, it may be possible to combine it in clinical practice with other acne treatments (see below).
How will clascoterone be used in clinical practice?
The trial data examined clascoterone as monotherapy twice daily for moderate-to-severe acne.6-8 There are currently no clinical trials that support its use in combination with other acne therapies. However, in clinical practice, it may be possible to use clascoterone as a twice-daily treatment in conjunction with other topical therapies that are often used at night, such as fixed-dose combination topical agents containing either a topical antibiotic/retinoid or benzoyl peroxide. It may even be possible to combine it with oral treatments. Although clascoterone is approved as a monotherapy, it will be up to the clinician whether to use it as monotherapy or in combination with other acne treatments as they see fit.
Because of its tolerability profile observed in clinical trials,clascoterone may be a good treatment option for patients with sensitive skin.6-8 As it is not a topical antibiotic, its usage does not confer any antibacterial resistance.
The aim of acne treatment is to treat as many pathogenic factors as possible, and it is significant that clascoterone is the only topical therapy that directly targets sebum production. The only other acne treatments that act this way are oral antiandrogens such as the combined oral contraceptives, spironolactone, cyproterone acetate and the oral retinoid, isotretinoin.
Clascoterone treatment may be considered in the following patients:
- patients aged 12 years and older of any gender with mild-to-moderate acne
- patients wishing to avoid oral medications such as the oral contraceptive, oral antibiotics or isotretinoin
- transmasculine patients in whom oral hormonal medication could interfere with gender-affirming care.
Conclusion
Clascoterone is available for use in patients aged 12 years and older with facial and truncal acne. It has been an eagerly awaited product because of its novel mode of action as a topical androgen-receptor blocker. It may be able to be combined with current treatment options. Prescription is a private cost; however, it may be a safe and effective treatment for patients who are unwilling to take oral medications. MT
COMPETING INTERESTS: Dr See has received consulting fees from Viatris, L’Oréal, Galderma, SunPharma and CSL; has received payment from Viatris, L’Oréal, Galderma, SunPharma and CSL; is co-chair of the All About Acne group; and is a member of the Public Affairs committee of the Australasian College of Dermatology.
This article is for general information purposes only, and the full Product Information should be consulted before prescribing any of the mentioned medications.
References
1. WINLEVI® (clascoterone). Australian Product Information. Sun Pharma ANZ, 2024. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2024-PI-01451-1&d=20240523172310101 (accessed August 2024).
2. Ferraboschi P, Legnani L, Celasco G, Moro L, Ragonesi L, Colombo D. A full conformational characterization of antiandrogen cortexolone-17α-propionate and related compounds through theoretical calculations and nuclear magnetic resonance spectroscopy. Med Chem Commun 2014; 5: 904-914.
3. Mazzetti A, Moro L, Gerloni M, Cartwright M. Pharmacokinetic profile, safety, and tolerability of clascoterone (cortexolone 17-alpha propionate, CB-03-01) topical cream, 1% in subjects with acne vulgaris: an open-label phase 2a study. J Drugs Dermatol 2019; 18: 563-568.
4. Rosette C, Agan FJ, Mazzetti A, Moro L, Gerloni M. Cortexolone-17 alpha-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol 2019; 18: 412-418.
5. Savage LJ, Layton AM. Treating acne vulgaris: systemic, local and combination therapy. Expert Rev Clin Pharmacol 2010; 13: 563-580
6. Herbert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream,1% for treatment in patients with facial acne. JAMA Dermatol 2020; 156: 621-630.
7. Herbert A, Eichenfield L, Thiboutot D, et al. Efficacy and safety of 1% Clascoterone cream in patients aged 12 years or more with acne vulgaris. J Drugs Dermatol 2023; 22: 174-181.
8. Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice-daily, in patients with acne vulgaris. J Am Acad Dermatol 2020; 83: 477-485.
9. Eichenfield L, Hebert A, Gold LS, et al. Long-term safety and efficacy of twice-daily topical clascoterone cream 1% in patients greater than or equal to 12 years of age with acne vulgaris. J Drugs Dermatol 2023; 22: 810-816.
10. Del Rosso JQ, Gold LS, Squittieri N, Thiboutot D. Is there a clinically relevant risk of hyperkalemia with topical clascoterone treatment? J Clin Aesthet Dermatol 2023; 16: 20-24.
11. US Food and Drug Administration Center for Drug Evaluation and Research. NDA 213433: Winlevi (clascoterone) cream, 1%. Multidisciplinary review and evaluation. October 12, 2018. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213433Orig1s000MultidisciplineR.pdf (accessed August 2024).
12. Bhatia N, Eichenfield LF, Mazzetti A, Moro L, Squittieri N, Hebert A. Hypothlamic-pituitary-adrenal axis response in patients with acne vulgaris treated with clascoterone. J Drugs Dermatol 2024; 23: 433-437.