Mild cognitive impairment and dementia: the treatment landscape

The treatment landscape for cognitive disorders continues to evolve. New disease-modifying therapies are available in many countries, but in Australia only symptomatic therapies are currently available for people with mild cognitive impairment and dementia. These symptomatic therapies, however, remain valuable and can cause a modest improvement in symptoms.
- Treatments for mild cognitive impairment and dementia are rapidly expanding.
- New therapies targeting the underlying disease processes are not yet approved by the TGA in Australia.
- Only therapies that improve symptoms are currently available in Australia for people with mild cognitive impairment and dementia. These therapies cause a modest improvement in memory loss, executive dysfunction, attention span, mood and personality changes and impaired function.
- GPs can initiate currently available therapies; however, they are often under-prescribed.
The treatment landscape for cognitive disorders continues to evolve. New disease-modifying therapies are available in many countries, but in Australia only symptomatic therapies are currently available for people with mild cognitive impairment and dementia. These symptomatic therapies, however, remain valuable and can cause a modest improvement in symptoms.
What do we want to achieve?
The ideal therapy for mild cognitive impairment (MCI) and dementia would restore brain function to the predisease state; However, this is impossible to achieve because too much damage has already occurred before symptoms begin. Disease-modifying therapies, which target the underlying pathology, are not currently available in Australia, but are expected to slow or delay the clinical progression of the disease in most people with dementia.
In Australia, only therapies that improve symptoms are available for people with MCI and dementia. These therapies cause a modest improvement in symptoms, including memory loss, executive dysfunction, attention, mood and personality changes and impaired function.1 These symptoms will, however, progress after time, because these symptomatic therapies are not targeting underlying disease processes. Nevertheless, a six- to 12-month period of reduced symptoms, achieved in some people, is worthwhile.
At what stage of dementia are therapies effective?
Preclinical stage
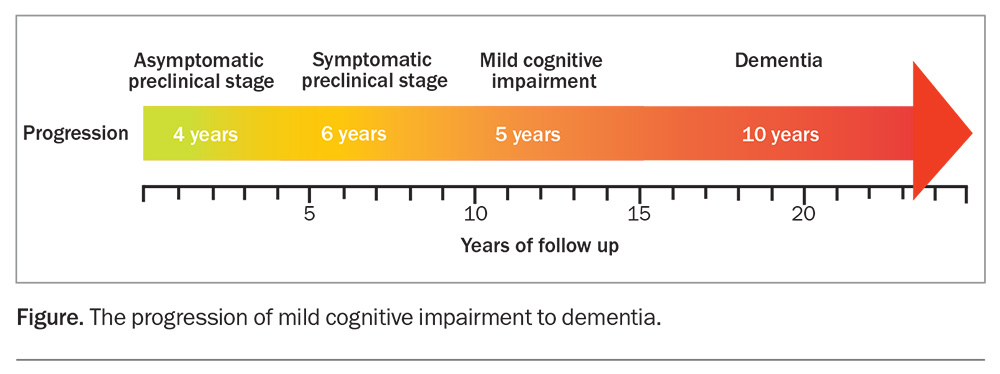
The pathological changes in neurocognitive diseases, such as Alzheimer’s disease, begin up to two decades before any symptoms appear (Figure). Yet, this preclinical stage is when disease-modifying therapies might prevent symptomatic disease, before too much synaptic damage has occurred. Trials of therapies that remove amyloid, and concurrently modify tau deposition, have so far failed but are still ongoing, using new monoclonal antibodies such as lecanemab and donanemab.2 The current symptomatic therapies do not have any benefits at this preclinical stage.
Mild cognitive impairment stage
MCI, in which there are cognitive and often behavioural changes but function is preserved, is an obvious target for both disease-modifying and symptomatic therapies. Symptomatic therapies have not shown benefit at this stage and, in fact, suggested harm through increased cardiovascular deaths.3 This may be because the trials were conducted 20 years or more ago when biomarker-based evidence of disease was not required. Thus, potentially up to 30% of trial participants did not have the disorder being tested, and thus, the potential of the drug to show benefit over placebo was reduced.4 These trials are unlikely to be repeated with better defined target populations because the drugs are now mostly generic; therefore, manufacturers have little incentive to fund the large, expensive trials that this would require.
Dementia stage
Cholinesterase inhibitors and memantine have shown benefit in the dementia stage of the disease. This is true even though the pivotal trials potentially included people without the diagnosis being targeted, suggesting these drugs may have shown greater effect if the population had been better defined. Cholinesterase inhibitors are most effective in the mild-to-moderate stage of dementia, but can have benefit if initiated in the more severe stage of dementia caused by Alzheimer’s disease. Memantine has shown benefit in the moderately severe stage of Alzheimer’s disease.1
How do we measure efficacy in clinical trials?
The trials of symptomatic therapies for MCI and dementia had endpoints that focused on memory and attention, overall clinical severity and functional status.1 Some also measured caregiver burden and patient quality of life. Most of the trials looked for changes compared with placebo by three months and extended to 18 to 24 months. Some clinicians, therefore, concluded that the drugs were only effective for up to 12 to 24 months, but this is incorrect as symptoms naturally begin worsening around this time. Some patients remained above the (projected) placebo arm beyond the trial endpoint; however, time to death has not been shown to be extended. Time to institutionalisation was positively impacted in some but not all trials.
In ongoing trials using disease-modifying therapies, the aim is to identify an increasing difference in the severity of measured cognitive and functional endpoints between placebo and therapy arms. In addition, reductions in the levels of biomarkers of the disease process, including amyloid and tau levels, are expected and have so far been found.
What therapies are currently available in Australia?
Souvenaid
Souvenaid, a medical food consisting primarily of polyunsaturated fatty acids, uridine, choline and other vitamins and minerals, has been formulated to enhance synaptic formation, which is important in a brain undergoing neurodegeneration where these precursors of the synapse are often deficient. It has shown benefit in a three-year randomised, placebo-controlled trial in people with biomarker-defined MCI caused by Alzheimer’s disease (prodromal Alzheimer’s disease).5 Significant reductions in decline were observed for the primary endpoint, the Neuropsychological Test Battery 5-item composite score, which translated to a ‘time saved’ metric of about nine months compared with the placebo group. The study was small and only a fraction reached the three-year point. In other smaller trials with Souvenaid, there has been a suggestion of benefit if initiated during the mild dementia stage of Alzheimer’s disease.6
It is the recommendation of this author that Souvenaid be started when a diagnosis of MCI caused by Alzheimer’s disease is made, and preferably when this diagnosis is supported by a biomarker such as 18F-fluorodeoxyglucose uptake on positron emission tomography. If initiated during the prodromal stage then it should be continued into the dementia stage. Souvenaid should be used daily for a minimum of three years to enhance the likelihood of benefit. It can be used safely with cholinesterase inhibitors and has very few adverse effects. It costs about $4 per day, but for patients with Department of Veterans’ Affairs Veteran Gold Card or Community Aged Care Packages, it is funded if prescribed or recommended by a doctor.
Cholinesterase inhibitors
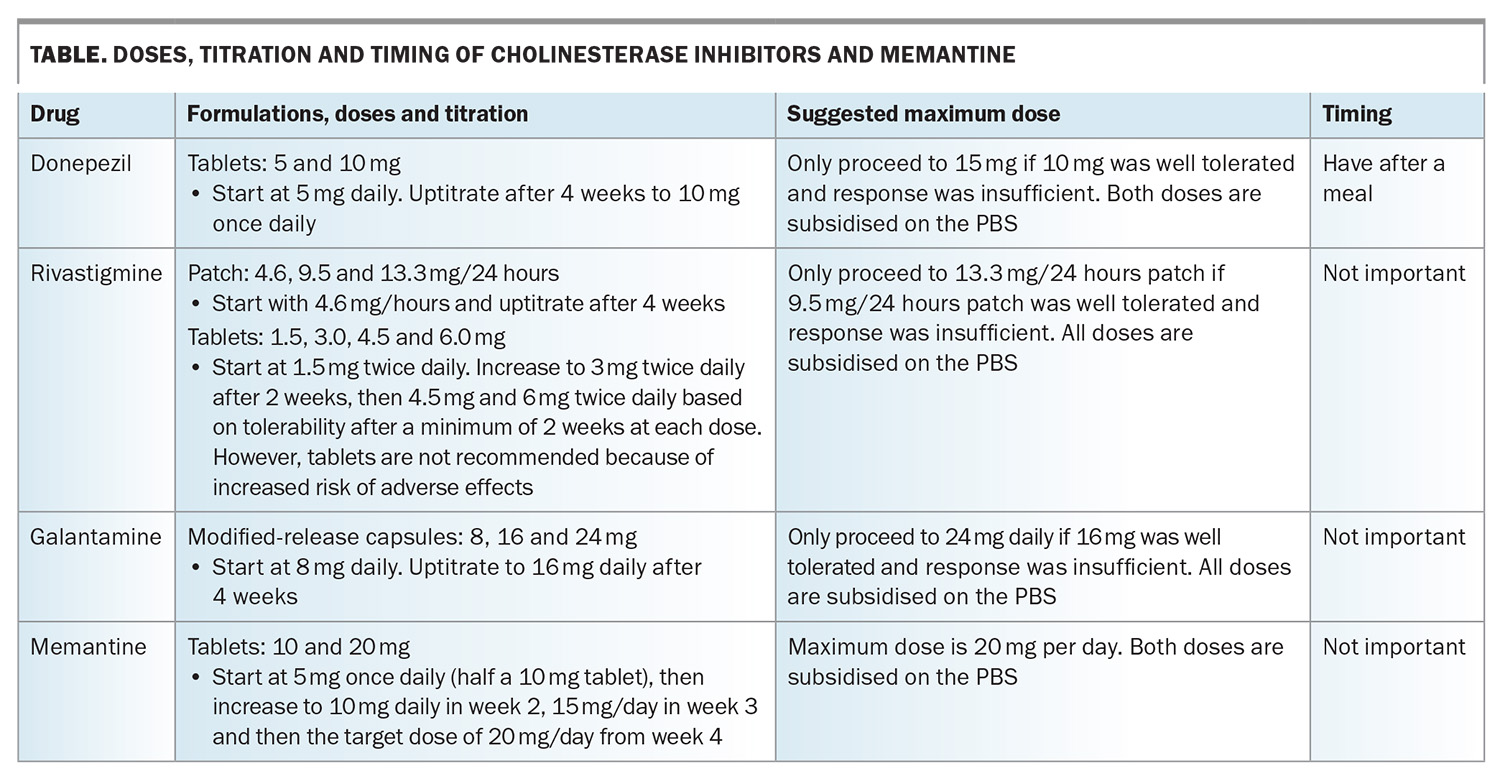
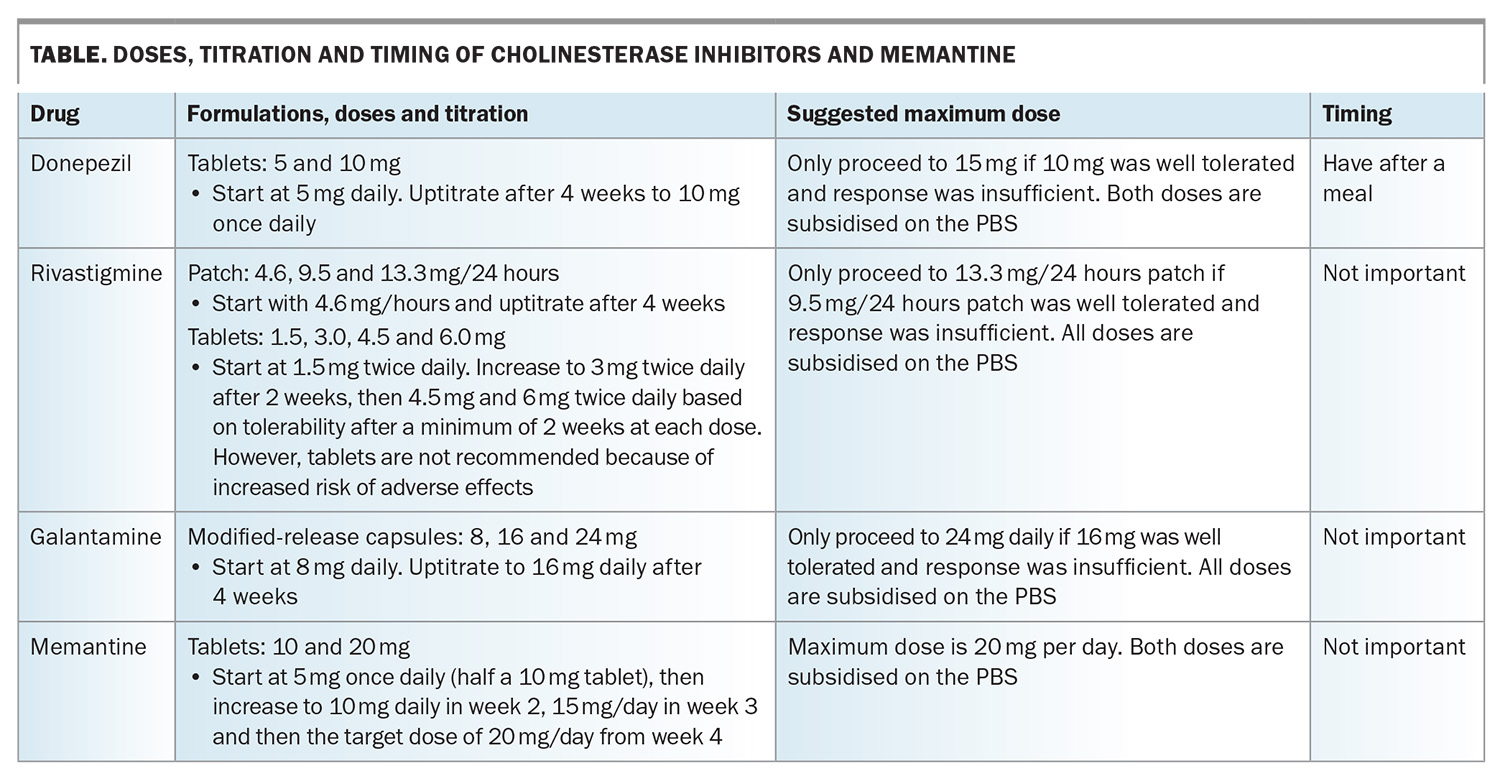
Cholinesterase inhibitors boost acetylcholine levels through inhibiting the enzyme, acetylcholinesterase, which breaks it down. This neurotransmitter is deficient in people with Alzheimer’s disease or Lewy body disease, and this deficiency likely contributes to the cognitive and behavioural symptoms. The three available cholinesterase inhibitors in Australia are donepezil, rivastigmine and galantamine (Table). All have been shown to be beneficial for people with mild-to-moderate dementia caused by Alzheimer’s disease, and donepezil has shown benefit when initiated in the severe stage of dementia caused by Alzheimer’s disease.7 Rivastigmine also boosts butyrylcholine levels; this neurotransmitter may play a greater role in more severe stages of the disease.
The main adverse effects are gastrointestinal, insomnia and cardiac (bradycardia effects), such that about 20% of patients cannot tolerate them. As such, only about 50% of people remain on these therapies beyond six months. Another cholinesterase inhibitor may be tolerated if one is not, so it is often worth trying at least two cholinesterase inhibitors if adverse effects occur.
Donepezil, rivastigmine and galantamine are available on the PBS for people with mild to moderately severe Alzheimer’s disease with a Mini-Mental State Examination (MMSE) score of 10 or above, or an MMSE score below 10 for reasons other than Alzheimer’s disease, such as prominent aphasia or an inability to communicate adequately because of a lack of competence in English (in people of non-English speaking background). After the initial six-month trial, a clinician needs to decide whether there has been a meaningful response before continuing subsidy on the PBS. The patient's response can include an improvement on the MMSE score, obvious symptom improvement or, more controversially, an assessment that the rate of decline has attenuated.
None of the cholinesterase inhibitors are subsidised for conditions other than Alzheimer’s disease, but there is trial evidence for benefits of cholinesterase inhibitors in people with dementia with Lewy bodies, Parkinson’s disease dementia and mixed Alzheimer’s or vascular dementia.8,9
Memantine
Memantine acts on the glutamate receptor and favourably attenuates the ‘signal to noise’ ratio, increasing the effectiveness of neurotransmitted signals. Other mechanisms have also been detected. In clinical trials, it has been found to be effective for moderately severe dementia caused by Alzheimer’s disease, not for the milder stages of dementia caused by Alzheimer’s disease.1 Common adverse effects include dizziness and agitation. It is best started at 5 mg once a day (halving the 10 mg tablets), then increased to 10 mg and then 20 mg daily, increasing every two to four weeks (Table). It can be used in combination with a cholinesterase inhibitor, but only one drug at a time can be subsidised by the PBS. It is preferable to have memantine subsidised, as it is usually the most expensive. Memantine is listed on the PBS for people with moderately severe Alzheimer’s disease with a baseline MMSE score of 10 to 14.
Atypical antipsychotics
Atypical antipsychotics include risperidone, olanzapine, quetiapine, aripiprazole and brexpiprazole. They can be effective for agitation, aggression and psychosis associated with Alzheimer’s disease and Parkinson’s disease, with some evidence also of benefit for hallucinations in Lewy body disease.10 Olanzapine, quetiapine and aripiprazole are all off-label uses for agitation, aggression and psychosis associated with Alzheimer’s disease. The greatest supportive evidence is for risperidone in people with Alzheimer’s disease.11,12 Atypical antipsychotics have a considerable adverse effect profile, including sedation, parkinsonism, stroke and cardiovascular events, but are much safer than the typical antipsychotics. They should be used after nonpharmacological approaches have failed, although they are often prescribed without such a trial. Indeed, there is great concern about their overuse, as chemical restraint, especially in residential care facilities.
The minimum dose should initially be used (e.g. 0.25 mg once or twice daily for risperidone), and maximum doses should be low (e.g. 2 mg/day for risperidone). Only risperidone is PBS listed for behavioural disturbances in patients with dementia of the Alzheimer type, but brexpiprazole is TGA approved for agitation in people with Alzheimer’s disease. They should be used for a maximum of 12 weeks, then a withdrawal trial is often successful.
Other psychoactive drugs
Benzodiazepines are sometimes used for anxiety and agitation, but evidence of benefit is lacking for people with cognitive disorders, and the risk of adverse effects, including oversedation, falls and injuries, is considerable.
Hypnotics are also best avoided, although slow-release melatonin, which can facilitate the effects of common hypnotics, is likely safe. Tricyclic antidepressants should not be used as hypnotics because they have anticholinergic activity.
Depression in people with cognitive disorders can be refractory to pharmacological therapy but many clinicians trial newer drugs such as escitalopram, sertraline and vortioxetine because they are safer and have less adverse effects. Counselling may be just as effective, even in people with cognitive disorders.
Other psychoactive drugs, including valproate, carbamazepine and pregabalin, sometimes used for challenging behaviours, have not been shown to be effective in people with MCI or dementia. Similarly, antiandrogen therapies have not been demonstrated to be effective for challenging sexual behaviours, although there is some evidence for effects of medroxyprogesterone acetate and cyproterone acetate as a third-line agent in aggressive and fantasy sexuality of dementia.
Can GPs initiate these drugs?
All drugs mentioned above can be initiated and repeat prescriptions given by GPs. Cholinesterase inhibitors and memantine require a specialist to confirm the diagnosis, but this can be by telephone call.
When to cease these therapies
Generally, when the dementia becomes severe, it is preferable to deprescribe, including drugs specifically initiated for symptoms of neurocognitive disorders.13 Patients who are bed bound, fail to recognise loved ones and have difficulty swallowing are unlikely to benefit from such therapies.
Other natural and nondrug therapies
There is little to no evidence to support the use of micronutrients and so-called ‘natural’ therapies. These include ginkgo biloba, Brahmi, ginseng and many other products on the shelves of health food and other shops. Poor nutrition should, however, be managed, and a Mediterranean diet has been shown to reduce the risk of dementia and may be useful in delaying symptom progression as a part of a ‘brain health’ program.14
A range of nondrug therapies is being trialed but these all lack evidence of sustained benefits. These include transcranial magnetic stimulation, transcranial electric stimulation, various light therapies and cranial ultrasound.
Conclusion
Ultimately, we will detect the first pathological changes of neurocognitive disorders well before symptom onset and will have therapies that remove and neutralise the effects of the toxic processes (including accumulation of proteins such as amyloid and tau) before symptoms arise. The therapies used in asymptomatic patients must be safe and ideally not required lifelong. Until then, modestly effective drugs are available and should be trialled in appropriate patients. MT
COMPETING INTERESTS: None.
References
1. Rodda J, Carter J. Cholinesterase inhibitors and memantine for symptomatic treatment of dementia. BMJ 2012; 344: e2986.
2. Wu W, Ji Y, Wang Z, et al. The FDA-approved anti-amyloid-β monoclonal antibodies for the treatment of Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Eur J Med Res 2023; 28: 544.
3. Matsunaga S, Fujishirob H, Takechia H. Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: a systematic review and meta-analysis. J Alzheimer’s Dis 2019; 71: 513-523.
4. Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer Disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010 J Neuropathol Exp Neurol 2012; 71: 266-273.
5. Soininen H, Solomon A, Visser PJ, et al. 36-month LipiDiDiet multinutrient clinical trial in prodromal Alzheimer’s disease. Alzheimer’s Dement 2021; 17: 29-40.
6. van Wijk N, Broersen LM, de Wilde MC, et al. Targeting synaptic dysfunction in Alzheimer’s disease by administering a specific nutrient combination. J Alzheimers Dis 2014; 38: 459-479.
7. Winblad B, Kilander L, Eriksson S, et al. Donepezil in patients with severe Alzheimer’s disease: double-blind, parallel-group, placebo-controlled study Lancet 2006; 367: 1057-1065.
8. Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; (3): CD006504.
9. Erkinjuntti T, Gauthier S, Bullock R, et al. Galantamine treatment in Alzheimer’s disease with cerebrovascular disease: responder analyses from a randomized, controlled trial (GAL-INT-6). J Psychopharmacol 2008; 22: 761-768.
10. Armstrong MJ, Weintraub D. The case for antipsychotics in dementia with Lewy bodies. Mov Disord Clin Pract 2016; 4: 32-35.
11. Ballard C, Creese B, Corbett A, Aarsland D. Atypical antipsychotics for the treatment of behavioral and psychological symptoms in dementia, with a particular focus on longer term outcomes and mortality. Expert Opin Drug Saf 2011; 10: 35-43.
12. Schneider LS, Tariot PN, Dagerman KS, et al. for the CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355: 1525-1538.
13. Reeve E, Farrell B, Thompson W, et al. Deprescribing cholinesterase inhibitors and memantine in dementia: guideline summary. Med J Aust 2019; 210: 174-179.
14. Livingston G, Huntley J, Liu KY, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024; 404: 572-628.