Snake envenoming – approach to suspected snakebites and delivering effective antivenom

Although rare, snake envenoming can occur in patients presenting to GPs or hospitals in rural areas, where specialists and laboratory resources may be limited. Improved treatment guidelines and access to expert telephone advice have enabled an improved approach to the treatment of snakebite, with a focus on early clinical assessment and identifying signs and symptoms of systemic envenoming, rather than the type of snake involved, to guide early decision-making about antivenom treatment.
- Snakebite is an urgent presentation, often requiring a decision to administer antivenom with limited investigation and test results.
- Expert advice is available 24 hours a day from the Poison Information Hotline on 13 11 26.
- In addition to placing a pressure bandage with immobilisation, providing immediate basic life support to patients with early collapse after snakebite is essential to increase their chance of survival.
- The most important change in antivenom treatment is a focus on whether or not patients are envenomed, rather than trying to determine the type of snake that caused the bite.
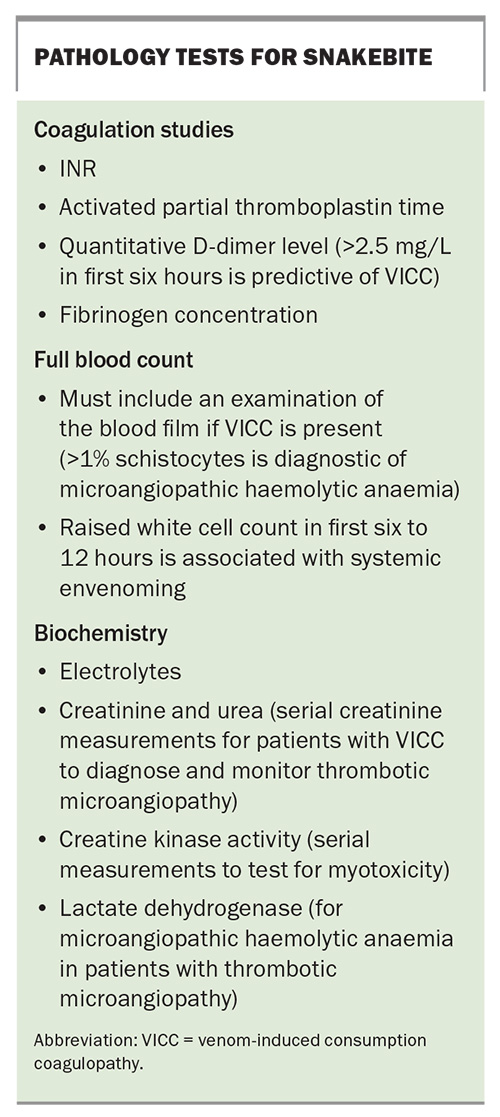
- All patients with systemic envenoming with venom-induced consumption coagulopathy require a blood film within 24 hours of the bite and serial creatinine measurements to identify thrombotic microangiopathy, which has an associated risk of acute kidney injury.
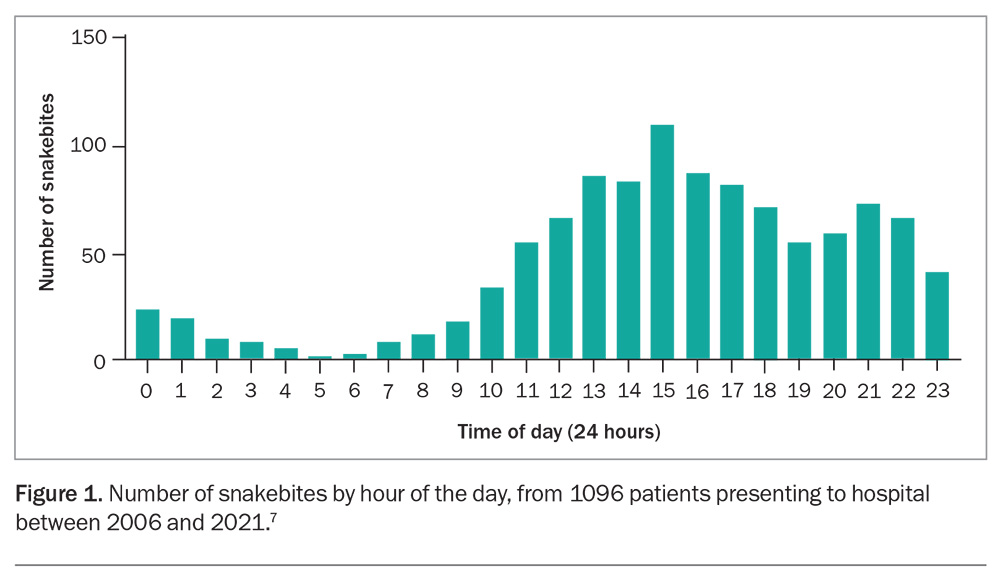
Australian snakebite is a fascinating medical topic and an oft feared clinical presentation. Snake envenoming is a rare clinical condition in Australia that may be more likely to occur in patients presenting to small or rural hospitals with limited pathology services, rather than to tertiary centres where specialist expertise and pathology services reside. Compounding these geographical constraints is the acuity of snake envenoming, which requires immediate assessment, and sometimes resuscitation and early intervention with antivenom. Bites often occur later in the day (Figure 1), so patients tend to present in the late afternoon or evening when there are fewer staff and laboratory resources available. Although this means that the treatment of snakebite is almost always stressful and unpredictable, the past 10 years have seen the publication of much improved clinical guidelines and, most importantly, the provision of telephone access to expert advice from the national network of poisons information centres via the Poison Information Hotline (13 11 26).1
There is increasing evidence that early intervention with antivenom will improve outcomes for patients with snakebite.2-4 Similar to the ‘golden hour’ for trauma or the ‘golden 60 minutes’ of newborn life, there is a ‘golden three hours’ after a snakebite in which the administration of antivenom to systemically envenomed patients is most effective.5,6 Clinical decision-making in this initial three-hour period, which is often reduced by delay in presentation to hospital, is crucial to preventing neurotoxicity and myotoxicity in patients with snakebite. Making an early diagnosis and seeking expert advice in this period are the most important parts of snakebite treatment. Recent research in Australia has shown that much of the delay in antivenom administration actually occurs in hospital, with most patients arriving within one to two hours of the bite.7
This review outlines an improved approach to the early treatment of snakebite, based on best available evidence, with the aim of administering antivenom to envenomed patients as early as possible within three hours of the bite.8 The rarity of snakebite necessitates improved and readily available information to demystify it, as well as access to national treatment guidelines and urgent specialist advice from the poisons information centres at the time of the bite.1
Australian venomous snakes
There are eight medically important types of venomous snakes in Australia:
- brown snakes (Pseudonaja spp.; Figure 2a)
- tiger snakes (Notechis spp.)
- rough-scaled snakes (Tropidechis carinatus; Figure 2b)
- taipans (Oxyuranus spp.)
- red-bellied black snakes (Pseudechis porphyriacus; Figure 2c)
- mulga snakes (Pseudechis australis)
- death adders (Acanthophis spp.; Figure 2d)
- broad-headed snakes (Hoplocephalus spp.).
Most of these are fast-moving, long, thin snakes (like most elapids worldwide) that will generally move away from people unless they are directly interfered with. Unfortunately, their common names, particularly ‘brown snakes’ and ‘black snakes’, do not always accurately represent their appearance. A brown-coloured snake could be a mulga snake (which is a black snake) or a tiger snake, and a black-coloured snake could be a brown snake or a tiger snake. Therefore, except for red-bellied black snakes, identification can be difficult. Death adders are unique in having a body form similar to that of vipers and being ambush predators, often remaining well camouflaged and only biting when someone treads on or near them (Figure 2d).
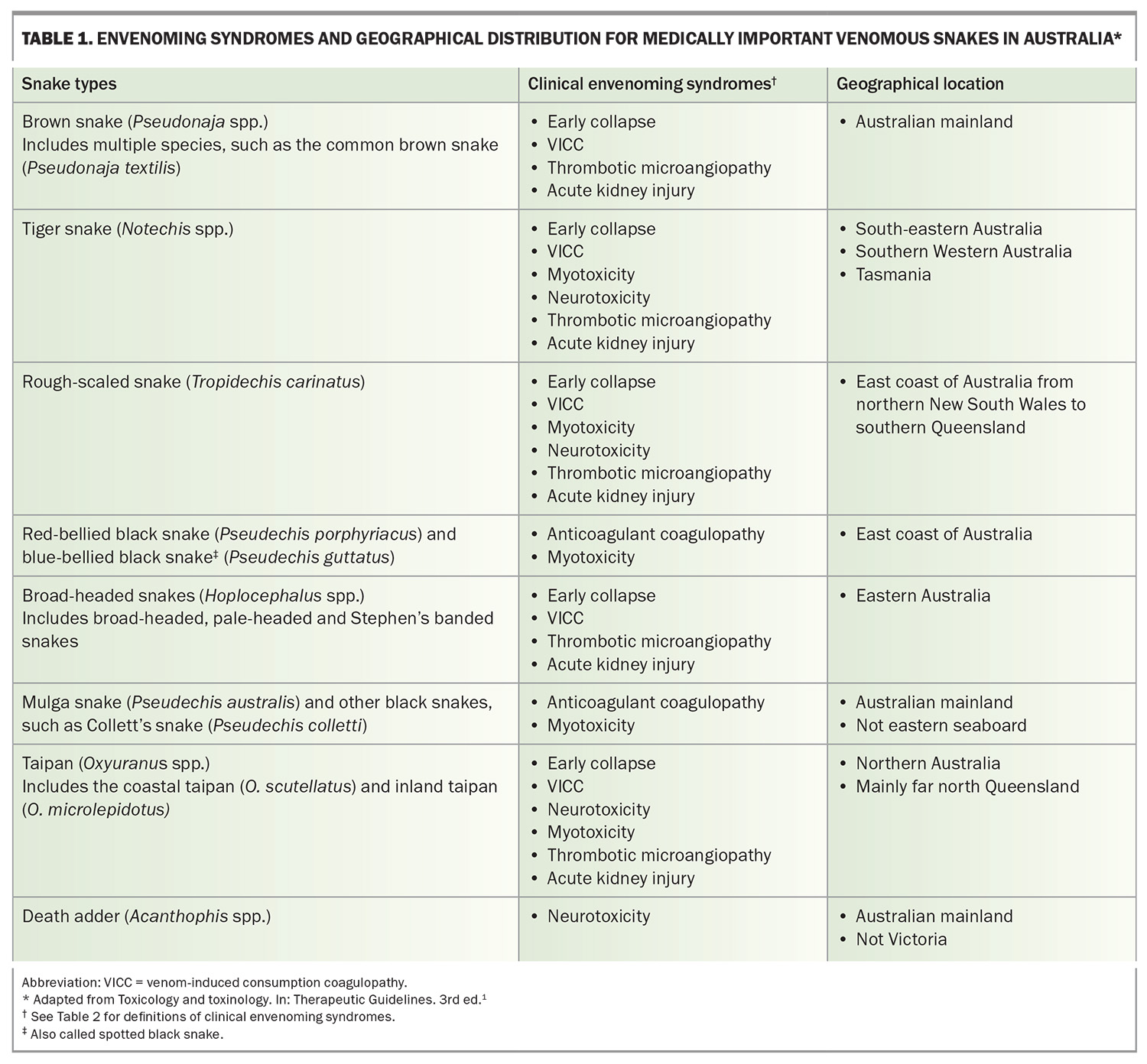
The distribution of these snakes around Australia determines which antivenoms should be kept in different regions and hospitals (Table 1).
Clinical effects of snakebite
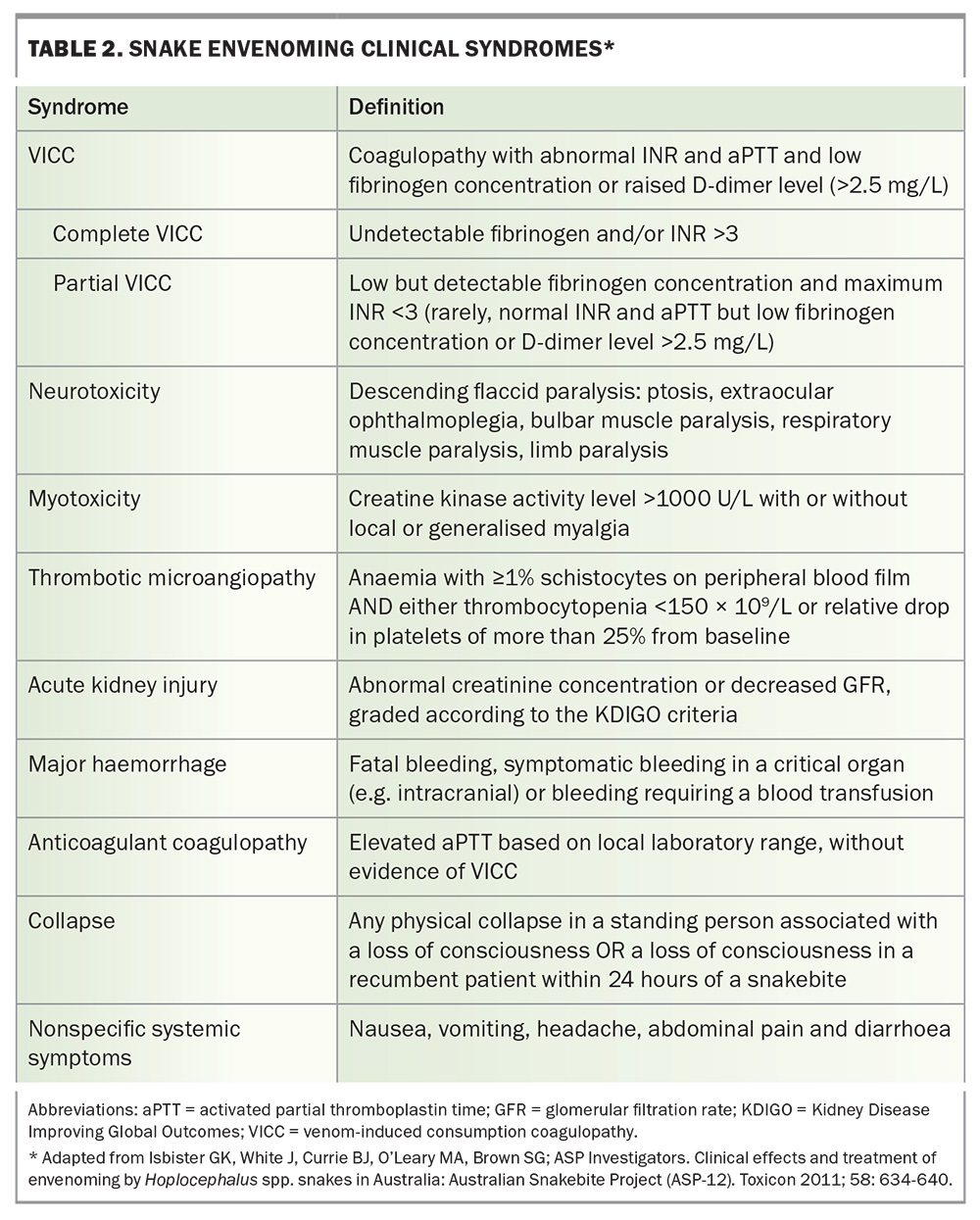
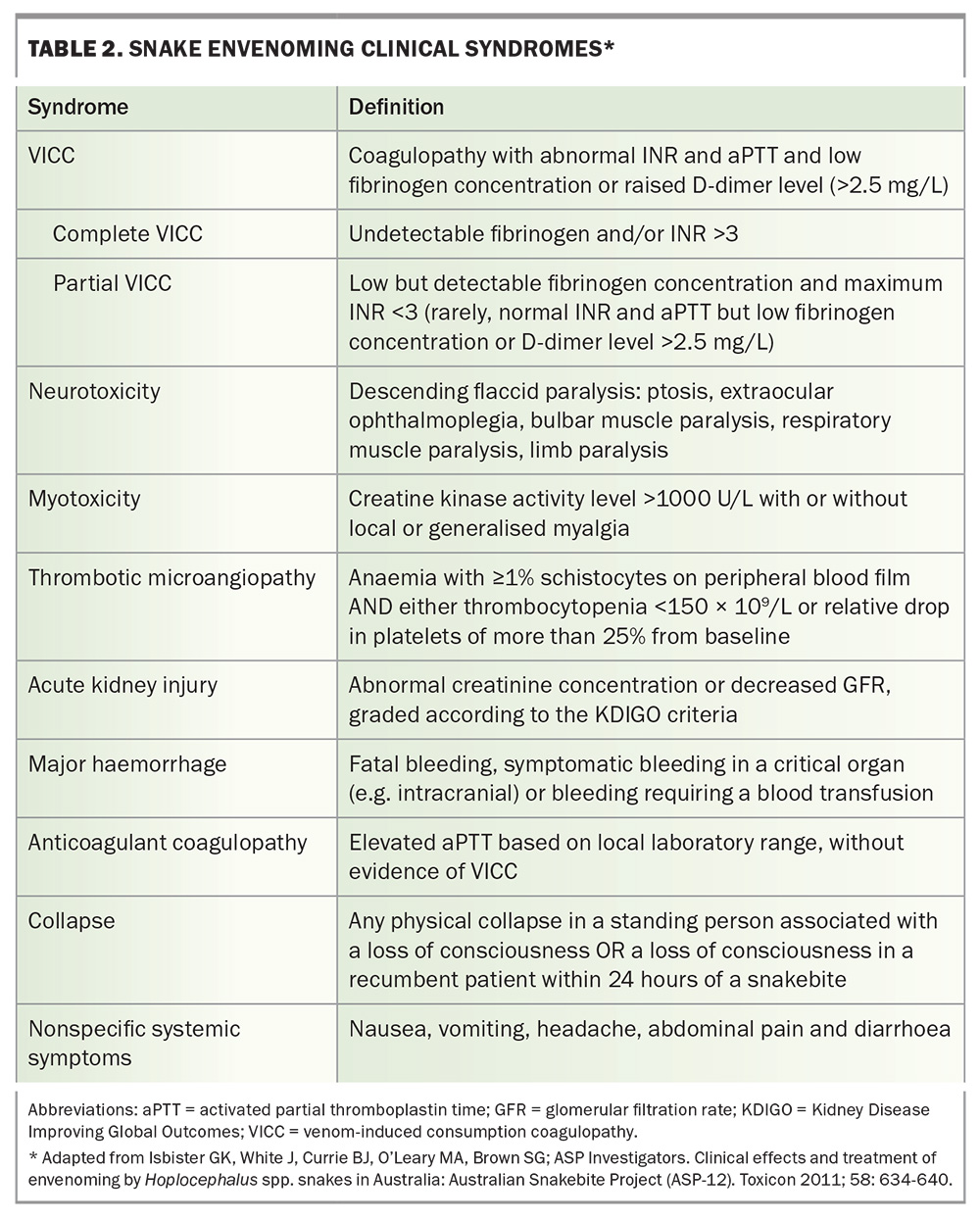
There are several different snake envenoming syndromes, determined by the toxin types in each snake venom (Table 2). The common important clinical syndromes that result from Australian elapid bites are venom-induced consumption coagulopathy (VICC), neurotoxicity (descending paralysis), myotoxicity, thrombotic microangiopathy and acute kidney injury (AKI).9 Other effects include nonspecific systemic symptoms from most bites, anticoagulant coagulopathy (from black snake bites) and local effects. Each of the medically important snakes causes a characteristic combination of these clinical syndromes (Table 1).
Venom-induced consumption coagulopathy
VICC is the most common important manifestation of systemic snake envenoming in Australia, accounting for three-quarters of cases.9 VICC can occur after brown snake, tiger snake, rough-scaled snake and broad-headed snake envenoming and is most severe in cases of brown snake envenoming.10 It results from the activation of the clotting pathway by procoagulant toxins in the snake venom.11 In Australian elapids, these are prothrombin activators, which convert prothrombin to thrombin, and thence fibrinogen to fibrin. In addition, there is activation of factor V to Va and factor VIII to VIIIa. Overall, this mass activation results in the consumption of factor V, factor VIII and fibrinogen, resulting in severe coagulopathy.
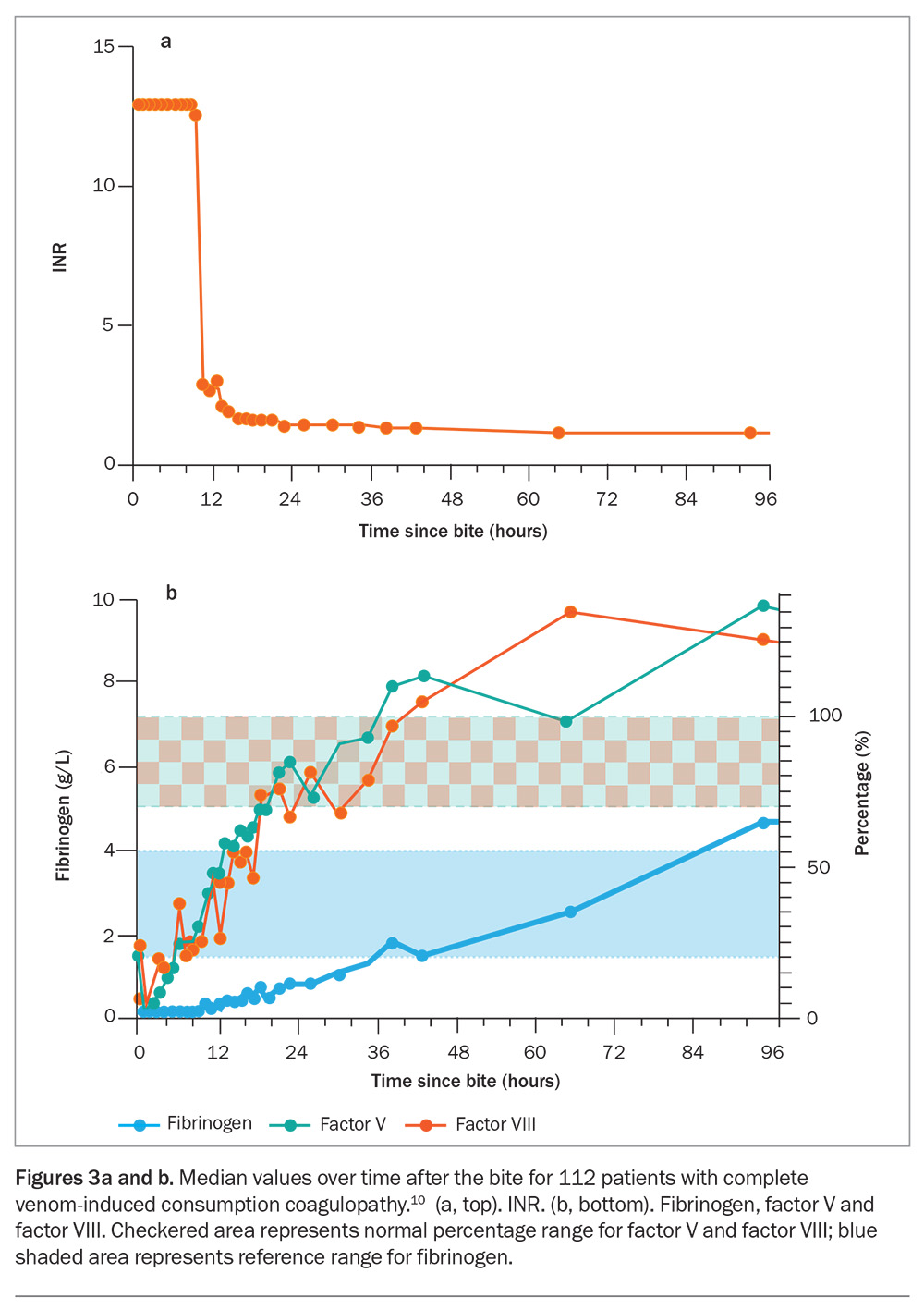
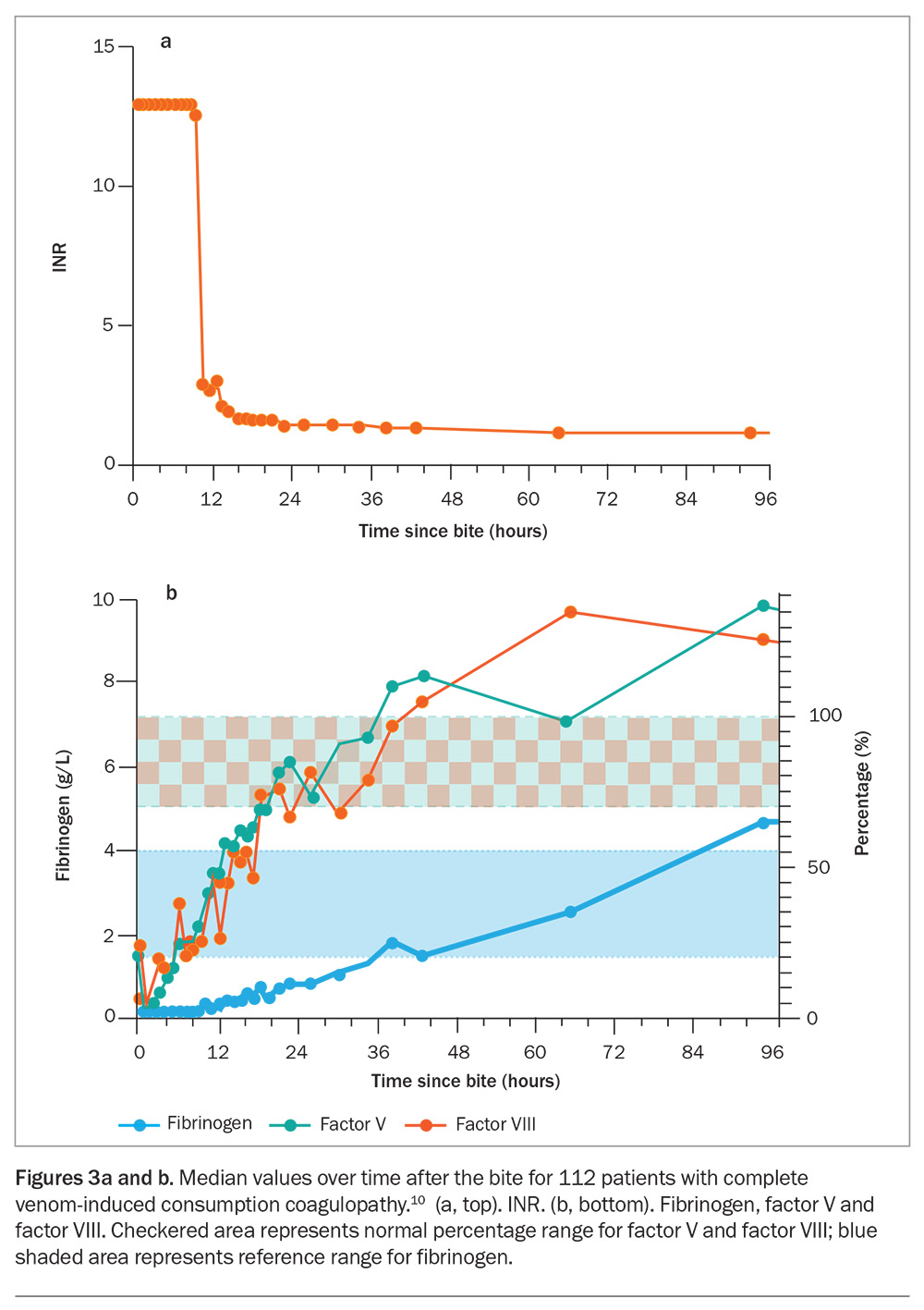
In most cases of VICC, there is complete consumption of fibrinogen, resulting in an undetectable fibrinogen concentration and an unrecordable INR (usually an INR greater than 10, but VICC is classified as complete if the INR is greater than 3) and a very high D-dimer level (greater than 100 g/L). VICC develops rapidly, and consumption of factors is usually complete within one hour after the bite (Figure 3). Less often, there is partial consumption or partial VICC, which can be more of a diagnostic challenge (Table 2).10 In this case, the fibrinogen concentration is low but measurable, and the INR will be abnormal but less than 3 (Table 2). Occasionally, there is minimal consumption, such that the INR may be normal, with only a mild elevation in D-dimer level (above 2.5 g/L) and a decrease in fibrinogen concentration. Unfortunately, the severity of VICC does not appear to correlate well with other toxidromes, such as thrombotic microangiopathy, which can still occur with partial VICC.
Recovery from VICC generally occurs over 12 to 36 hours (as measured by the INR), mainly due to rapid recovery (via hepatic synthesis) of factors V and VIII (Figure 3a). The fibrinogen concentration takes longer to recover and may still be slightly abnormal or low normal when the INR has normalised (Figure 3). The recovery of VICC does not appear to be influenced by the time at which antivenom is administered.12
Myotoxicity
Myotoxicity, although less common with Australian snakebite, is nevertheless the major toxidrome in black snake envenoming and an important part of tiger snake envenoming. It results from the action of phospholipase A2 myotoxins, which cause direct muscle injury that develops over a period of hours to days. Localised or generalised myalgia and muscle tenderness are the most common clinical effects.
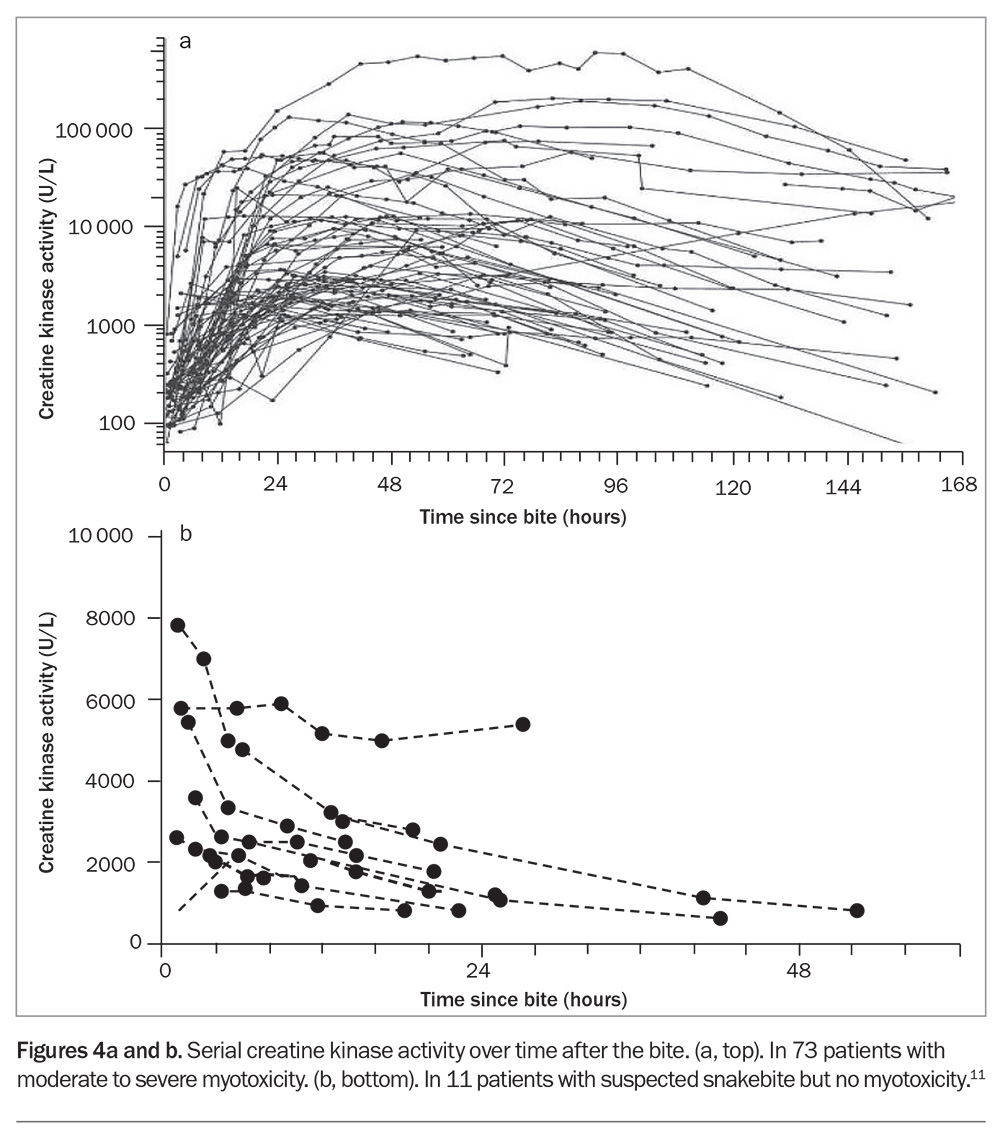
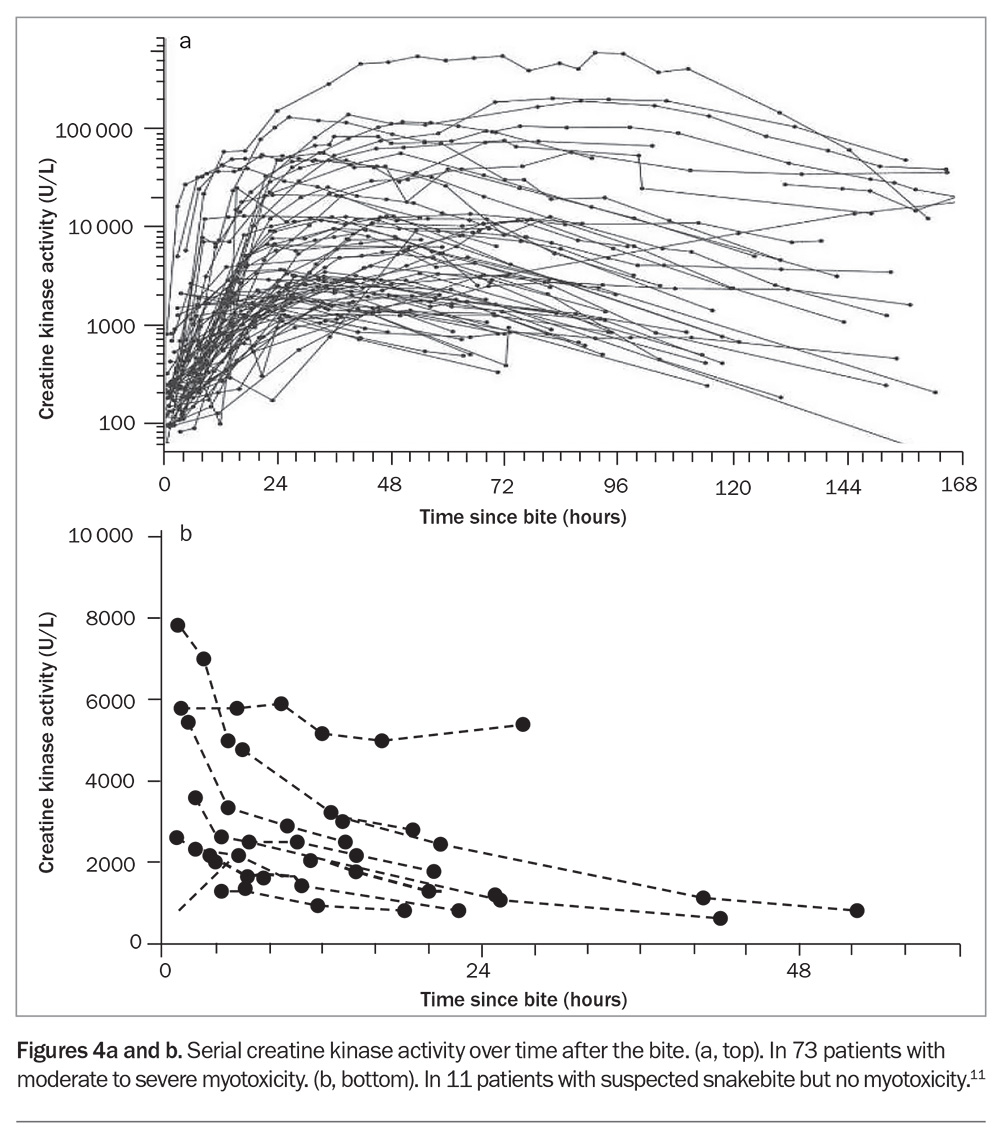
Elevated creatine kinase (CK) activity is the defining biomarker of myotoxicity, although its time course is delayed compared with the actual muscle injury. Snakebite myotoxicity is defined as a CK activity level greater than 1000 U/L, but in severe cases this will rise to the 10,000s or 100,000s, and the condition is then better referred to as rhabdomyolysis (Figure 4a).13 AKI rarely occurs, even if the CK activity level is in the 100,000s. In severe life-threatening cases, hypocalcaemia and hyperkalaemia are the most important complications.
There is good evidence from one randomised controlled trial and several prospective observational studies that early antivenom administration will prevent myotoxicity in patients with red-bellied black snake envenoming (if given within six hours of the bite) and mulga snake envenoming (within three hours of the bite).4,14 In addition, administration of antivenom before the appearance of the first abnormal CK activity level (above 500 U/L) is associated with less severe myotoxicity.13 Although CK activity is the best clinical marker of myotoxicity, the median time to recording the first abnormal CK activity level was found to be 11 hours (and 34 hours to the peak level), meaning that antivenom treatment needs to be given before elevations in the CK activity level are seen.13
Problematically, it is not uncommon for the CK activity to be elevated in non-envenomed patients, typically in the first blood sample collected. This initial elevation in CK activity is too early for myotoxicity and decreases over time, indicating that it is due to another cause, such as physical exertion (Figure 4b).13
Neurotoxicity
Neurotoxicity is an uncommon manifestation of systemic envenoming from Australian snakebite and occurs mainly with death adder and taipan envenoming. In most cases, it is due to presynaptic phospholipase A2 neurotoxin that causes irreversible injury. It always presents as a descending paralysis, proceeding from ptosis to involvement of the extraocular muscles, followed by bulbar, diaphragmatic and limb paralysis. In mild to moderate cases, there may only be ptosis and eye involvement. The important stage in its progression is bulbar paralysis, after which patients are unable to protect their airway and require intubation. Further paralysis involves respiratory muscles, and ventilation is required to prevent respiratory depression. Administration of antivenom within three to four hours of the bite has been shown to prevent neurotoxicity, including the requirement for intubation.2,15 Once generalised paralysis has developed, recovery may take days to weeks.
Thrombotic microangiopathy and acute kidney injury
AKI occurs in 12% of cases of Australian snakebite with systemic envenoming and is almost always associated with thrombotic microangiopathy.9 Thrombotic microangiopathy occurs in about 15% of Australian snake envenoming cases and only from snakes that cause VICC. It develops over the first 24 hours after the bite and is characterised by schistocytes (greater than 1%) and red cell fragments on the blood film, thrombocytopenia and AKI.16 Most cases involve only stage 1 or 2 AKI, without oliguria or a requirement for renal replacement therapy. In more severe cases, there is stage 3 AKI requiring renal replacement therapy, moderate to severe thrombocytopenia and microangiopathic haemolytic anaemia. Patients with microangiopathic haemolytic anaemia will also have a high lactate dehydrogenase level.
The association between VICC and thrombotic microangiopathy means that all patients with VICC require a blood film within 24 hours of the bite and serial creatinine measurements. Although antivenom is almost always administered to these patients based on their presentation, there is limited evidence to suggest that antivenom prevents thrombotic microangiopathy.17
Early hypotensive collapse
Early collapse after snakebite occurs in about 12% of snake envenoming cases in Australia, is associated with severe envenoming and can progress to cardiac arrest and death. It is the most common cause of death from snakebite in Australia.9 It only occurs from bites that cause VICC and is most common in brown snake envenoming.18 Early collapse occurs on average 20 minutes after the bite and within one hour in almost all cases. Collapse most often occurs before presentation to hospital, and immediate administration of basic life support is likely to improve patient outcomes.
Local effects
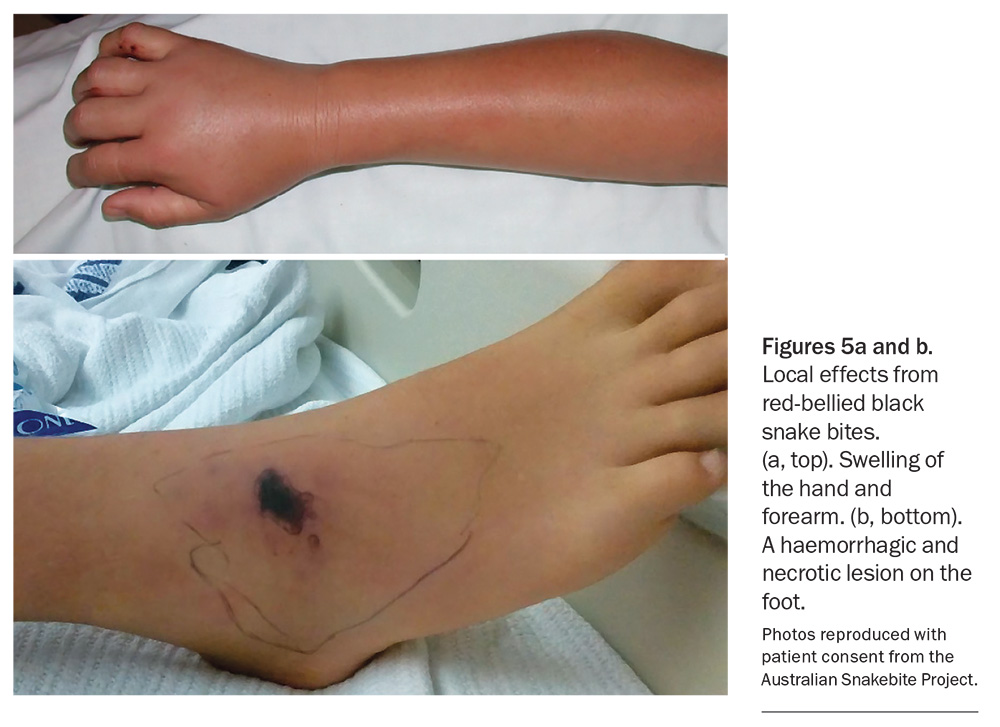
Significant local effects are less common with Australian snakebite than with bites from snakes in other countries. For some snakes, typically brown snakes, there may be little indication of a bite, with no visible mark or only scratches from the fangs. More significant redness and swelling can occur with bites from black snakes, tiger snakes and rough-scaled snakes (Figure 5a). Rarely, local necrosis may develop at the bite site hours to days after the bite; this is most common with red-bellied black snakes (Figure 5b).
Other effects
An unusual but uncommon effect, mainly reported with bites from black snakes and tiger snakes, is a loss of or change in the senses of taste and smell. Onset often occurs more than 24 hours after the bite and in many cases is not documented or realised by the patient while in hospital. Follow up of some patients suggests it can continue for weeks to months but resolves in most cases. The cause and the effectiveness of antivenom for treating this effect remain unclear.
Clinical assessment of suspected snakebite
History
A focused history, including the circumstances of the bite (suspected or not), whether the patient saw the snake and whether they felt or saw the snake biting them, is essential for the clinical assessment of snakebite. Although most patients presenting with suspected snakebite require observation and serial blood tests, a definite bite from an observed snake makes interpretation of clinical symptoms and early blood test results much more definitive.
A history of events in the hour immediately after the bite is essential for determining whether there is systemic envenoming. This includes any nonspecific systemic symptoms – nausea, vomiting, headache, abdominal pain and diarrhoea – as well as a history of early collapse, as an important feature of severe envenoming.18
Physical examination
The physical examination needs to include:
- checking the bite site for fang or scratch marks, erythema and swelling
- haematological assessment, which involves checking for bleeding or oozing at the bite site, at cannula sites, from any open lacerations and from the mouth
- neurological assessment, with cranial nerve examination for ptosis, ophthalmoplegia, bulbar weakness, limb weakness and respiratory muscle weakness
- assessment for localised or generalised myalgia or muscle tenderness, suggesting myotoxicity.
Local necrosis at the bite site is rare with Australian snakebite and will take hours to develop. However, in some cases there may be no obvious bite site or evidence of a bite; this is particularly important with brown snakes, because they have very short fangs. The draining lymph nodes are often painful in patients with systemic envenoming.
Serial examination for bulbar weakness is important for assessing the airway, and respiratory muscle weakness for assessing breathing and respiratory depression. Signs of neurotoxicity, particularly ptosis and bulbar weakness, may be difficult to assess in sleeping patients, so it is important that patients are woken and properly assessed.
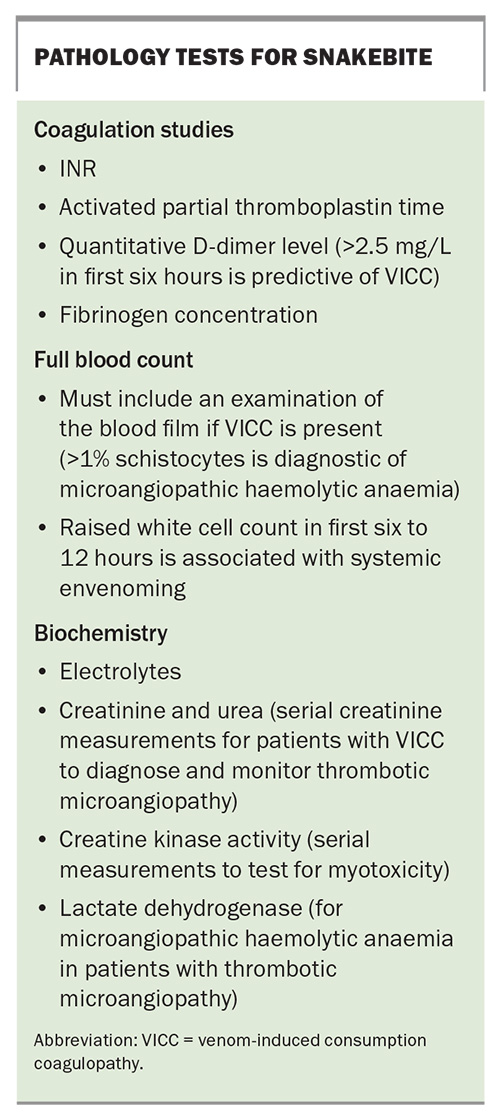
Laboratory investigations and early diagnostic tests
There are several key investigations for suspected or confirmed snakebite (Box). Initial laboratory tests may assist in the early diagnosis of systemic envenoming but should not delay the decision to treat and administer antivenom to patients with symptoms and signs of systemic envenoming.7 Whereas early testing aims to diagnose systemic envenoming, later testing is used to assess recovery (e.g. coagulopathy) or monitor for complications (e.g. AKI or thrombotic microangiopathy).
All investigations for snakebite require a laboratory, and this is often the main reason for transferring patients to a hospital with a laboratory for coagulation studies. Early diagnosis would be greatly improved if bedside tests were available to detect systemic envenoming. The 20-minute whole blood clotting test has been used extensively in resource-poor regions but has a poor sensitivity to VICC.19 Point-of-care INR and D-dimer tests have also been used in Australia but have been shown to be unreliable, with false-positive and false-negative results.20 The currently available point-of-care devices should not be used for snakebite.
The snake venom detection kit (SVDK) is no longer recommended for the management of snakebite in Australia.1 Results from the Australian Snakebite Project showed that, from 1060 uses, the SVDK was inappropriately used for 364 non-envenomed patients (a false-positive rate of 36%) and resulted in nine non-envenomed patients receiving antivenom. Among 597 envenomed patients, the SVDK result was incorrect, negative or inconclusive in a sixth of cases.9 Therefore, the SVDK added little to diagnosis, delayed decision-making and resulted in inappropriate treatment decisions in many cases.
First aid
First aid for snakebite includes the application of a pressure bandage with immobilisation (PBI) to any person with a suspected snakebite and basic life support for patients with early collapse. Immobilisation includes both the limb and the patient, preferably on a stretcher. The bandage should be applied over the bite site, then continued to cover the entire limb, preferably using an elasticised bandage at about the same pressure as for a sprained ankle.21 If patients present to hospital without a pressure bandage or immobilisation, a PBI should be applied to any patient presenting within four hours of the bite.
The pressure bandage can be removed once there is no evidence of envenoming, based on the absence of nonspecific systemic symptoms and normal results of laboratory investigations.22 For patients with systemic envenoming, the bandage should be removed after the completion of antivenom administration. There are rare cases of envenoming developing when the bandage is removed, but in these cases the bandage is usually acting as a venous or arterial tourniquet. Therefore, observation immediately after the removal of the bandage is essential for all patients. There are reports of adverse outcomes in patients with tight pressure bandages left in place for many hours, due to long hospital transfer times.23 It is therefore important that the tightness of the bandage is assessed, and limb observations are taken.
Potentially more important than placing a PBI is providing basic life support to patients with early collapse after snakebite. Survival of patients with early collapse is associated with immediate resuscitation, compared with delayed or no life support.9
Management of suspected snakebite
An improved approach to snake envenoming
As snake envenoming is rare, and the evidence for treatment continues to improve, it is usually appropriate to discuss the treatment, particularly the decision to give early antivenom within three hours of the bite, with a clinical toxicologist on the Poison Information Hotline (13 11 26) or at a local or regional toxicology service, if available.
There are three key components required for the treatment and observation of patients with snake envenoming:
- availability of appropriate antivenom
- an onsite 24-hours/day laboratory that can perform coagulation studies (INR, activated partial thromboplastin time [aPTT] and quantitative D-dimer testing)
- critical care facilities where antivenom can be administered and anaphylaxis safely treated.
Occasionally in cases of severe envenoming with neurotoxicity and myotoxicity, patients will need to be managed in an intensive care unit to allow mechanical ventilation and, in some cases, renal replacement therapy for AKI.
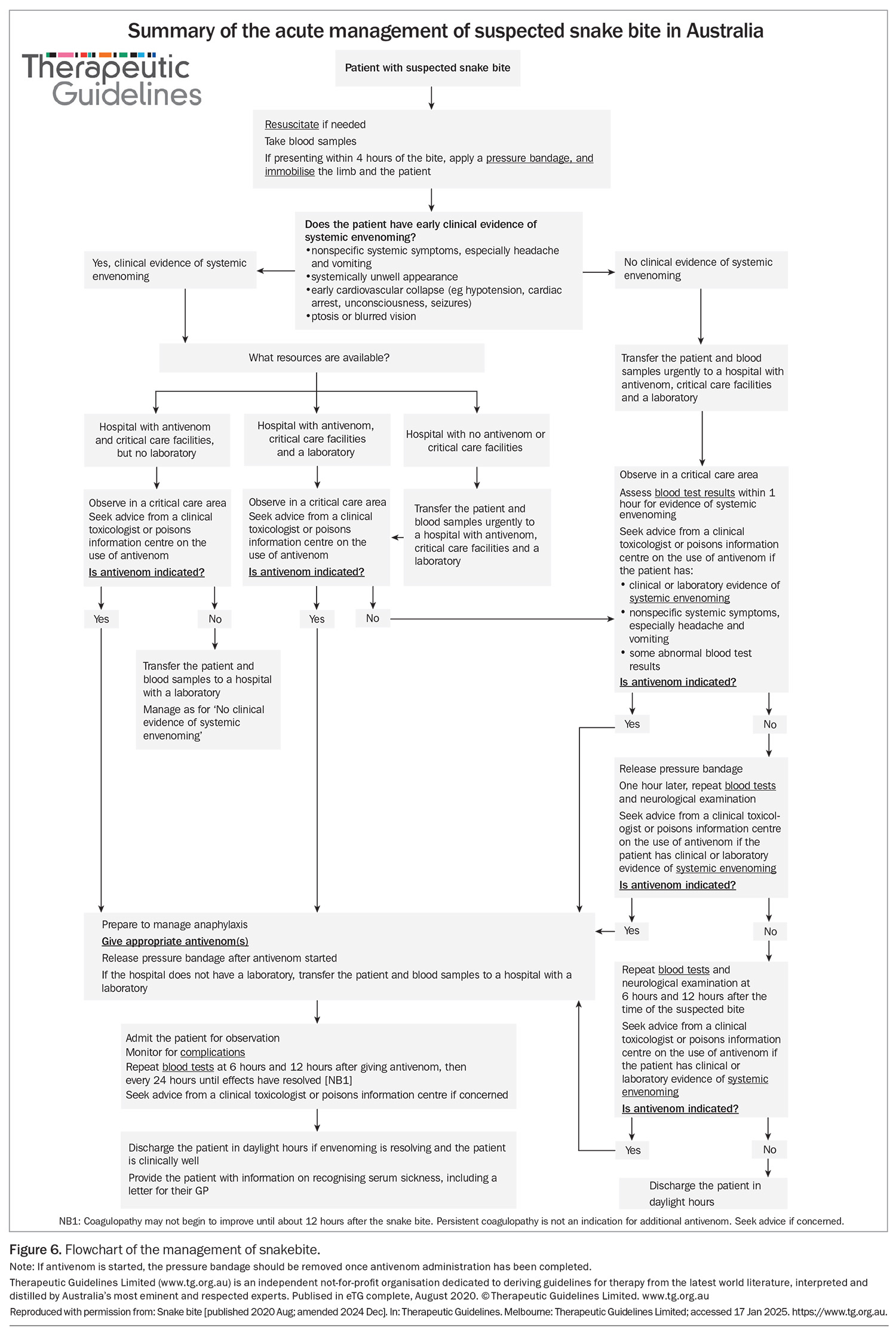
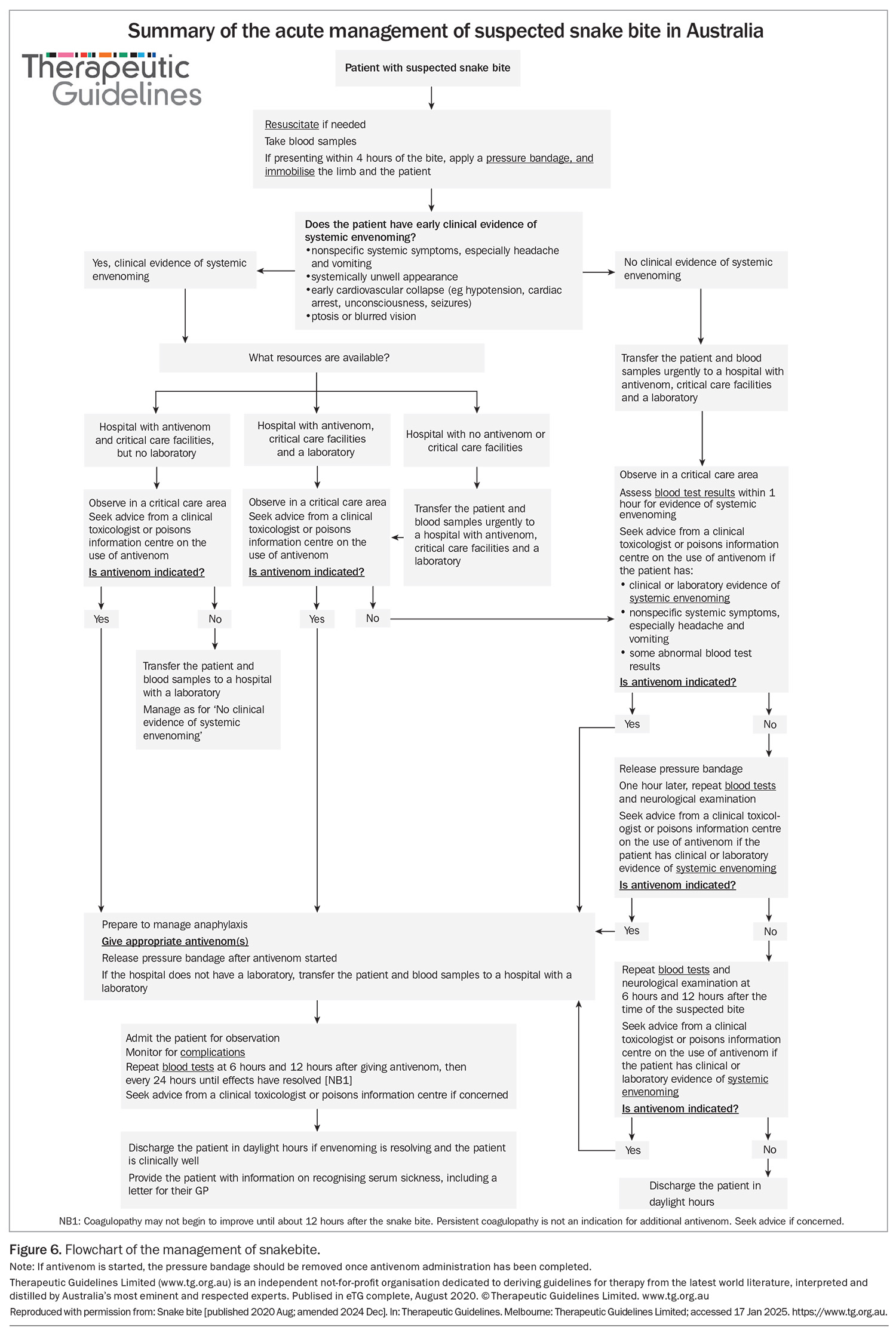
As antivenom is effective in preventing neurotoxicity and myotoxicity when given within three hours of the bite for most snakes, early assessment and decision-making are crucial for effective treatment of snakebite (Figure 6).2-4 This means that clinical assessment and a decision as to whether antivenom should be administered within three hours should precede laboratory testing. In patients with snakebite, early administration of antivenom should be considered if the patient has either of:
- an early cardiovascular collapse (e.g. hypotension, cardiac arrest or seizure)
- nonspecific systemic symptoms (e.g. vomiting, headache or abdominal pain).
All patients with snakebite, definite or suspected, should be triaged as category 2 (urgent, requiring assessment within 10 minutes). This allows an early decision of whether to administer antivenom based on the history and clinical presentation. Simultaneously, blood samples should be collected and sent for urgent testing (Box). If these blood test results are abnormal, most often with abnormal coagulation studies, then antivenom should be administered. For patients with no clinical features of envenoming (i.e. no headache, vomiting, abdominal pain or diarrhoea) and normal results of admission laboratory tests, the clinician should follow the suspected snakebite pathway (observation and repeat blood tests; Figure 6).
Any patient who receives antivenom should be admitted for observation, with their level of care based on the severity of the initial envenoming (e.g. collapse with cardiac arrest) or the potential for complications (e.g. myotoxicity). Most patients, who will only have VICC, can be observed in an emergency short-stay unit until the results of coagulation studies normalise. However, these patients need a blood film performed in the first 24 hours, and monitoring of urine output and creatinine concentration. Ultimately, the main goal is to prevent trauma, particularly falls in patients with severe coagulopathy, and to exclude thrombotic microangiopathy.
Exclusion of envenoming
Most cases of suspected snakebite do not result in systemic envenoming and do not require antivenom. These patients will have no clinical features of envenoming or abnormalities in their admission laboratory test results. After this initial assessment, they need to be observed for 12 hours, with serial laboratory tests (INR, aPTT and CK activity) and neurological examinations for ptosis, extraocular muscle weakness, bulbar palsy and respiratory paralysis. The pressure bandage can be removed in a critical care area after the initial assessment, and repeat blood tests should be performed one hour after bandage removal (Figure 6). The patient can then be observed in a general clinical area and have blood samples collected for testing at six and 12 hours after the bite.22
Data from the Australian Snakebite Project showed that all cases of severe envenoming were evident based on results of laboratory investigations by 12 hours after the bite, irrespective of when the bandage was applied or removed.22 If results of any of the laboratory investigations in this time are abnormal or if neurotoxicity develops, then antivenom should be considered based on the time since the bite and the severity of the effects; this can be discussed with a clinical toxicologist. The exact timing of these blood tests may be modified slightly, such as delaying the 12-hour post-bite tests to the following morning so that after-hours laboratory staff do not need to be called in. Often the tests required at one hour after bandage removal and six hours after the bite coincide to within an hour, reducing the number of blood samples that need to be taken.
Antivenom treatment
Recommendations for antivenom administration
There have been major changes in the approach to and use and administration of antivenom in the past 10 years.1,9 These changes include:
- using polyvalent antivenom – either the two monovalent antivenoms (brown snake and tiger snake) as a ‘low-dose’ polyvalent antivenom, or the commercial polyvalent antivenom, which is a much higher volume dose
- reducing the dose of antivenom to one vial
- no redosing of antivenom
- not administering antivenom more than 12 hours after the bite.
The most important change in antivenom treatment is a focus on whether or not patients are envenomed, rather than attempting to determine the specific snake type that caused the bite. For patients in whom systemic envenoming is clinically apparent, or where there is laboratory evidence of envenoming, antivenom should be administered. In most parts of Australia, one vial of brown snake antivenom and one vial of tiger snake antivenom will cover all types of snakes, including brown snakes, tiger snakes, rough-scaled snakes, broad-headed snakes and red-bellied black snakes. This combination is a total of 4000 U of antivenom.
However, in a small proportion of cases, namely envenoming from death adders, taipans and mulga snakes, the combination of brown snake and tiger snake antivenom may be an insufficient volume to bind all the venom present, as these snakes deliver much larger amounts of venom. For confirmed systemic envenoming from these three snakes, or any circumstance in which one of these snakes is likely based on the clinical presentation and geography, polyvalent antivenom is recommended. Compared with the individual monovalent antivenoms for each of these three snakes, polyvalent antivenom is far more cost-effective, with no increased risk of antivenom reactions.24 There are several specific suspected envenoming situations in which polyvalent antivenom is recommended:
- suspected death adder envenoming with isolated neurotoxicity, which can occur in most parts of mainland Australia, except Victoria
- suspected taipan envenoming in patients with VICC, with or without neurotoxicity, in far northern Australia
- suspected mulga snake, taipan or death adder bites in snake handlers anywhere in Australia
- suspected mulga snake bites based on location and nonspecific effects (e.g. nausea, vomiting, headache, abdominal pain or diarrhoea), with or without anticoagulant coagulopathy.
Evidence from the Australian Snakebite Project has shown that one vial of the appropriate (snake-specific) antivenom is sufficient to bind all venom in patients with systemic envenoming from all major Australian snakes.2,4,18,25,26 However, with the change to diagnosing systemic envenoming, rather than identifying the specific snake involved, and to ensure that the administered antivenom covers all major snake types based on geography and clinical effects, the approach is now to administer either two vials of monovalent antivenom or a polyvalent antivenom. In addition, studies showed that there is no role for further doses or redosing of antivenom, which was particularly prevalent in patients with VICC. Redosing was often based on abnormal results of coagulation studies after administration of antivenom, as the abnormal results were interpreted as a failure of the antivenom. However, an improved understanding of VICC and its recovery has shown that it takes 12 to 36 hours for results of coagulation studies to normalise, irrespective of antivenom administration.10 The role of repeat coagulation studies is simply to determine when it is safe to discharge the patient.
Finally, delayed or late administration of antivenom is highly unlikely to be beneficial or effective in systemic envenoming and exposes the patient to the risk of immediate and delayed hypersensitivity reactions. Current evidence is that antivenom needs to be administered within three hours for most snake types, and within six hours for red-bellied black snake envenoming, to prevent myotoxicity and neurotoxicity.2,4,14,26 Antivenom administered between six and 12 hours after the bite is unlikely to be effective but should still be considered. Antivenom should not be administered more than 12 hours after a snakebite because there is little benefit and a risk of adverse reactions, including severe anaphylaxis.
Antivenom reactions
Reactions to antivenom are well recognised and include both early systemic hypersensitivity reactions and delayed reactions, known as serum sickness.24,27 Mild to moderate immediate reactions to antivenom, including rash or urticaria, flushing, itchiness and gastrointestinal effects, occur in 20 to 25% of administrations of commercial antivenom in Australia. Severe anaphylaxis, almost always with hypotension, occurs in 3 to 5% of antivenom administrations.24 This underscores the need to administer antivenom in a critical care location and highlights the difficulties of balancing the benefits of early antivenom administration for patients with systemic envenoming against the risk of these potentially severe adverse effects. Of 755 patients with snakebite recruited to the Australian Snakebite Project over 10 years who were given antivenom, 49 were not envenomed and, of these, one in five unnecessarily developed an allergic reaction.9
Treatment of antivenom reactions includes the cessation of antivenom and the administration of intravenous fluids and adrenaline in cases of anaphylaxis. In most cases, antivenom administration can continue at a slower rate, with or without adrenaline. Either intramuscular administration of adrenaline or a diluted intravenous adrenaline infusion is appropriate and should be consistent with the treatment of any cause of anaphylaxis.28
Serum sickness occurs in just over a third of patients who receive antivenom.27 It occurs any time from five to 10 days after antivenom administration and is characterised by an influenza-like illness, with rash, myalgia, arthralgia, fever and gastrointestinal effects. Treatment is with oral prednisone, 25 to 50 mg daily, for seven days. There is no evidence that prophylactic corticosteroids will prevent serum sickness, and it is essential that patients are warned of the potential for its development.
Ongoing management of envenomed patients
The ongoing management of patients with systemic snake envenoming depends on the type of snake and the toxidrome, clinical effects and complications that result. The most common important complications of snake envenoming are thrombotic microangiopathy and AKI, severe rhabdomyolysis, severe neuromuscular paralysis and major bleeding. Any patient with one or more of these complications will need to be admitted to an intensive care unit or high dependency ward. In all such cases, there should be consultation with clinical toxicology staff or a poisons information centre and a haematology specialist, as well as a renal medicine specialist for patients with thrombotic microangiopathy.
VICC
For patients with VICC, serial measurements of INR, aPTT and fibrinogen concentrations should be done until the INR and aPTT are normal, usually 24 to 48 hours after the bite. Blood sample collections at six, 12, 24 and 36 hours, and thence daily, would be sufficient. The fibrinogen concentration takes longer to return to the normal range and in itself is not a reason to prevent hospital discharge. More importantly in patients with VICC, serial measurements of creatinine concentration should be performed and a blood film examined within 24 hours of the bite for schistocytes, red cell fragments and platelet count. The percentage of schistocytes should be measured, with greater than 1% being indicative of thrombotic microangiopathy. A normal blood film, creatinine concentration and platelet count at 24 hours are highly suggestive that thrombotic microangiopathy will not occur, and the patient can be discharged.
Investigation for occult bleeding should be undertaken as clinically indicated, such as a cerebral CT scan for intracranial bleeding or imaging for intra-abdominal bleeding. In particular, older patients with a history of hypertension or antihypertensive treatment may be at increased risk of an intracranial haemorrhage.29 Any patient with a history of significant trauma around the time of the bite should also be appropriately investigated for bleeding.
Thrombotic microangiopathy and AKI
The occurrence of thrombotic microangiopathy is the most common reason for an extended hospital stay and intensive care admission for patients with snakebite in Australia. It will become evident eight to 24 hours after the bite in patients with VICC. Ongoing treatment is dictated by the severity of the AKI. Although there may be moderate to severe thrombocytopenia and microangiopathic haemolytic anaemia, neither of these require treatment with blood products or result in significant complications.
AKI associated with snakebite should be managed similarly to AKI from other causes, with attention given to fluid status, serial measurement of creatinine concentration and renal replacement therapy when indicated. In severe cases, the patient may have oliguria or anuria from the time of the bite and require two weeks to two months of renal replacement therapy. This is usually best done as continuous renal replacement therapy in intensive care, transitioning to inpatient and then outpatient intermittent haemodialysis.
There is no good evidence for using therapeutic plasma exchange in patients with snakebite-associated thrombotic microangiopathy, and it is resource intense and costly, using large volumes of blood donor plasma.17,30 In a large cohort of patients with snakebite-associated thrombotic microangiopathy followed up for at least 90 days, there were no cases of end-stage renal failure. However, 13 of 25 patients (52%) with stage 3 AKI requiring dialysis developed stage 3 or higher chronic kidney disease, compared with only one of 29 patients (3%) with AKI not requiring dialysis.31
Major bleeding
Major bleeding is rare in patients with Australian snakebite. It results from associated trauma near the time of the bite (e.g. early collapse) or from occult intracranial or abdominal bleeding while the patient is still coagulopathic. The use of blood products in patients with snakebite with active bleeding and coagulopathy is complicated by the risk of further consumption of factors if given too early. A randomised controlled trial of fresh frozen plasma (FFP) for snakebite coagulopathy in Australia found that there was a rapid improvement in the INR (to less than 2) within six hours, but no decrease in length of stay.32 However, it was found that early administration of FFP (within eight hours of the bite) in a subgroup of patients did not result in correction of the coagulopathy, with evidence of persistent consumption.32 This means that FFP should potentially be delayed until at least six hours after the bite, unless there is immediately life-threatening active bleeding. FFP is the most appropriate factor replacement (rather than cryoprecipitate or multifactor replacement products) because it contains fibrinogen, factor V and factor VIII, and the correction of the latter two appears to be the most important.
Neuromuscular paralysis
Occurrence of neuromuscular paralysis with either bulbar involvement (airway) or respiratory muscle involvement requires intubation and ventilation until the paralysis has resolved. The duration of mechanical ventilation will depend on the severity of the paralysis and how early antivenom was administered, but it may be required for two to three weeks, with associated risks and requirements for prolonged ventilation. Patients with mild neurotoxicity, isolated ptosis or extraocular muscle involvement can be discharged once this is resolving and there is no risk to the airway, although they should be informed that the resolution may be slow.
Rhabdomyolysis
Rhabdomyolysis develops 24 to 48 hours after the bite. In severe cases, it causes electrolyte abnormalities – hypocalcaemia and hyperkalaemia – requiring fluid and electrolyte treatment and sometimes dialysis. Rhabdomyolysis is usually rapidly progressive, almost always occurs from tiger snake or occasionally rough-scaled snake bites and is one of the causes of death or severe morbidity with prolonged intensive care unit admission for patients with Australian snakebite.25,33
In contrast to severe rhabdomyolysis, most patients with myotoxicity will have an uncomplicated course and can be discharged once their CK activity is decreasing and they have normal renal function.1,13 In some cases, particularly with bites from red-bellied black snakes, there may be associated severe local and regional pain and swelling. This should be managed supportively, including tetanus prophylaxis as required, with little role for antibiotics.
Snake handlers
Snake handlers are an important group of patients with snakebite and make up about 10% of snake envenoming cases in Australia.34 True exotic snakebite (i.e. bites from non-Australian snakes) is exceedingly rare in Australia, but most bites of snake handlers are from snakes not naturally occurring in the same geographical location as the handler. For example, about half of all bites from taipans and mulga snakes occur outside of these snakes’ normal distribution.2,4 Snake handlers are sometimes reluctant to receive antivenom, despite their risk of anaphylaxis being similar to that of the general population. What is more important is that snake handlers may develop hypersensitivity to the venom, and venom anaphylaxis is an important and life-threatening condition in this group.34
Conclusion
In Australia, snakebite, and particularly snake envenoming, is a rare occurrence, but one that is likely to occur in patients presenting to rural and regional hospitals. The attending healthcare workers may have limited experience with snakebite and limited access to laboratory resources. These limitations can be compounded by the acuity of the injury, which may be high, requiring immediate resuscitation. Fortunately, in the past decade, clinical guidelines have improved, and immediate access to expert advice is now available via the national Poison Information Hotline (13 11 26). The focus of snakebite management has moved to early clinical assessment, so that a decision about antivenom administration can be made within three hours, before the results of laboratory tests are available. In addition, antivenom treatment is now based on identifying systemic envenoming rather than determining the type of snake responsible for the bite. MT
COMPETING INTERESTS: Professor Isbister and Associate Professor Berling have received payment from Emergency Trauma Management Pty Ltd for consultation, writing and presenting of educational toxicology-specific material for the ETM Online toxicology course.
References
1. Toxicology and toxinology. In: Therapeutic Guidelines. 3rd ed. Melbourne: Therapeutic Guidelines Ltd; 2020.
2. Johnston CI, Ryan NM, O’Leary MA, Brown SGA, Isbister GK. Australian taipan (Oxyuranus spp.) envenoming: clinical effects and potential benefits of early antivenom therapy – Australian Snakebite Project (ASP-25). Clin Toxicol (Phila) 2017; 55: 115-122.
3. Churchman A, O’Leary MA, Buckley NA, et al. Clinical effects of red-bellied black snake (Pseudechis porphyriacus) envenoming and correlation with venom concentrations: Australian Snakebite Project (ASP-11). Med J Aust 2010; 193: 696-700.
4. Johnston CI, Brown SG, O’Leary MA, et al. Mulga snake (Pseudechis australis) envenoming: a spectrum of myotoxicity, anticoagulant coagulopathy, haemolysis and the role of early antivenom therapy – Australian Snakebite Project (ASP-19). Clin Toxicol (Phila) 2013; 51: 417-424.
5. Gondek S, Schroeder ME, Sarani B. Assessment and resuscitation in trauma management. Surg Clin North Am 2017; 97: 985-998.
6. Sharma D, Sharma P, Shastri S. Golden 60 minutes of newborn’s life: Part 2: Term neonate. J Matern Fetal Neonatal Med 2017; 30: 2728-2733.
7. Isbister GK, Chiew AL, Buckley NA, et al. Real world delays in antivenom administration: patient, snake or hospital factors (ASP-33). Clin Toxicol (Phila) 2025; 63: 10-15.
8. Isbister GK. Antivenom availability, delays and use in Australia. Toxicon X 2022; 17: 100145.
9. Johnston CI, Ryan NM, Page CB, et al. The Australian Snakebite Project, 2005–2015 (ASP-20). Med J Aust 2017; 207: 119-125.
10. Isbister GK, Scorgie FE, O’Leary MA, Seldon M, Brown SG, Lincz LF; ASP Investigators. Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10). J Thromb Haemost 2010; 8: 2504-2513.
11. Isbister GK. Procoagulant snake toxins: laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin Thromb Hemost 2009; 35: 93-103.
12. Isbister GK, Duffull SB, Brown SG; ASP Investigators. Failure of antivenom to improve recovery in Australian snakebite coagulopathy. QJM 2009; 102: 563-568.
13. Johnston CI, Isbister GK. Australian snakebite myotoxicity (ASP-23). Clin Toxicol (Phila) 2021; 59: 611-618.
14. Isbister GK, Jenkins S, Downes MA, Fakes K, Buckley NA. A randomized controlled trial and prospective cohort investigating antivenom for red-bellied black snake envenomation. Clin Toxicol (Phila) 2024; 62: 343-351.
15. Lalloo DG, Trevett AJ, Korinhona A, et al. Snake bites by the Papuan taipan (Oxyuranus scutellatus canni): paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am J Trop Med Hyg 1995; 52: 525-531.
16. Noutsos T, Currie BJ, Isoardi KZ, Brown SGA, Isbister GK. Snakebite-associated thrombotic microangiopathy: an Australian prospective cohort study [ASP30]. Clin Toxicol (Phila) 2022; 60: 205-213.
17. Noutsos T, Currie BJ, Lek RA, Isbister GK. Snakebite associated thrombotic microangiopathy: a systematic review of clinical features, outcomes, and evidence for interventions including plasmapheresis. PLoS Negl Trop Dis 2020; 14: e0008936.
18. Allen GE, Brown SG, Buckley NA, et al; ASP Investigators. Clinical effects and antivenom dosing in brown snake (Pseudonaja spp.) envenoming — Australian Snakebite Project (ASP-14). PLoS One 2012; 7: e53188.
19. Wedasingha S, Silva A, Siribaddana S, Seneviratne K, Isbister GK. Comparison of bedside clotting tests for detecting venom-induced consumption coagulopathy following Sri Lankan viper envenoming. Clin Toxicol (Phila) 2022; 60: 1328-1335.
20. O’Rourke KM, Correlje E, Martin CL, Robertson JD, Isbister GK. Point-of-care derived INR does not reliably detect significant coagulopathy following Australian snakebite. Thromb Res 2013; 132: 610-613.
21. Canale E, Isbister GK, Currie BJ. Investigating pressure bandaging for snakebite in a simulated setting: bandage type, training and the effect of transport. Emerg Med Australas 2009; 21: 184-190.
22. Ireland G, Brown SG, Buckley NA, et al; ASP Investigators. Changes in serial laboratory test results in snakebite patients: when can we safely exclude envenoming? Med J Aust 2010; 193: 285-290.
23. Little M. Harm due to the use of pressure bandage immobilisation in patients bitten by snakes in Australia. Clin Toxicol (Phila) 2023; 61: 611-612.
24. Isbister GK, Brown SG, MacDonald E, White J, Currie BJ; ASP Investigators. Current use of Australian snake antivenoms and frequency of immediate-type hypersensitivity reactions and anaphylaxis. Med J Aust 2008; 188: 473-476.
25. Isbister GK, O’Leary MA, Elliott M, Brown SG. Tiger snake (Notechis spp) envenoming: Australian Snakebite Project (ASP-13). Med J Aust 2012; 197: 173-177.
26. Johnston CI, O’Leary MA, Brown SG, et al; ASP Investigators. Death adder envenoming causes neurotoxicity not reversed by antivenom – Australian Snakebite Project (ASP-16). PLoS Negl Trop Dis 2012; 6: e1841.
27. Ryan NM, Kearney RT, Brown SG, Isbister GK. Incidence of serum sickness after the administration of Australian snake antivenom (ASP-22). Clin Toxicol (Phila) 2016; 54: 27-33.
28. Anaphylaxis: emergency management for health professionals. Aust Prescr 2018; 41(2): 54.
29. Berling I, Brown SG, Miteff F, Levi C, Isbister GK. Intracranial haemorrhages associated with venom induced consumption coagulopathy in Australian snakebites (ASP-21). Toxicon 2015; 102: 8-13.
30. Noutsos T, Isbister GK. Snakebite-associated thrombotic microangiopathy: a spotlight on pharmaceutical interventions. Expert Rev Clin Pharmacol 2023; 16: 559-574.
31. Noutsos T, Currie BJ, Isoardi KZ, Brown SGA, Isbister GK. Snakebite-associated thrombotic microangiopathy: an Australian prospective cohort study [ASP30]. Clin Toxicol (Phila) 2022; 60: 205-213.
32. Isbister GK, Buckley NA, Page CB, et al; ASP Investigators. A randomized controlled trial of fresh frozen plasma for treating venom-induced consumption coagulopathy in cases of Australian snakebite (ASP-18). J Thromb Haemost 2013; 11: 1310-1318.
33. Magee A, Isbister GK, Isoardi KZ, Holmes P, Dorofaeff T. Rough-scaled snake envenomation. J Paediatr Child Health 2022; 58: 699-701.
34. Isbister GK, Brown SG; ASP Investigators. Bites in Australian snake handlers—Australian snakebite project (ASP-15). QJM 2012; 105: 1089-1095.