Gastrointestinal nematode infections: common Australian encounters

GPs may encounter Strongyloides, Enterobius vermicularis (pinworms), Ascaris and hookworm infections in Australian clinical settings. These nematodes primarily infect the gastrointestinal tract but may cause varied clinical symptoms on infection.
- Ascaris, hookworm and Enterobius (also known as pinworm or threadworm) infections are transmitted through the faecal–oral route and ingestion of infective eggs.
- Strongyloides stercoralis infections can be chronic, with the risk of disseminated disease and hyperinfection in immunocompromised hosts. Clinical presentations can vary and depend on the parasite life cycle stage and burden of infection. Patients may be asymptomatic.
- Microscopy for ova, larvae or adult worms in stool is the mainstay of diagnosis of intestinal nematode infections. Ideally, three stool samples on separate days should be sent for laboratory assessment in fixative to optimise the diagnostic yield of stool microscopy. For strongyloidiasis, fresh stool is required for microscopy, larval culture and polymerase chain reaction assays, as well as serology.
- Testing algorithms for parasites vary between laboratories. It is important to provide relevant clinical details (e.g. travel history, immunocompromised state, presence of eosinophilia) to ensure laboratories perform appropriate testing. Consider speaking to the microbiologist to obtain advice regarding collection and ensure appropriate testing is conducted.
- Strongyloidiasis should be excluded in at-risk patients before immunosuppression, and other at-risk groups.
- Treatment includes the use of antiparasitic agents such as ivermectin, albendazole, mebendazole and pyrantel, which are generally well tolerated.
Helminths, derived from the Greek word helmins for worms, are large, multicellular organisms that are characterised by their anatomical structures.1 The major groups of parasitic helminths include trematodes (flukes), cestodes (tapeworms) and nematodes (roundworms). Nematodes are the second largest phylum in the animal kingdom, with intestinal roundworm infections forming the largest group of helminthic diseases in humans.2 Globally, one billion people are infected with at least one species of helminth.3 In Australia, although nematode infections in the animal population are well studied, there are limited data on the epidemiology in humans.
This article reviews common roundworm infections that may be encountered in Australian clinical settings, including Strongyloides, Enterobius vermicularis (pinworms), Ascaris and hookworm infections. The clinical presentation, nematode life cycle, diagnosis and treatment of these infections are discussed.
Strongyloides
Strongyloides stercoralis is an intestinal nematode that causes strongyloidiasis. The infection is considered one of the most neglected tropical diseases.4 People living in environments with poor sanitation, an unsafe water supply, overcrowding and poor healthcare infrastructure have the highest risk of infection.4,5 The Strongyloides genus includes more than 50 species, with two species known to infect humans.6Strongyloides fuelleborni is prevalent in Papua New Guinea and Africa, whereas S. stercoralis is endemic throughout Southeast Asia, sub-Saharan Africa, parts of southeastern United States, southern Europe and the northern two-thirds of Australia.7
Epidemiology
The prevalence of strongyloidiasis in Australia is unknown as epidemiological data are not routinely collected. However, the infection appears to be widespread among Aboriginal communities in tropical regions of Australia, including Queensland, the Northern Territory, Western Australia and Northern New South Wales.1-3 Strongyloidiasis is also endemic in Australia’s neighbouring countries.5 With the return of pre-pandemic levels of migration, including a significant proportion of people migrating from Asia, it is imperative to be aware of diseases endemic to these regions.6
Life cycle
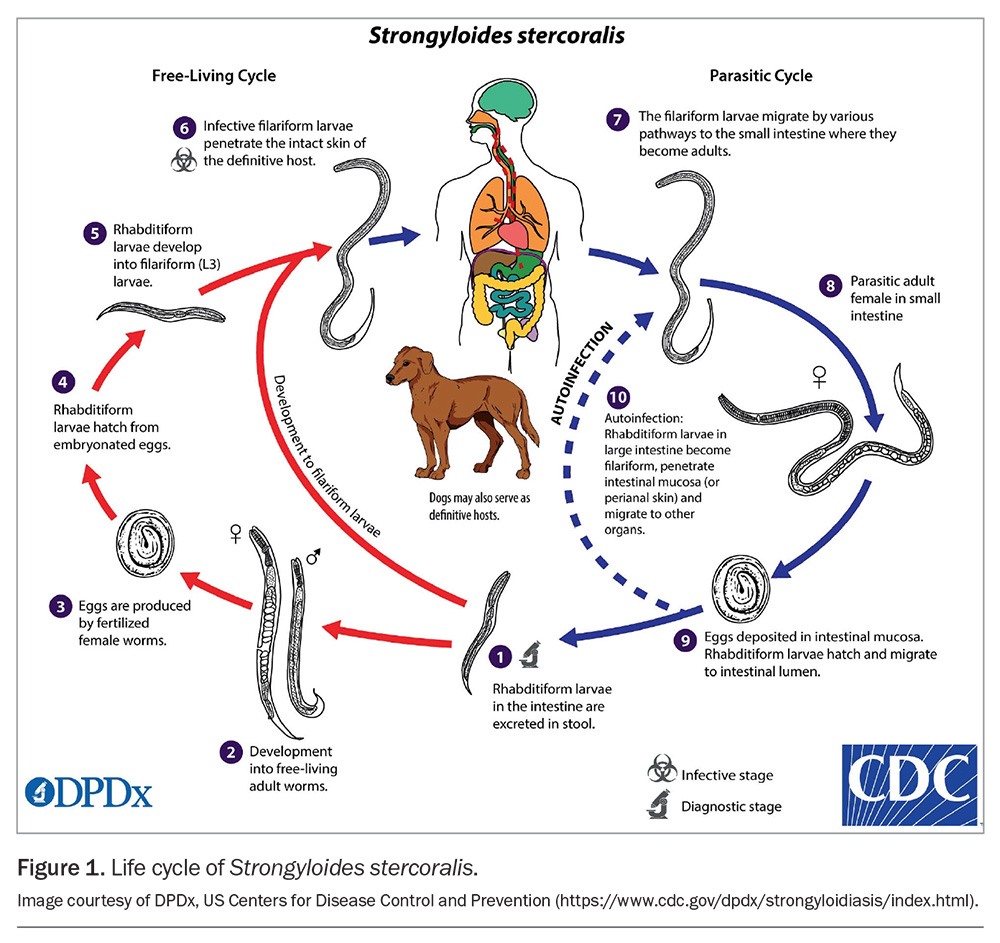
The life cycle of S. stercoralis is complicated, involving a free-living cycle, a parasitic cycle and autoinfection (Figure 1). In the free-living cycle, the rhabditiform larvae are passed in the stools of an infected host. The larvae then develop into either infective filariform larvae or free-living adult males and females that mate and produce eggs. Rhabditiform larvae hatch from the eggs and become infective filariform larvae. These filariform larvae can survive for up to two to three weeks in optimal environmental conditions.
To continue the life cycle, the filariform larvae in soil penetrate human skin on contact with soil, initiating the parasitic phase. Following skin penetration, they migrate to the small intestine via various pathways, including the bloodstream and lymphatics, to the lungs, where they are coughed up and swallowed. Filariform larvae also appear capable of migrating to the intestine through abdominal viscera or connective tissue. In the small intestine, they mature into adult male and female worms.
A peculiar feature of Strongyloides is the ability of adult female worms to produce eggs via parthenogenesis. In this case, offspring can develop from the egg or female gamete without prior fertilisation by the male gamete. Rhabditiform larvae hatch from the eggs within the intestine and pass through the stools. It is these larvae that are detected on microscopy in the diagnostic stage.
The autoinfection cycle occurs when the developing rhabditiform larvae transform into the infective filariform stage before being shed, penetrate the large intestinal wall or perianal skin and migrate back via the bloodstream or through tissues to the small intestine. Autioinfection is why strongyloidiasis can persist as chronic infection for many years.7-9 When immune control of the autoinfection lifecycle is lost, large numbers of larvae are produced and their dissemination can lead to life-threatening complications.
Clinical manifestations
The clinical manifestations of strongyloidiasis vary, depending on whether the infection is acute, chronic or disseminated. Acute and chronic strongyloidiasis can also be asymptomatic.10
In acute infection, a pruritic, erythematous rash at the skin site of larvae penetration can occur. The erythematous serpiginous lesions can move rapidly, about 2 to 10 cm/hour.11 This rash can also appear intermittently. Diarrhoea, bloating or abdominal pain can occur as the larvae penetrate the intestinal mucosa.12 As larvae migrate through the body to parenteral sites, such as the lungs, pulmonary symptoms such as cough, haemoptysis, bronchopneumonia and dyspnoea can occur.11,13
Chronic infections are often clinically asymptomatic and generally diagnosed after an incidental finding of peripheral blood eosinophilia.12 A migrating serpiginous rash, larva currens, may be seen intermittently, often appearing on the buttocks, perineum or thighs, representing larvae migrating through the skin during autoinfection cycles.7
Disseminated strongyloidiasis and hyperinfection syndrome can be fatal. Known risk factors for these include corticosteroid treatment, immunosuppressive clinical conditions, transplants, human T-lymphotropic virus 1 infection, alcohol misuse and malnutrition.14 Clinical presentations include Gram-negative bacteraemia or meningitis following the invasion of larvae through the gastrointestinal mucosa and introduction of enteric organisms into the bloodstream or meninges, with associated complications of meningitis or septic shock. Pulmonary inflammation with widespread pneumonitis and haemoptysis, abdominal distension and ileus can also occur.12 The mortality rate of hyperinfection syndrome in an immunocompromised host can be as high as 60 to 85%.15
Enterobius
E. vermicularis, commonly known as pinworm or threadworm, is the causative agent of enterobiasis. As one of the most common helminth infections, it has a worldwide distribution and is frequently encountered in Australia. Humans are thought to be the only natural host for this infection.16 Adult female worms are about 8 to 13 mm in length, with a pointed tail resembling a pin.17 Male worms are comparably much smaller, at about 2.5 mm in length and rarely seen.17 Transmission occurs through the faecal–oral route via ingestion of infective eggs. Children are more commonly affected, with infections associated with crowding occurring in households and institutionalised groups.12,17 As such, infections are prevalent among children attending daycare, preschools and primary schools.18
Life cycle
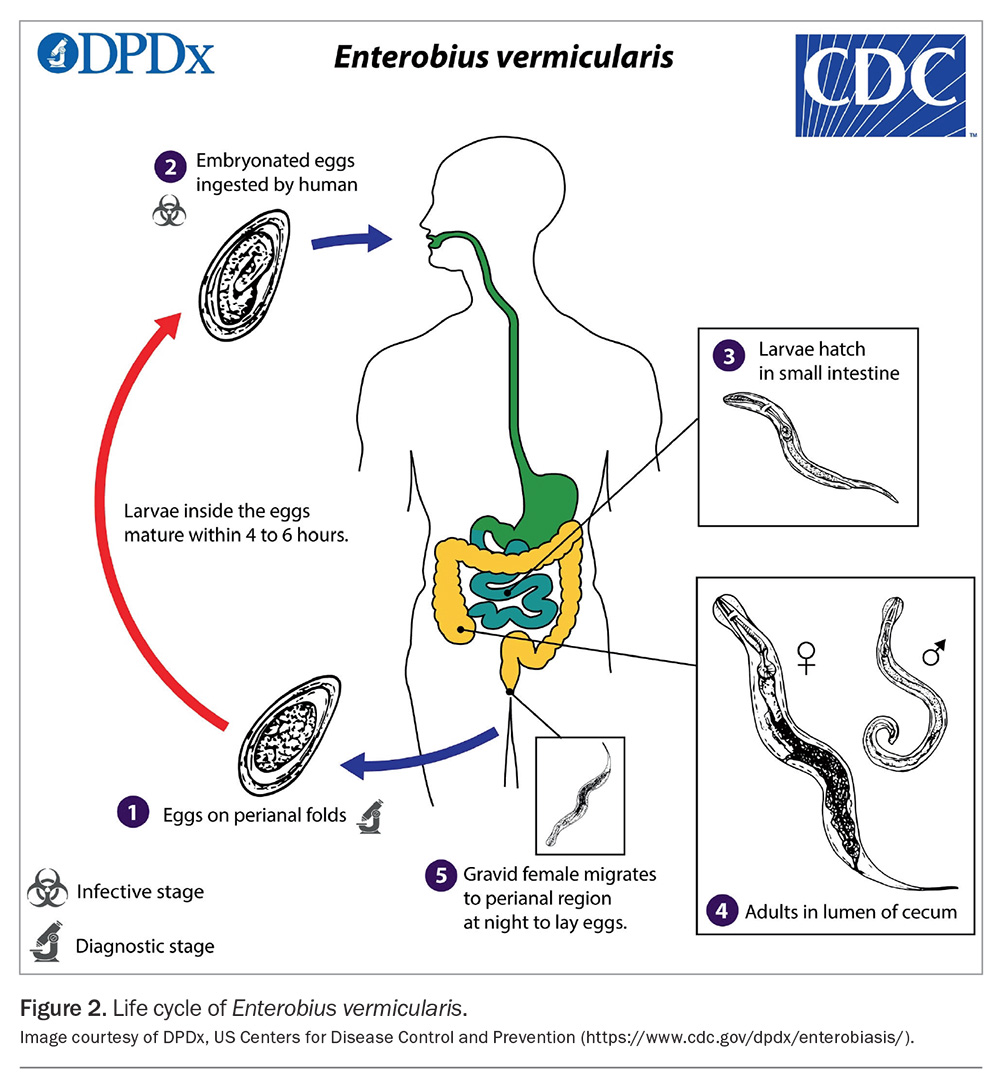
The adult female worm migrates through the colon, depositing her eggs on the perianal and perineal skin (Figure 2).15 Thousands of eggs may be laid, taking about six hours to become infective and remaining viable for up to five days.17 Transmission occurs through the ingestion of infected eggs, either via the hands of the patient – often introduced under the fingernails through scratching the perianal area (autoinfection) – or through exposure to eggs on contaminated surfaces in the environment, such as bed linens and clothes.17 Upon ingestion, the eggs hatch in the small intestine, and the larvae subsequently migrate to the large intestine where they mature into adult worms and reside in the caecum, appendix and ascending colon.17 Retroinfection, wherein hatched larvae from the anal skin migrate back into the rectum, can also occur. The time from egg ingestion to egg production (prepatent period) is about three to four weeks.17
Clinical manifestations
Infections are often asymptomatic. When symptoms are present, the most typical presentation is perineal or perianal pruritus, which is worse at night.16 Excoriation and secondary bacterial infection as complications from scratching may occur.16 Rarely, migration of parasites results in ectopic disease, including invasion of the female genital tract, resulting in vulvovaginitis, as well as pelvic or peritoneal granulomas. There have also been several case reports describing presentations of pinworm infections mimicking appendicitis.19 Significant eosinophilia or elevated serum immunoglobulin E levels are not usually seen with pinworm infections.12
Hookworms
Hookworms can reside in the small intestine for many years and cause hookworm disease if there are moderate to high numbers of adult worms causing iron-deficiency anaemia. These parasites thrive in tropical and subtropical zones with almost 500 million infected people in developing tropical countries.20 The overall prevalence of hookworm infections in Australia is low, given that they can be controlled by effective sanitation and access to clean water.21Necator americanus is the predominant hookworm infection globally and is common in southern China, Southeast Asia, the Americas and most of Africa. Ancylostoma duodenale is more endemic in the Mediterranean region, in northern regions of India and China and in North Africa.21 Both of these species have been present in the Australian population since the early 1900s, mainly found in Queensland and New South Wales.22Ancylostoma ceylanicum and Ancylostoma caninum are also found in Australia in domestic and wild canids and pose a public health risk to Indigenous communities, residents and tourists in the Wet Tropics of Queensland.23 The infection can be seen in returning travellers.
Life cycle
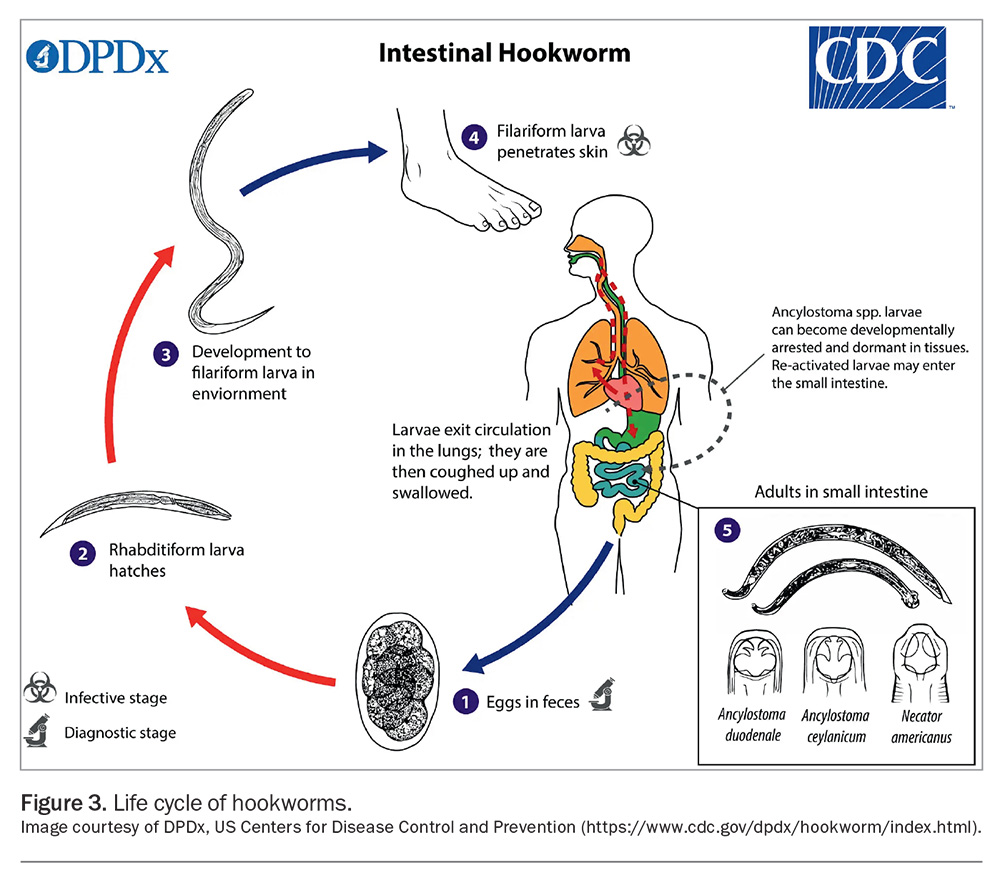
Hookworm eggs hatch and develop in soil, moulting twice before becoming infective filariform larvae, which can survive in the environment for three to four weeks (Figure 3). Filariform larvae are about 0.5 to 0.6 mm in length. They penetrate the skin and then enter the bloodstream.20 The larvae are carried through the blood vessels to the heart, and then to the lungs, where they migrate into the alveoli.24 They then ascend the trachea to the pharynx, where they enter the digestive tract and migrate to the small bowel, where they mature into adults. Once in the duodenum, the hookworms lacerate the mucosa and anchor themselves to avoid ejection via gut peristalsis and to facilitate feeding.20 As the juvenile worms feed on blood, they mature into adult parasites, which are usually eliminated after one to two years.24 Male and female hookworms mate, producing up to 10,000 eggs per day. The eggs are then passed via faeces.
Clinical manifestations
The symptoms of hookworm infections vary based on the location of the larvae. When the infectious larvae penetrate the skin, a serpiginous or vermicular rash can form. Gastrointestinal symptoms typically involve abdominal pain and diarrhoea. As hookworms lacerate the mucosa and feed on blood, a large worm burden can cause iron-deficiency anaemia. Fatigue, weakness and oedema because of hypoproteinaemia secondary to blood loss can also occur.12 Children with hookworm disease can present with growth and cognitive delays.
Ascaris
Ascaris lumbricoides is the primary species involved in human ascariasis; however, the closely related Ascaris suum in pigs may also infect humans (whether this is a truly distinct species is currently contentious).25 Worldwide, as a common cause of soil-transmitted helminth infections, around 700 million people are affected, being more common among those living in tropical and subtropical climates.17 Ascariasis acquired in Australia is rare.26,27 Of note, A. suum has been identified in pigs in Australia.28 Although distributed worldwide, A. lumbricoides is found more commonly in tropical and subtropical climates.17 The worms can also survive in cooler, temperate climates.29Ascaris worms are very large and are commonly mistaken for the common earthworm. Occasionally, earthworms or other nonpathogenic worms are sent for identification to the laboratory, having been found in the toilet bowl. However, adult Ascaris worms are the only human roundworm pathogen of this size. Adult worms are light brown to pink in colour, with female worms about 20 to 35 cm in length and males ranging from 15 to 30 cm in length.17,25 Transmission occurs via the faecal–oral route following the ingestion of infective eggs. Infections usually peak in childhood or early adolescence.29
Life cycle
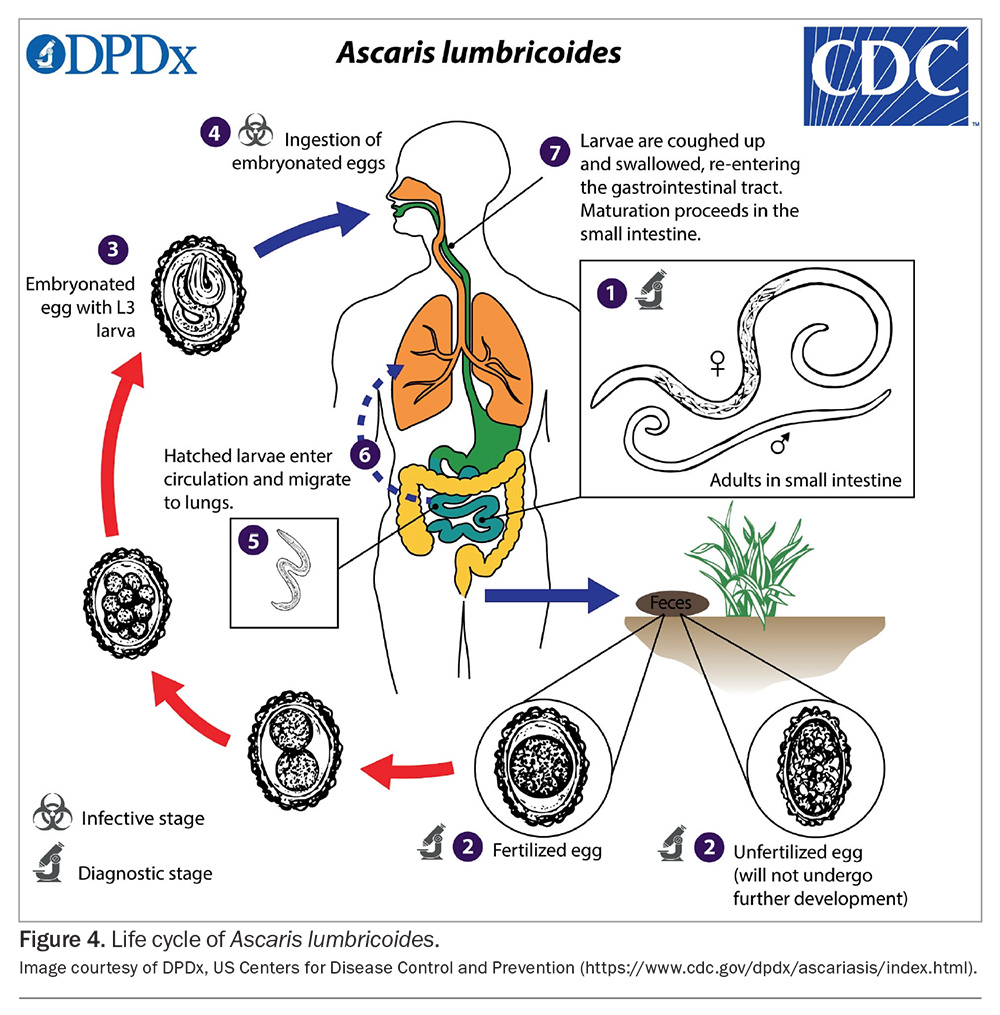
Adult worms live in the small intestine, with females passing many thousands of eggs in faeces (Figure 4).25 In the soil, depending on the environmental conditions, the eggs develop into infective embryonated eggs and may survive in this state for many years.17 Once ingested, the larvae hatch and invade the intestinal mucosa, entering venous circulation and arriving at the lungs.25 After maturation, the larvae migrate to the upper airways and are swallowed. They finally mature into adult worms in the small intestine, where they can live for one to two years.25 The prepatent period from egg ingestion to egg production is two to three months.25
Clinical manifestations
Most patients are asymptomatic.12 Symptoms, when they are present, are associated with the worm life cycle and depend on the intensity of the infection (i.e. the number of parasites present within the human).30 During the migration of larvae through the lungs, around 10 to 14 days after infection, patients may develop pneumonitis associated with peripheral eosinophilia and transient pulmonary infiltrates, known as Loeffler’s syndrome.26,27 Occasionally, presentation in low-burden countries involves the passage of a large adult roundworm in faeces, or oral or nasopharyngeal regurgitation in young children. With adult infection, symptoms are associated with the degree of worm burden and can range from asymptomatic to mild, nonspecific abdominal symptoms and, in severe cases, may result in obstruction.17 Extraintestinal manifestations because of worm migration may result in hepatobiliary obstruction.17 In children with a heavy burden of infection (i.e. a high number of parasites), malnutrition can occur.17
Diagnosis of nematode infections
Testing algorithms for parasites vary between laboratories. It is important to provide relevant clinical details (e.g. travel history, immunocompromised state, presence of eosinophilia) to ensure laboratories perform appropriate testing. Consider speaking to the microbiologist to obtain advice regarding collection and ensure appropriate testing is conducted.
Microscopy
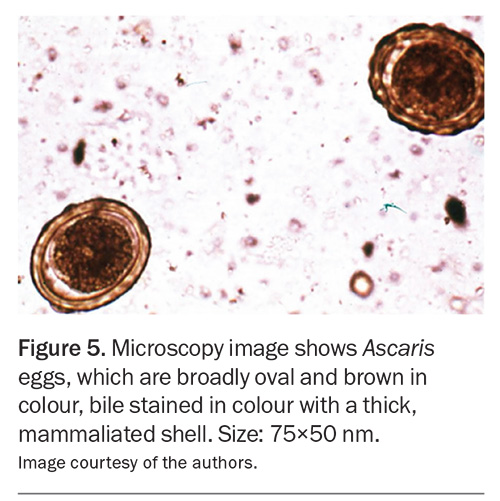
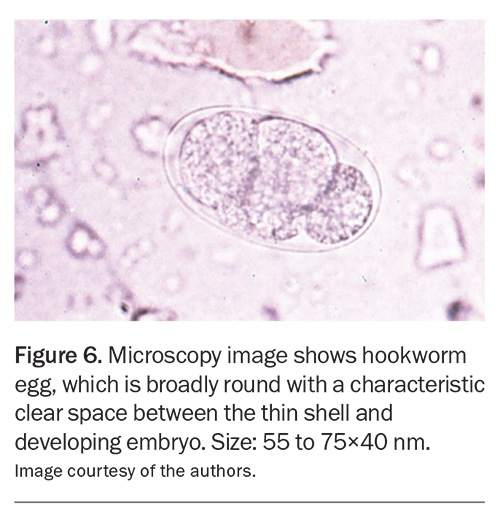
The diagnosis of intestinal helminth infections usually relies on the microscopic identification of eggs, larvae or adult worms. It is generally recommended that three stool specimens be collected on separate days over a 10-day period considering the intermittent shedding of larvae and eggs. As Medicare currently rebates two stool specimens within a seven-day period, the third sample must be collected in the subsequent week. Stool samples should be submitted in sterile containers as close to the time of collection as possible. In addition, specimen jars containing fixatives are provided by some laboratories to preserve parasite and egg morphology until laboratory examination. It is useful to check local laboratory processes and collection recommendations before testing. The diagnosis of Ascaris, hookworm and Enterobius infections can be achieved through microscopic identification of the distinctive egg appearance (Figure 5 and Figure 6).16,24 Lugol's iodine staining may also be used to identify rhabditiform larvae in stool.5
The diagnosis of strongyloidiasis is made by the microscopic identification of S. stercoralis rhabditiform (and occasionally, filariform) larvae or S. fuelleborni eggs in stool.31 Occasionally, adult Ascaris worms may be shed in stool, and E. vermicularis may be visible around the perianal area.16,25 In Strongyloides hyperinfection, other specimens including sputum samples, upper gastrointestinal aspirates and cerebrospinal fluid may be relevant for microscopic examination.
Although pinworm eggs may be found in the stool, the ‘sticky tape test’ is considered the method of choice to detect E. vermicularis eggs. This simple bedside test should be performed in the morning before defecation or washing. Instructional resources are available to help with the technique of this test.32 A sticky tape is attached over a wooden tongue depressor (to use as a handle), and the tape is pressed firmly against the perianal skin, with the intention of capturing any eggs present. The sticky tape is then transferred on to a microscope slide and sent for examination.33
Culture
As the sensitivity of stool microscopic examination for both Strongyloides and hookworm infection is low, culture tests can be considered to improve the diagnostic yield, especially in cases of high clinical suspicion. Fresh faecal specimens are required for these tests.
Serology
Antibody detection is available for Strongyloides infection and is often the mainstay of diagnosis, as the sensitivity is better than that of faecal testing, and the relatively high negative predictive value is useful in excluding S. stercoralis infections.34 Serology can also serve as a screening tool for at-risk, asymptomatic patients before undergoing immunosuppression.15 The limitations include cross-reactivity with other nematode infections and an inability to distinguish between current and past infections (although antibody levels usually decline over time after treatment).32,34 However, caution should also be exercised in the interpretation of serology in immunosuppressed patients who may have false-negative test results.32,34 The presence of peripheral eosinophilia is neither specific nor sensitive, and should not be used as a standalone test in a patient with a suspected helminth infection.35
Molecular diagnosis
Both in-house and commercial molecular panels, usually polymerase chain reaction panels, are increasingly available and more sensitive than phenotypic diagnosis. Polymerase chain reaction assays are used in some Australian laboratories and include many of the more common nematodes, with a sensitivity of about 90%.
Treatments for nematode infections
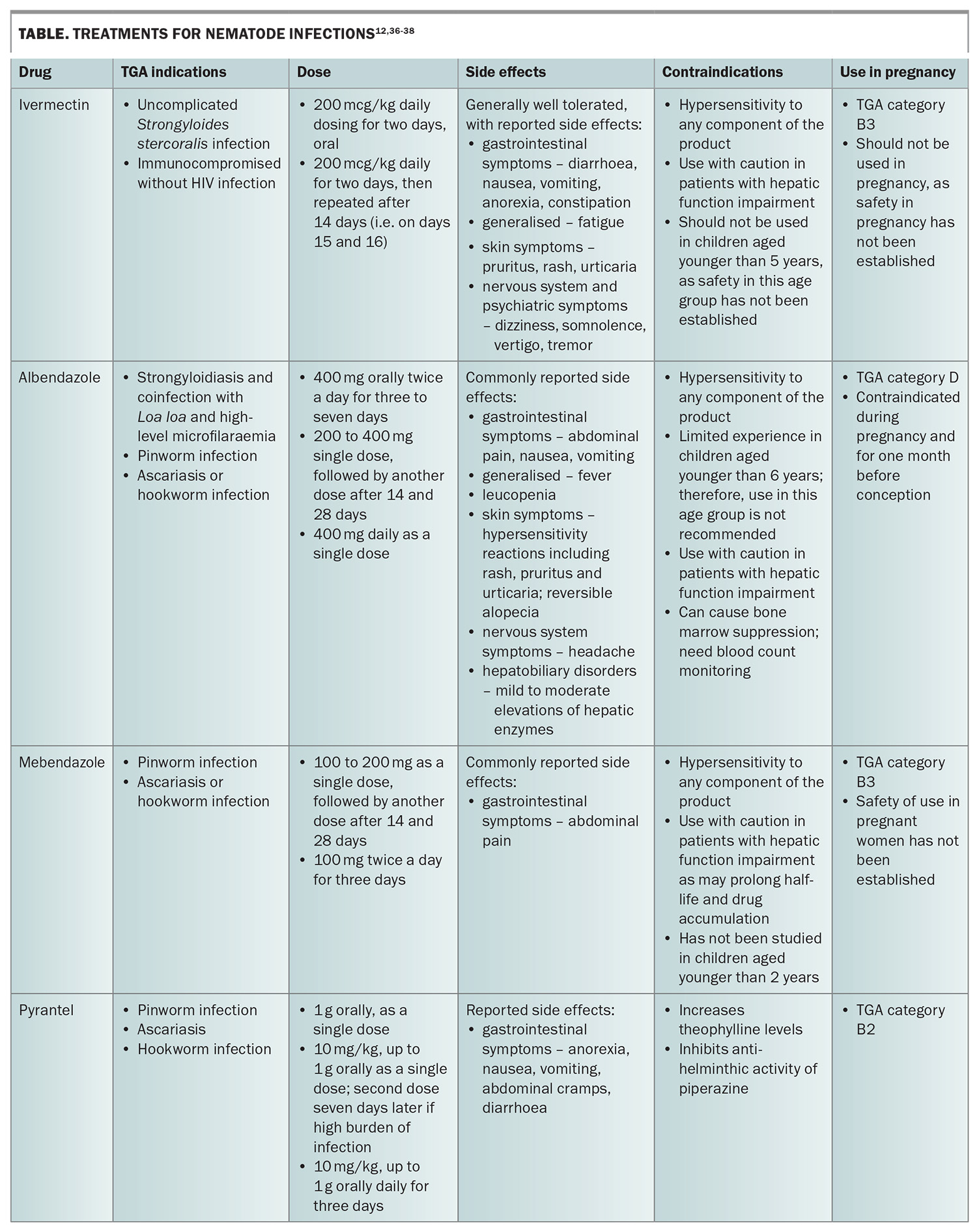
An overview of the treatments for nematode infections are provided in the Table.12,36-38
Strongyloidiasis
Ivermectin is the drug of choice for the treatment of strongyloidiasis and is well tolerated.39 In patients who are immunocompromised without HIV infection, a four-dose course is recommended.40 Following treatment, immunocompromised patients who live or visit Strongyloides endemic areas (e.g. remote Aboriginal and Torres Strait Islander communities) should receive ongoing prophylaxis every three months.
Patients who have lived or travelled to areas of West and Central Africa where Loa loa is endemic should be screened for coinfection with this organism, as ivermectin can cause severe reactions in patients with loiasis and high microfilarial levels.10 Albendazole is a good alternative for those with coinfection with L. loa and high-level microfilaraemia.41 Post-treatment serology should be performed at six months and 12 months after treatment to ensure a decrease in titre.42
Pinworm infection
The two benzimidazole derivates, mebendazole and albendazole, are considered to be the most effective drugs for pinworm infections, as they are both adulticidal and ovicidal.43 Repeated doses on day 0, day 14 and day 28 can be given because of high rates of autoinfection from eggs remaining in the environment. Pyrantel is also an option and given in a single dose.
Ascariasis
Albendazole, mebendazole and pyrantel have high efficacy against A. lumbricoides. These anti-helminths have been shown to increase cure rates and egg reduction rates.44 A second dose of pyrantel can be administered seven days after the initial dose if there is a heavy burden of infection.40 If there is worm obstruction of the biliary tree or pancreatic duct, surgical or endoscopic removal of the worms may be required.26
Hookworm infections
Albendazole, mebendazole and pyrantel are the recommended treatment options for hookworm infections. The benzimidazole anti-helminths inhibit microtubule polymerisation in invertebrates, thereby killing adult worms.20
Conclusion
Nematodes encompass soil-transmitted roundworms that primarily infect the gastrointestinal tract, but depending on their life cycle, can present with varied clinical symptoms on infection. Untreated infections can lead to complications, such as malnutrition in children or hyperinfection in people with strongyloidiasis. A solid understanding of risk factors, endemicity, diagnostic tools and available treatment options is essential to manage these infections in the Australian population. MT
COMPETING INTERESTS: Dr Ung, Dr Baskar and Ms Phan: None. Dr McKew is Member of Strongyloides Australia Inc., a not-for-profit advocacy group aimed at improving the diagnosis and treatment of strongyloidiasis in Australia and making it a notifiable disease with the aim of eradication.
References
1. Robertson GJ, Koehler AV, Gasser RB, Watts M, Norton R, Bradbury RS. Application of PCR-based tools to explore Strongyloides infection in people in parts of northern Australia. Trop Med Infect Dis 2017; 2: 62.
2. Speare R, Miller A, Page WA. Strongyloidiasis: a case for notification in Australia? Med J Aust 2015; 202: 523-524.
3. Shield J, Braat S, Watts M, et al. Seropositivity and geographical distribution of Strongyloides stercoralis in Australia: a study of pathology laboratory data from 2012-2016. PLoS Negl Trop Dis 2021; 9: e0009160.
4. Paltridge M, Smith S, Traves A, et al. Rapid progress toward elimination of Strongyloidiasis in north Queensland, tropical Australia, 2000-2018. Am J Trop Med Hygiene 2020; 102: 339-345.
5. Ericsson CD, Steffen R, Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 2001; 33: 1040-1047.
6. Australian Bureau of Statistics (ABS). Overseas migration. Canberra: ABS; 2023. Available online at: https://www.abs.gov.au/statistics/people/population/overseas-migration/latest-release (accessed October 2024).
7. Centers for Disease Control and Prevention (CDC). About Strongyloidiasis. Atlanta, GA: CDC; 2019. Available online at: https://www.cdc.gov/strongyloides?CDC_AAref_Val=https://www.cdc.gov/parasites/strongyloides/gen_info/faqs.html (accessed October 2024).
8. Loukas A, Maizels RM, Hotez PJ. The yin and yang of human soil-transmitted helminth infections. Int J Parasitol 2021; 51: 1243-1253.
9. Wilson A, Fearon D. Paediatric Strongyloidiasis in central Australia. Trop Med Infect Dis 2018; 3: 64.
10. Marie C, Petri WA. Strongyloidiasis (threadworm infection). MSD Manual. Rahway, NJ: Merck & Co; 2022. Available online at: https://www.msdmanuals.com/home/infections/parasitic-infections-nematodes roundworms/trichinosis#Prevention_v14459194 (accessed October 2024).
11. Page W, Speare R. Chronic strongyloidiasis - don’t look and you won’t find. Aust Fam Physician 2016; 45: 40-44.
12. Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2019.
13. Caumes E, Keystone JS. Acute Strongyloidiasis: a rarity. Chronic Strongyloidiasis: a time bomb! J Travel Med 2011; 18: 71-72.
14. Buonfrate D, Requena-Mendez A, Angheben A, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis 2013; 13: 78.
15. L SKK, Basavraj GK, Papireddy CKR. Strongyloides stercoralis hyper infection syndrome. Indian J Surg 2021; 83: 582-586.
16. Centers for Disease Control and Prevention (CDC). Enterobiasis. Atlanta, GA: CDC; 2019. Available online at: https://www.cdc.gov/dpdx/enterobiasis/ (accessed October 2024).
17. Jorgensen JH, Carroll KC, Funke G, et al. Manual of Clinical Microbiology. 11th ed. ASM Press; 2015.
18. Gunaratna GP, Dempsey S, Ho C, Britton PN. Diagnosis of Enterobius vermicularis infestations. J Paediatr Child Health 2020; 56: 1994.
19. Sousa J, Hawkins R, Shenoy A, et al. Enterobius vermicularis-associated appendicitis: a 22-year case series and comprehensive review of the literature. J Paediatr Surg 2021; 57: 1494-1498.
20. Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers 2016;2: 16088.
21. Raw C, Traub RJ, Zendejas-Heredia PA, Stevenson M, Wiethoelter A. A systematic review and meta-analysis of human and zoonotic dog soil-transmitted helminth infections in Australian Indigenous communities. PLoS Negl Trop Dis 2022; 16: e0010895.
22. Prociv P, Luke RA. The changing epidemiology of human hookworm infection in Australia. Med J Aust 1995; 162: 150-154.
23. Smout FA, Skerratt LF, Butler JRA, Johnson CN, Congdon BD, Thompson RCA. The hookworm Ancylostoma ceylanicum: an emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health 2017; 3: 66-69.
24. Centers for Disease Control and Prevention (CDC). Hookworm (intestinal). Atlanta, GA: CDC; 2019. Available online at: https://www.cdc.gov/dpdx/hookworm/ (accessed October 2024).
25. Centers for Disease Control and Prevention (CDC). Ascariasis. Atlanta, GA: CDC; 2019. Available online at: https://www.cdc.gov/dpdx/ascariasis/ (accessed October 2024).
26. Victorian Government Department of Health. Ascariasis (roundworm infection). Melbourne: Victorian Government: 2024. Available online at: https://www.health.vic.gov.au/infectious-diseases/ascariasis-roundworm-infection (accessed October 2024).
27. Holland C, Sepidarkish M, Deslyper G, et al. Global prevalence of Ascaris infection in humans (2010-2021): a systematic review and meta-analysis. Infect Dis Poverty 2022; 11: 113.
28. Gordon CA, Kurscheid J, Jones MK, Gray DJ, McManus DP. Soil-transmitted helminths in tropical Australia and Asia. Trop Med Infect Dis 2017; 2: 56.
29. Garcia LS. Diagnostic Medical Parasitology. 6th ed. Washington, DC: Wiley; 2016.
30. Else KJ, Keiser J, Holland CV, et al. Whipworm and roundworm infections. Nat Rev Dis Primers 2020; 6: 44.
31. Centers for Disease Control and Prevention (CDC). Strongyloidiasis. Atlanta, GA: CDC; 2019. Available online at: https://www.cdc.gov/dpdx/strongyloidiasis/index.html (accessed October 2024).
32. Kucik CJ, Martin GL, Sortor BV. Common intestinal parasites. Am Fam Physician 2004; 69: 1161-1168.
33. Genchi M, Potters I, Kaminsky RG, Montresor A, Magnino S. Bench aids for the diagnosis of intestinal parasites. 2nd ed. Geneva: World Health Organisation; 2019.
34. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology 2017; 144: 263-273.
35. Leder K, Weller PF. Eosinophilia and helminthic infections. Baillieres Best Pract Res Clin Haematol 2000; 13: 301-317.
36. Therapeutic Goods Administration. Product Information for Ivermectin. Canberra: Australian Government; 2023. Available online at: https://www.tga.gov.au/resources/artg/181338 (accessed October 2024).
37. Therapeutic Goods Administration. Product Information for Eskazole. Canberra: Australian Government; 2024. Available online at: https://www.tga.gov.au/resources/artg/97504 (accessed October 2024).
38. MedSafe. Vermox. Wellington; New Zealand Medicines and Medical Devices Safety Authority: 2018. Available online at: https://www.medsafe.govt.nz/profs/datasheet/datasheet.htm#V (accessed October 2024).
39. Krowlewiecki A, Nutman TB. Strongyloidiasis: a neglected Neglected Tropical Disease (NTD). Infect Dis Clin North Am 2019; 33: 135-151.
40. Therapeutic Guidelines (TG). Gastrointestinal helminths (worms). Melbourne: Therapeutic Guidelines Limited; 2019.
41. Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis 2012; 25: 458-463.
42. Requena-Mendez A BD, Gomez-Junyet J, Zammarchi L, Bisoffi Z, Munoz J. Evidence-Based Guidelines for Screening and Management of Strongyloidiasis in non-endemic countries. Am J Trop Med Hygiene 2017; 97: 645-652.
43. Wendt S, Trawinski H, Schubert S, Rodloff AC, Mossner J, Lubbert C. The diagnosis and treatment of pinworm infection. Deutsches Arzteblatt Int 2019; 116: 213-219.
44. Moser W, Schindler C, Keiser J. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta-analysis. BMJ 2017; 358: j4307.