Actinic keratoses – a guide to treatment with 5-fluorouracil cream

Actinic keratosis (AK) is a common condition that develops as scaly lesions on chronically sun-exposed skin. Areas of significant sun damage with multiple AKs can be effectively treated with topical 5-fluorouracil (most commonly as cream). Awareness of the expected clinical course of treatment, contraindications and potential adverse effects can help clinicians guide patients through successful treatment.
Actinic keratosis (AK), also known as solar keratosis, is one of the most common skin conditions in Australia. Medicare billings for the treatment of AKs have increased significantly over the years, with $19.6 million in Medicare benefits paid out in 2012 for cryotherapy.1 The greatest risk factor for the development of AK is exposure to ultraviolet (UV) radiation, predominantly from sunlight. Sun protection measures, such as physical barriers and sunscreen, are essential in preventing the development of AKs.2
Several options are available for treating AKs. For solitary lesions, cryotherapy is effective. For patients with significant sun damage and multiple AKs, field treatment is preferred. Field treatment options include 5-fluorouracil (5-FU) topical cream (5-FU 5% or 5-FU 4%),3,4 a fluorouracil and salicylic acid solution, imiquimod cream, diclofenac 3% in hyaluronan 2.5% gel and photodynamic therapy. This article provides guidance on using 5-FU creams as field treatment for AKs.
Clinical features
Actinic keratosis is a scaly lesion found in areas of sun-damaged skin, often on frequently sun-exposed areas such as the face, scalp, hands and forearms. Risk factors for developing AK include having fair skin, a history of sunburns, long-term sun exposure, ageing, an immunocompromised state and certain medications. Lesions can appear as flat or elevated papules or plaques, and can be white, yellow, skin coloured, pink, red or pigmented. They may or may not be tender.
Potential for transformation to squamous cell carcinoma
Actinic keratoses are classified as pre-cancerous lesions. They carry a risk of transformation to squamous cell carcinoma (SCC), the rate of which varies significantly between studies. A systematic analysis of 24 eligible studies found rates of progression ranged from 0 to 0.075% per lesion-year, increasing to 0.53% in those with a history of previous nonmelanoma skin cancers.5 A study of a high-risk population reported a 0.6% risk of transformation to SCC at one year and 2.57% risk at four years.6 Another study showed the risk of progression from AK to SCC was 0.025 to 16% per year.7 Finally, a study in Sweden found patients with AKs had a five times higher risk of developing any type of skin cancer within a 10 year follow-up period than a control group without AKs.8 Because this risk of transformation does exist, a biopsy of the AK should be performed to exclude invasive cutaneous carcinoma if the lesion becomes tender, thickened, ulcerated or is enlarging.
Treatment with topical 5-fluorouracil
Mechanism of action
Although the exact mechanism of action is not fully understood, 5-FU inhibits DNA and RNA synthesis with subsequent growth inhibition and cell death, particularly in rapidly dividing cells such as those of AKs and cutaneous malignancies.9,10 When applied to the skin, this process causes erythema, followed by erosion, necrosis and, finally, epithelialisation.3 The preferential uptake tends to create a ‘spotty’ reaction since the rapidly dividing cells are more greatly affected. On occasion, unintendedly excessive reactions may result in vesiculation or ulceration, particularly on severely sun-damaged skin.
Potential for preventing SCC
A randomised, double-blinded, placebocontrolled trial of 932 patients compared twice daily application of topical 5-FU 5% cream or a control cream to the face and ears for four weeks.11 The 5-FU group had fewer AKs (3 vs 8.1, p<0.001) six months after starting treatment and for the entire study duration (mean follow-up duration 2.6 years), a higher 100% lesion clearance rate at six months (38% vs 17%, p<0.01), needed fewer spot treatments at each six-month visit, and developed fewer hypertrophic AKs than the control group. By extension, it is assumed the reduction in AK numbers translates to an overall lower risk of SCC development.
Similar findings were reported in a randomised, double-blind, placebo-controlled trial of high-risk American Veterans, predominantly of Fitzpatrick skin phototypes I to III.12 Participants had a median of six baseline AKs on their face and ears, and at least two keratinocyte carcinomas in the previous five years, with at least one of these being on the face or ears. Those who applied a two- to four-week course of topical 5-FU 5% field treatment twice a day to the face and ears had a 75% reduction in the risk of developing an SCC in one year compared with the control group, who applied a placebo.12
Relapse is expected
Occasionally, a repeat or a longer treatment course of 5-FU is needed. Some patients with actinic damage may need to apply a yearly field treatment of 5-FU on their most sun damaged areas, such as the scalp. This is often done during winter, when patients are able to avoid sun exposure more easily.
How to use 5-fluorouracil
5-FU is a white cream that is generally well tolerated and is available as 4 or 5% formulations. It is also available as a clear liquid solution of 0.5% 5-FU in combination with 10% salicylic acid. This solution is used for up to 10 mild to moderate lesions within an area of up to 25 cm2 and, although the skin reactions seen may be similar, is not discussed further in this article.
We recommend that patients with AKs apply a thin layer of 4% or 5% 5-FU cream to the affected area once daily, aiming for a total of four weeks of therapy, or 5% 5-FU twice a day for three weeks, ideally during winter months.3 As discussed below, on occasion, temporarily pausing or even stopping treatment will be necessary. No overlying dressing is needed. Hands should be washed thoroughly after use, particularly if gloves have not been used for application. Patients, particularly first-time users, should be reviewed after two weeks of therapy to assess the risk of adverse reactions and the degree of inflammatory response.
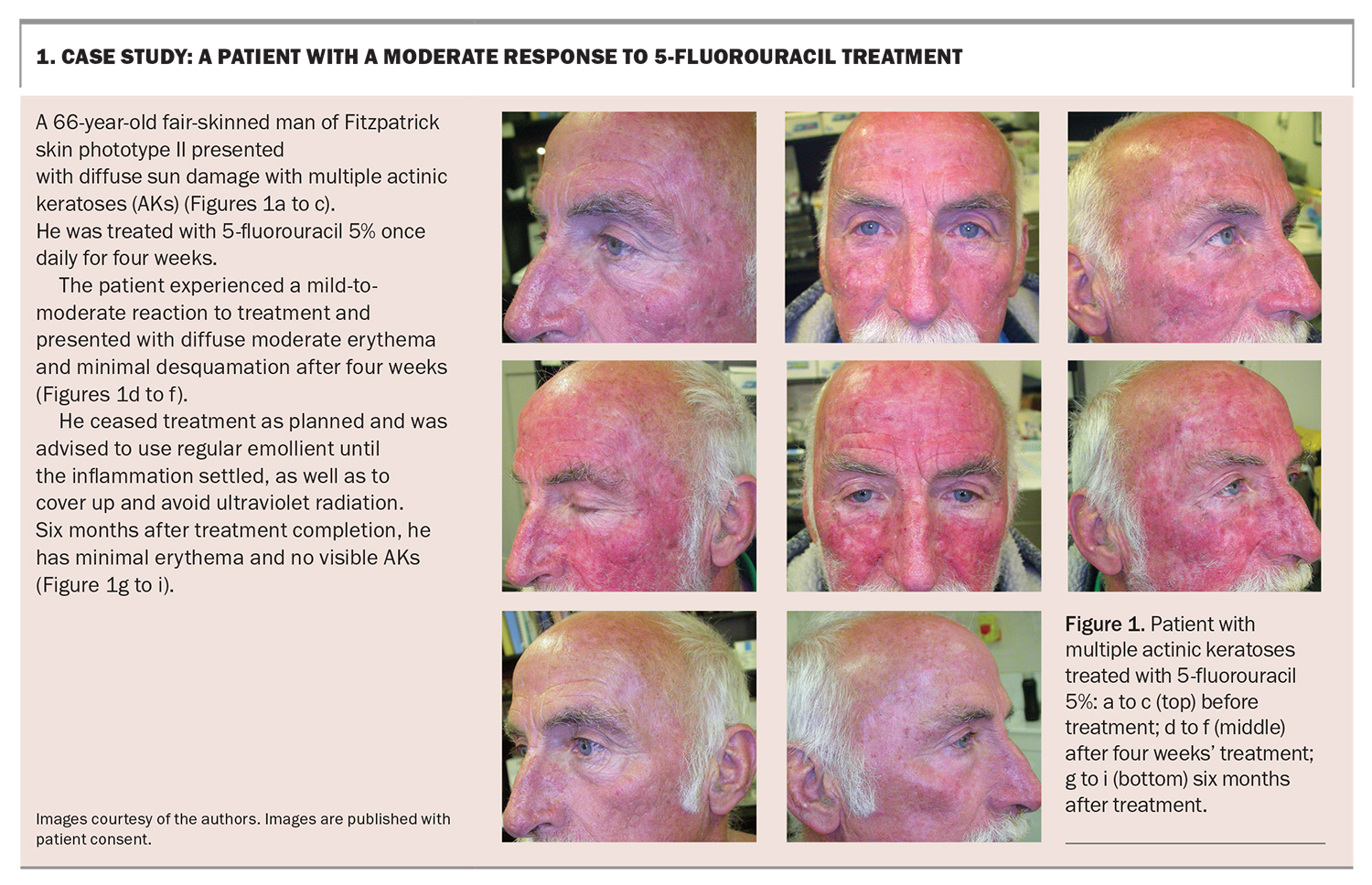
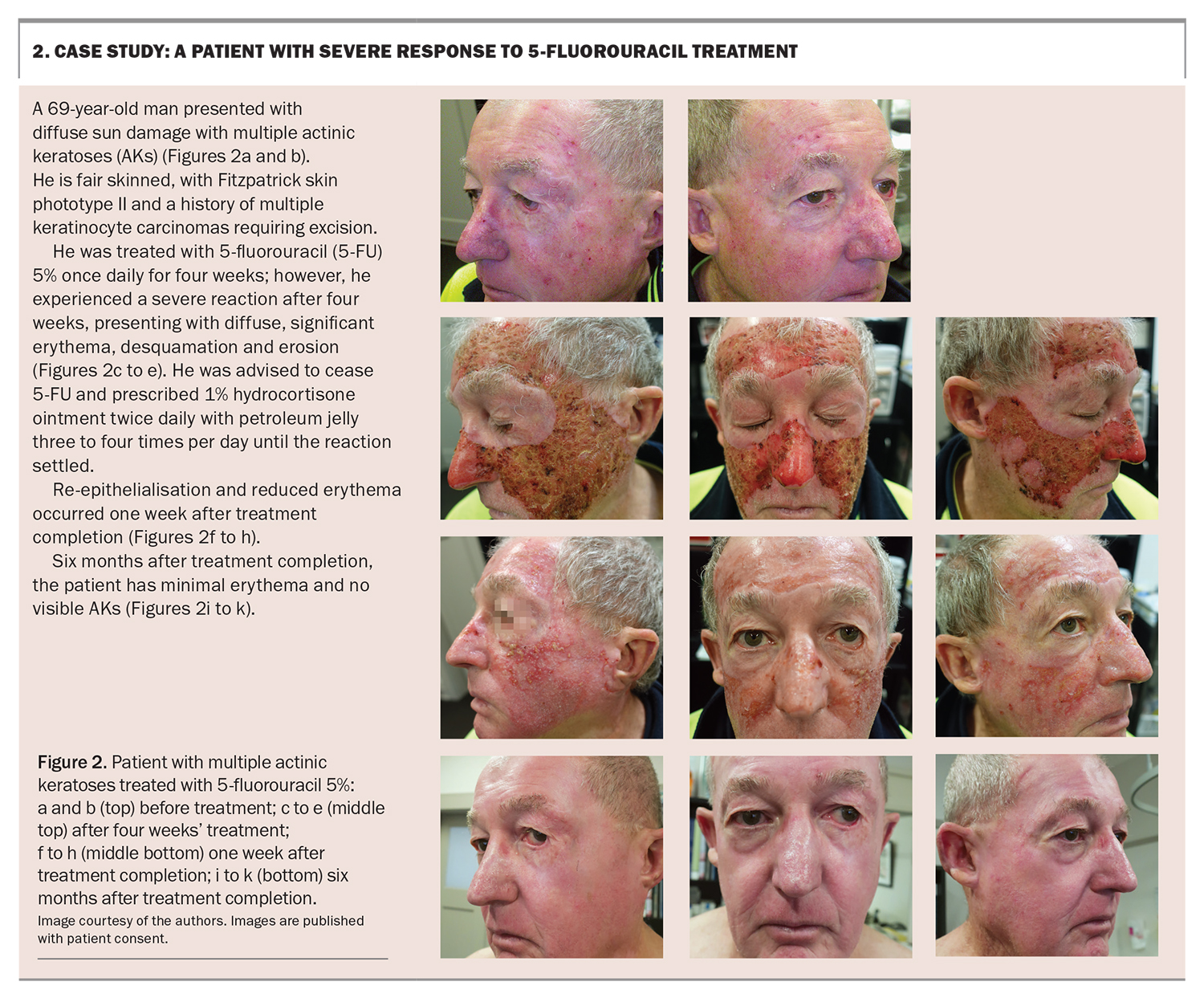
Within three to five days, the skin will become erythematous and perhaps blotchy red, followed by the development of blistering, peeling and cracking within 11 to 14 days. The aim of treatment is to achieve erosion and cause the treated skin to flake away.11 Finally re-epithelialisation, or healing, occurs. Patients should be educated on the phases to expect and that they may experience pain, blistering and ulceration. Case studies of a patient with a mild-to-moderate reaction to 5-FU treatment and a patient with a severe inflammatory reaction to 5-FU treatment are presented in Box 1 and Box 2, respectively.
The maximum area treated should be 500 cm2, or 23 × 23 cm, in a single session. The cream should not be used in close proximity to mucous membranes as it can cause irritation, local inflammation, ulceration and, potentially, increased absorption through ulcerated skin. Therefore, patients should avoid or use caution when treating the perioral area or nasolabial fold. After treatment patients should avoid UV radiation, such as sunlight or tanning salons, to prevent a diffuse phototoxic response. Other topical medications or cosmetics should also be avoided.
Because of its teratogenicity, 5-FU is contraindicated in pregnancy or for women who may become pregnant during treatment. It is also contraindicated during breastfeeding as it is unknown whether it is excreted in breastmilk. 5-FU is also contraindicated in those with the uncommon dihydropyrimidine dehydrogenase (DPD) enzyme deficiency. Because 5-FU is largely catabolised by DPD, it can cause toxicities, including stomatitis, diarrhoea, neutropenia and neurotoxicity, and is potentially life threatening. 5-FU is not available on the PBS; however, is available through the RPBS to those with a Veteran Gold Card.
Managing skin reactions to treatment
Inflammation that persists after treatment can be settled with regular emollient and once daily application of a mild topical corticosteroid, such as 1% hydrocortisone ointment, then allowing the area time to heal. Although complications are uncommon, the following techniques can be used to manage the most common skin reactions and adverse effects.
- Pain. Simple analgesia such as paracetamol or ibuprofen can be used to manage pain resulting from treatment. If the pain becomes moderate to severe, treatment should be reduced in frequency or stopped.
- Redness. Redness experienced from treatment is inevitable and is known as postinflammatory erythema; however, precautionary measures, such as avoiding sun exposure and tanning beds, will help reduce this. Ideally, physical barriers, such as wearing wide-brimmed hats and clothing and seeking shade, will help reduce the need to apply sunscreen to already inflamed skin. Treating AKs during winter months will further help reduce redness from photosensitivity while patients are on treatment. Certain areas, such as the upper lip, may experience a more brisk inflammatory reaction. Special care should be taken in patients with conditions such as rosacea, as they are at higher risk of experiencing persistent erythema.
- Bleeding or oozing. Oozing may occur if the skin is very inflamed but should be self-limiting; however, frank bleeding should not occur. If oozing persists or bleeding occurs, targeted pressure should be applied to the site for 10 to 15 minutes. If bleeding persists beyond this, the patient should present to their GP or dermatologist, who can achieve haemostasis by various means, such as applying aluminium chloride hexahydrate solution or alginate dressings.
- Crusting. The drying out of excessive exudate at the site of 5-FU application may result in crusting. This can be treated with saline soaks, followed by gentle wiping away of the crusts, and application of petrolatum ointment, three times per day, until resolution.
- Infection. If infection is suspected, the patient should be treated promptly by their GP or dermatologist. If the area of erythema is limited to where the treatment has been applied, there is no purulent discharge and the patient is otherwise well, this is likely a normal response to treatment. If erythema extends beyond the area of treatment or if other signs of infection (e.g. purulent discharge or an abscess) or systemic symptoms or signs (e.g. fever) are present, treatment should be suspended and the patient should be prescribed antibiotics with adequate coverage for common skin pathogens such as Staphylococcus aureus. Cephalexin or flucloxacillin are most appropriate as long the patient is not allergic to these medications. Topical mupirocin is appropriate if infection is superficial and localised. Secondary infection is rare.
- Allergic reaction. Allergic contact dermatitis is a rare but possible adverse effect. It may be difficult to distinguish from the expected skin changes of 5-FU treatment. Patients should be referred for patch testing if allergic contact dermatitis is suspected.
Due to the nature of actinic field change, it is important to follow up with patients to ensure the expected response to treatment is achieved, usually around three to six months after a course of therapy. At this time, any lesions that persist despite treatment should be reviewed for consideration for cryotherapy or repeat treatment with 5-FU, or biopsied if any concerning features of invasive malignancy are detected.
Conclusion
5-FU 4% or 5% alone, or 0.5% in combination with salicylic acid, offer effective field treatment options for AKs. It is important to be aware of the expected clinical course and common local inflammatory skin reactions. Patients should be educated on these to ensure adherence to treatment and to safely navigate any local skin reactions that are not well tolerated. MT
COMPETING INTERESTS: Dr Adamson and Associate Professor Chong: None. Associate Professor Foley has received consultation fees from ASLAN, Bristol Myers Squibb, Eli Lilly, GenesisCare, Hexima, Janssen, Leo Pharma, Mayne Pharma, Novartis, Pfizer and UCB; received honoraria from AbbVie, Amgen, Eli Lilly, Galderma, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, Sun Pharma and Boehringer Ingelheim; and been on Advisory Boards for AbbVie, Amgen, ASLAN, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, GenesisCare, Hexima, Janssen, Leo Pharma, Mayne Pharma, Novartis, Pfizer, Sanofi and Sun Pharma.
References
1. Perera E, McGuigan S, Sinclair R. Cost for the treatment of actinic keratosis on the rise in Australia. F1000Res 2014; 3: 184.
2. Green A, McBride P. Squamous cell carcinoma of the skin (non-metastatic). BMJ Clin Evid 2014; 2014: 1709.
3. Therapeutic Goods Administration. Australian Product Information. Efudix (fluorouracil) cream. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2013-PI-01673-1&d=20220630172310101 (accessed September 2022).
4. Therapeutic Goods Administration. Australian Product Information. Tolak 4% once daily (fluorouracil) cream. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2021-PI-01964-1 (accessed September 2022).
5. Werner RN, Sammain A, Erdmann R, Hartmann V, Stockfleth E, Nast A. The natural history of actinic keratosis: a systemic review. Br J Dermatol 2013; 169: 502-518.
6. Criscione VD, Weinstock MA, Naylor MF, Luque C, Eide MJ, Bingham SF; Department of Veteran Affairs Topical Tretinoin Chemo-prevention Trial Group. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs topical tretinoin chemoprevention trial. Cancer 2009; 115: 2523-2530.
7. Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol 2000; 42(1 Pt 2): 23-24.
8. Guorgis G, Anderson CD, Lyth J, Falk M. Actinic keratosis diagnosis and increased risk of developing skin cancer: a 10-year cohort study of 17,651 patients in Sweden. Acta Derm Venereol 2020; 100: adv00128.
9. Drugbank. Fluorouracil. Available online at: https://go.drugbank.com/drugs/DB00544 (accessed September 2022).
10. Ceilley RI. Mechanisms of action of topical 5-fluorouracil: review and implications for the treatment of dermatological disorders, J Dermatolog Treat 2012; 23: 83-99.
11. Pomerantz H, Hogan D, Eilers D, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) Trial Group. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol 2015; 151: 952-960.
12. Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) Trial Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol 2018; 154: 167-174.