Finerenone: a new option for diabetic kidney disease
Finerenone is a novel nonsteroidal mineralocorticoid receptor antagonist that became available on the PBS in July 2023 for selected patients with chronic kidney disease and type 2 diabetes. It is indicated as an addition to standard care to delay progressive decline of kidney function and reduce the risk of cardiovascular mortality and morbidity in these patients.
Globally, 500 million people are estimated to have diabetes, with over 90% of these having type 2 diabetes.1 In Australia, type 2 diabetes affects almost 1.2 million people, and 125 new cases are diagnosed per day.2 Diabetic kidney disease is the most common cause of kidney failure, accounting for 37% of incident cases in 2021, with a five-year survival rate of less than 50%.3 In diabetic kidney disease, albuminuria is associated with an increased risk of decline in kidney function and cardiovascular events.4 As early chronic kidney disease (CKD) is often asymptomatic, with an estimated 80% of people with stage 3 CKD not yet diagnosed, there is a strong need to proactively screen at-risk patients in primary care.5
The standard of care to slow progression of CKD with albuminuria is blockade of the renin-angiotensin system (RAS) with an ACE inhibitor or angiotensin receptor blocker (ARB). However, this is only partially effective, and it has long been hypothesised that blockade of mineralocorticoid receptors in addition to RAS inhibition may enhance treatment to slow CKD progression.6-10
Finerenone is a novel nonsteroidal mineralocorticoid receptor antagonist (MRA) that was approved by the TGA in 2021 to slow progression of CKD and reduce cardiovascular risk in selected patients with CKD and type 2 diabetes.11 It was made available on the PBS from 1 July 2023.12 This review provides a practical update on the role of finerenone in the management of patients with diabetic kidney disease.
What are mineralocorticoid receptor agonists?
The mineralocorticoid hormone aldosterone is produced by the adrenal glands in response to stimuli such as reduced renal perfusion, increased levels of the hormone angiotensin II and hyperkalaemia. It has a central role in maintaining homeostasis by upregulating blood pressure and total body water and sodium. Aldosterone binds to mineralocorticoid receptors in the collecting duct cells of the renal cortex, leading to luminal sodium reabsorption and potassium excretion. Mineralocorticoid receptors are also expressed in vascular, endothelial and inflammatory cells and fibroblasts. In experimental studies, aldosterone blockade reduced proteinuria and nephrosclerosis in preclinical models of CKD, independent of the RAS.6-10 In addition, long-term RAS blockade with an ACE inhibitor or ARB incompletely suppresses serum aldosterone levels (termed ‘aldosterone escape’), and overactivation of mineralocorticoid receptors results in myocardial and renal fibrosis.6-10
The classical steroidal MRAs spironolactone and eplerenone have been available in Australia since 1960 and 2005, respectively. Spironolactone has a half maximal inhibitory concentration (IC50) for mineralocorticoid receptor blockade about 40-fold lower than that of eplerenone, whereas eplerenone has much less off-target affinity than spironolactone for glucocorticoid, androgen and progesterone receptor inhibition. Eplerenone is therefore deemed a selective MRA, with a lower risk of gynaecomastia, impotence, decreased libido, breast pain and menstrual irregularities.13
Multiple clinical trials have shown that both spironolactone and eplerenone reduce mortality related to heart failure with reduced ejection fraction (HFrEF).14,15 They also reduce albuminuria in patients with CKD and are effective in treating patients with resistant hypertension and primary hyperaldosteronism. However, no long-term trials have evaluated kidney-related outcomes with steroidal MRAs, and the increased risk of hyperkalaemia and acute kidney injury in patients with CKD have reduced enthusiasm for their clinical use in this population.4,16
What is finerenone?
Finerenone is a novel selective nonsteroidal MRA with an IC50 for mineralocorticoid receptor inhibition similar to that of spironolactone, but reduced affinity for glucocorticoid, androgen and progesterone receptor inhibition similar to eplerenone. Studies to date have not found the same sex hormone-related adverse events with finerenone as are found with steroidal MRAs. This has made it an appealing candidate to evaluate the role of concurrent mineralocorticoid receptor blockade in cardiorenal protection in patients with diabetic kidney disease.6,8,11
What is the evidence supporting finerenone?
The effect of finerenone in patients with type 2 diabetes-related CKD and albuminuria was investigated in two recent multicentre randomised controlled clinical trials (Table 1).6,17,18 In the FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) trial, treatment with finerenone resulted in an 18% lower incidence of the primary kidney composite outcome of kidney failure, sustained decrease of 40% or more in the estimated glomerular filtration rate (eGFR) or death from renal causes.6 In the FIGARO- DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) trial, finerenone resulted in a 13% lower risk of the primary cardiovascular composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke or hospitalisation for heart failure.4,6,16-18
A post-hoc analysis of the two trials, FIDELITY (Finerenone in Chronic Kidney Disease and Type 2 Diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial Programme Analysis), pooled data from both trials to perform a prespecified individual patient-level combined analysis, which included 13,171 participants.18 It showed that finerenone use resulted in an 18% reduction in the composite cardiovascular outcome and a 23% reduction in a composite outcome of doubling of creatinine level (or a sustained decrease in the eGFR of 57% or more), kidney failure (defined by initiation of long-term dialysis or kidney transplantation) or kidney-related death. Additionally, there was a 20% reduction in dialysis initiation and a 22% reduction in heart failure-related hospitalisations.4,16,18
Principles of finerenone use in diabetic kidney disease
Indications to commence finerenone
As a result of high-quality evidence from the pivotal trials, the TGA approved finerenone to delay progressive decline of kidney function and to reduce the risk of cardiovascular mortality and morbidity in adults with CKD (with albuminuria) associated with type 2 diabetes, in addition to standard of care.11 However, since completion of these trials, concurrent trials of SGLT-2 inhibitors have led to these agents being strongly recommended as a first-line drug therapy to prevent CKD progression and adverse cardiovascular outcomes in patients with diabetic kidney disease by the American Diabetes Association and Kidney Disease: Improving Global Outcomes (KDIGO).4,16 PBS criteria for finerenone require that it be considered as a second-line agent only after RAS inhibition and SGLT-2 inhibitors have been introduced, unless medically contraindicated (Flowchart).12
Evidence on combining finerenone and SGLT-2 inhibitors
Data on the combination of finerenone and SGLT-2 inhibitors are limited. In the two pivotal trials, only 877 participants (5 to 10% of the total participants) received combined treatment. Subgroup analyses in FIDELITY suggested the renoprotective benefits of combined treatment were similar to those of finerenone alone (hazard ratio [HR] 0.63; 95% confidence interval [CI] 0.40 to 1.00), and combined treatment also reduced hyperkalaemia risk.4,16,18
A post-hoc analysis of the EMPEROR (Cardiovascular and Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction) trial had similar results. In this study, use of an MRA did not change the protective effect of empagliflozin on kidney and cardiovascular outcomes, and the risk of hyperkalaemia was reduced (HR 0.70; 95% CI 0.47 to 1.04).19 Further clinical trials are in progress comparing the combination of empagliflozin and finerenone versus monotherapy with either drug alone in diabetic kidney disease (ClinicalTrials.gov identifier NCT05254002).
Steroidal vs nonsteroidal MRAs
Steroidal MRAs have established clinical indications in patients with HFrEF, primary hyperaldosteronism or refractory hypertension.4 Although the FIDELIO-DKD and FIGARO-DKD trials showed the cardiovascular and renal benefits of finerenone use in patients with CKD and type 2 diabetes, they did not compare finerenone with steroidal MRAs, and patients with HFrEF were excluded from the trials. To date, only two randomised controlled phase II trials (n=1524) have compared the safety and tolerability of finerenone with those of spironolactone and eplerenone in patients with HFrEF and CKD.20,21 There are insufficient data to recommend using nonsteroidal MRAs in this patient population.22
Thus, if patients are already receiving a steroidal MRA for another indication, they should not be switched to a nonsteroidal MRA, and steroidal and nonsteroidal MRAs should not be used in combination. In patients with diabetic kidney disease who are not receiving any MRA, the more clinically significant indication should drive the choice (i.e. risk of mortality with HFrEF versus albuminuria).4
Patients with minimal albuminuria
Patients with diabetic kidney disease and minimal albuminuria may not benefit from a nonsteroidal MRA. More than 85% of the patients in the FIDELIO-DKD trial had a urine albumin-to-creatinine ratio (ACR) greater than 33.9 mg/mmol, with a median of 96.2 mg/mmol (as the trial was designed to detect the effect of finerenone on kidney composite outcomes). Subgroup analysis of patients with a lower baseline urine ACR did not show a significant difference in the kidney composite outcome with finerenone use: HR of 0.92 (95% CI 0.49 to 1.72) for ACR 3.4 to 33.9 mg/mmol (n=685); and HR of 0.97 (95% CI 0.75 to 1.25) for ACR of 96.2 mg/mmol or lower.6
Adverse effects of finerenone
Hyperkalaemia
Hyperkalaemia was the most frequently reported adverse reaction in the FIDELIO-DKD and FIGARO-DKD studies, with a frequency of 10% or more. Mild to moderate hyperkalaemia (an increase of about 0.2 mmol/L) that occurred in the first month of treatment and tended to stabilise had a twofold higher incidence in the finerenone group compared with the placebo group.6,17,23
The primary risk factors for hyperkalaemia include:9,11,23
- reduced eGFR (less than 45 mL/min/1.73 m2)
- baseline serum potassium level greater than 4.5 mmol/L
- concurrent use of RAS blockers (three- to eightfold increased risk)
- previous episodes of hyperkalaemia
- combined treatment with medications that may increase potassium levels, such as potassium-sparing diuretics (which should not be used with finerenone), potassium supplements and trimethoprim-containing antibiotics.
Clinical management to prevent hyperkalaemia is mandatory. This includes laboratory surveillance, patient education, dietary counselling and awareness of concomitant medications that may increase the risk of hyperkalaemia.9,23
Reduction in eGFR
The FIDELIO-DKD study found that an initial decrease in eGFR (mean of 2 mL/min/1.73 m2) occurred more often with finerenone use than with placebo (6.3% vs 4.7%).24 This decrease attenuated with time and was shown to be reversible after medication discontinuation.
Other common adverse effects
Other common adverse effects, with a frequency of 1 to 10%, include hyponatraemia and mild asymptomatic elevations in the serum urate level of 0.02 mmol/L.6,17
Important precautions with finerenone use
Monitoring of potassium at baseline and follow up
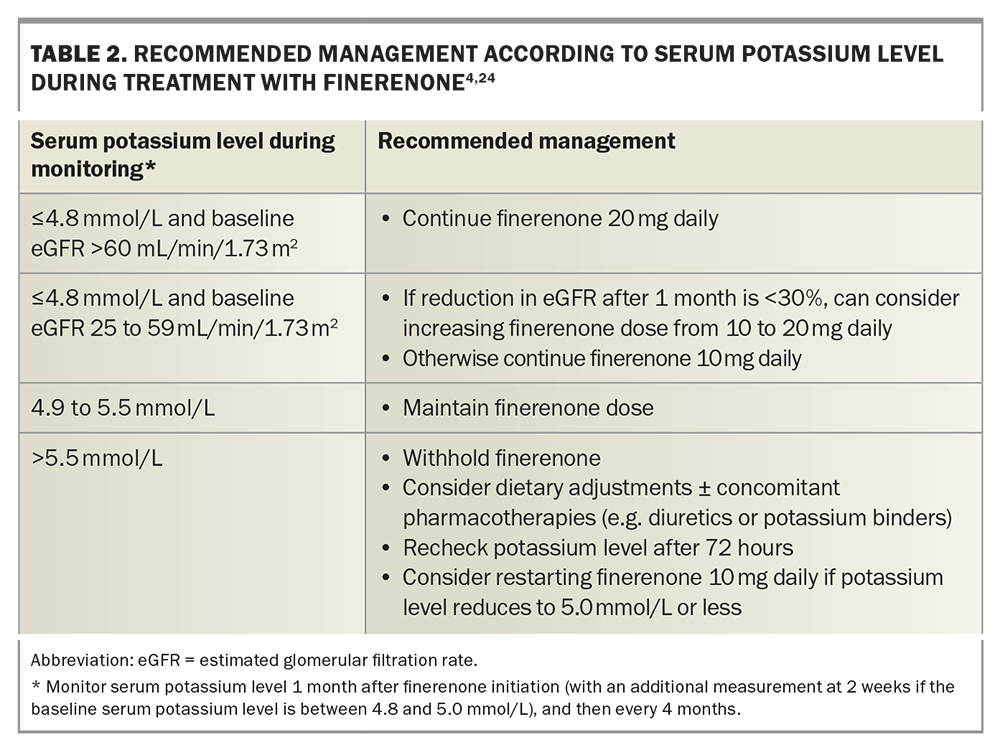
It is mandatory to measure the serum potassium level at baseline and indefinitely at follow up while a patient is taking finerenone. Finerenone is contraindicated if the baseline serum potassium level is over 5.0 mmol/L. The eGFR should be monitored along with the serum potassium level.4
The standard frequency of potassium monitoring in the FIDELIO-DKD and FIGARO-DKD trials included measurements at one, three and every four months thereafter. If the baseline serum potassium level is between 4.8 and 5.0 mmol/L, an additional, earlier measurement should be performed at two weeks.
Management according to the serum potassium level at follow up is summarised in Table 2. During follow-up treatment, finerenone should be withheld if the serum potassium level is greater than 5.5 mmol/L, and restarted at a lower dose if the potassium level is reduced to 5.0 mmol or lower on rechecking at 72 hours.16,23,24 The risk of hyperkalaemia is increased by concomitant use of potassium-sparing diuretics (e.g. amiloride, spironolactone), potassium supplements and trimethoprim-containing antibiotics, and these should be minimised or avoided.24
The importance of appropriate monitoring and management of hyperkalaemia outside a clinical trial setting was highlighted in a Canadian postmarketing surveillance of the RALES (Randomized Aldactone Evaluation Study). After publication of the trial in 1999, the rate of spironolactone prescription increased almost fivefold, with a subsequent threefold increase in the hospitalisation rate, from 2.4 to 11.0 per 1000 patients, and in the rate of hyperkalaemia-associated in-hospital death, from 0.7 to 2.0 per 1000 patients.25 Similarly to spironolactone, finerenone will likely be prescribed to an older patient population with more comorbidities than those included in the initial trials, and it is important to remain vigilant for a similar increase in adverse events with increased finerenone prescribing.
Drug interactions
Finerenone is a sensitive substrate of the cytochrome P450 3A4 enzyme (CYP3A4). Strong CYP3A4 inhibitors are therefore contraindicated, including grapefruit and medications such as itraconazole, ketoconazole, ritonavir, nelfinavir, cobicistat, clarithromycin, telithromycin and nefazodone. Patients who take moderate or weak CYP3A4 inhibitors require monitoring of the serum potassium level. Strong or moderate CYP3A4 inducers should be avoided.24
Special considerations
Considerations in prescribing finerenone in specific populations include the following.
- Older people. No dose adjustment is required in older people.
- Paediatric population. The safety and efficacy of finerenone have not been studied in patients younger than 18 years of age. Finerenone is therefore not recommended in this population.
- Pregnancy and lactation. Finerenone may be teratogenic and should be avoided during pregnancy and lactation. Women of childbearing age should be advised to use effective contraception while taking finerenone.
- Stage IV to V CKD. Finerenone can be continued if the eGFR falls to less than 25 mL/min/1.73m2 if the drug is otherwise tolerated and the serum potassium level remains within acceptable limits (Table 2). Finerenone initiation is not recommended if the eGFR is below 25 mL/min/1.73m2, because of the risk of hyperkalaemia.
- End-stage kidney disease. There is no clinical experience in the continuation of finerenone in patients with end-stage kidney disease, and this use is not recommended.
- Hepatic impairment. Finerenone should be avoided in patients with severe hepatic impairment (Child-Pugh class C). In those with mild to moderate hepatic impairment (Child-Pugh class A or B), no dose adjustment is required; however, additional monitoring of the serum potassium level and eGFR should be considered.16,24
Conclusion
For almost two decades, the treatment options for diabetic kidney disease have been confined to lifestyle management, improving diabetic control and drug treatment with an ACE inhibitor or ARB. Thus, the addition of finerenone to the PBS for the prevention of kidney failure as well as cardiovascular disease associated with diabetic kidney disease represents a major milestone, especially following so soon after the PBS listing of the SGLT-2 inhibitor dapagliflozin for the treatment of CKD.
However, many questions remain to be answered about the use of finerenone in CKD. First, based on published evidence, finerenone is appropriately a second-line treatment for patients with refractory albuminuria resistant to a maximum-dose ACE inhibitor or ARB in combination with an SGLT-2 inhibitor. It remains to be seen what proportion of patients with diabetic kidney disease will ultimately require and be eligible to receive triple therapy with an ACE inhibitor or ARB, SGLT-2 inhibitor and finerenone in real-world clinical practice, as well as the impact of increased polypharmacy on adherence.
Secondly, with regard to hyperkalaemia, further studies are needed to determine whether SGLT-2 inhibitor cotherapy reduces the risk of hyperkalaemia in the real world, as well as how this risk compares with that of the established and less costly MRA spironolactone. Thirdly, there are limited data on the use of steroidal versus nonsteroidal MRAs for both kidney and cardiovascular outcomes. At present, finerenone has not yet been tested in patients with HFrEF, and steroidal MRAs such as spironolactone and eplerenone remain the agents of choice to improve cardiovascular outcomes. Lastly, long-term data on the effect of these agents on reducing the risk of dialysis-dependent kidney failure and cardiovascular mortality due to diabetes are eagerly awaited. MT
COMPETING INTERESTS: None.
This article is for general information purposes only, and the full Product Information should be consulted before prescribing any of the mentioned medications.
References
1. International Diabetes Federation. Facts & figures. Available online at: https://idf.org/about-diabetes/facts-figures/ (accessed October 2023).
2. Australian Institute of Health and Welfare (AIHW). Diabetes: Australian facts. Type 2 diabetes. Available online at: https://www.aihw.gov.au/reports/diabetes/diabetes-australian-facts/contents/how-common-is-diabetes/type-2-diabetes (accessed October 2023).
3. Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry. ANZDATA 45th annual report 2022. Chapter 1. Incidence of kidney failure with replacement therapy. Adelaide: ANZDATA Registry. Available online at: https://www.anzdata.org.au/report/anzdata-45th-annual-report-2022-data-to-2021/ (accessed October 2023).
4. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2022; 102(5S): S1-S127.
5. Australian Institute of Health and Welfare (AIHW). Chronic kidney disease: Australian facts. Last updated 30 June 2023. Available online at: https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease/contents/treatment-and-management-of-chronic-kidney-disease/general-practice-and-primary-health-care (accessed October 2023).
6. Bakris GL, Agarwal R, Anker SD, et al; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219-2229.
7. Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 2014; (4): CD007004. Update in: Cochrane Database Syst Rev 2020; 10: CD007004.
8. Shavit L, Lifschitz MD, Epstein M. Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: current concepts and emerging treatment paradigms. Kidney Int 2012; 81: 955-968.
9. Barrera-Chimal J, Lima-Posada I, Bakris GL, Jaisser F. Mineralocorticoid receptor antagonists in diabetic kidney disease - mechanistic and therapeutic effects. Nat Rev Nephrol 2022; 18: 56-70.
10. Epstein M. Aldosterone blockade: an emerging strategy for abrogating progressive renal disease. Am J Med 2006; 119: 912-919.
11. Therapeutic Goods Administration, Australian Government Department of Health and Aged Care. Public summary. Summary for ARTG entry: 350772 KERENDIA finerenone 10 mg film-coated tablet blister pack. Canberra: Commonwealth of Australia (accessed October 2023).
12. Pharmaceutical Benefits Scheme. Finerenone. Available online at: https://www.pbs.gov.au (accessed October 2023).
13. Bärfacker L, Kuhl A, Hillisch A, et al. Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem 2012; 7: 1385-1403.
14. Pitt B, Zannad F, Remme WJ, et al; Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709-717.
15. Montalescot G, Pitt B, Lopez de Sa E, et al; REMINDER Investigators. Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: the Randomized Double-Blind Reminder Study. Eur Heart J 2014; 35: 2295-2302.
16. de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2022; 102: 974-989.
17. Pitt B, Filippatos G, Agarwal R, et al; FIGARO-DKD Investigators. Cardiovascular events with finerenone in kdney disease and type 2 diabetes. N Engl J Med 2021; 385: 2252-2263.
18. Agarwal R, Filippatos G, Pitt B, et al; FIDELIO-DKD and FIGARO-DKD investigators. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022; 43: 474-484.
19. Ferreira JP, Zannad F, Pocock SJ, et al. Interplay of mineralocorticoid receptor antagonists and empagliflozin in heart failure: EMPEROR-Reduced. J Am Coll Cardiol 2021; 77: 1397-1407.
20. Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 2013; 34: 2453-2463.
21. Filippatos G, Anker SD, Böhm M, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J 2016; 37: 2105-2114.
22. Chung EY, Ruospo M, Natale P, et al. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 2020; 10: CD007004.
23. Rossing P, Caramori ML, Chan JCN, et al. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney Int 2022; 102: 990-999.
24. Bayer Australia Ltd. Australian product information. Kerendia (finerenone) film-coated tablets. Sydney: Bayer Australia Ltd; 2023.
25. Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004; 351: 543-551.