Preventing diabetic kidney disease progression: an update
It is important to implement treatments to delay and prevent diabetic kidney disease progression by aiming for tight metabolic and blood pressure control and simultaneously addressing cardiovascular risk factors aggressively. Using pharmacological agents with cardiovascular and renal benefits, including renin–angiotensin system inhibitors and sodium-glucose cotransporter-2 inhibitors, is paramount. Glucagon-like peptide-1 receptor agonists may also be renoprotective.
Correction
A correction for this article appears in the September 2023 issue of Medicine Today (see link here). The online version and the full text PDF of this article have been corrected.
Update
An update of this article has been published in the November 2023 issue of Medicine Today and is available here.
- In patients with type 2 diabetes, screen for diabetic kidney disease (DKD) by measuring the albumin to creatinine ratio in an early-morning spot urine sample and measuring the serum creatinine level to calculate the estimated glomerular filtration rate (eGFR).
- A diagnosis of DKD should be made if repeat testing confirms an elevated albumin to creatinine ratio (greater than 2.5 mg/mmol in men or greater than 3.5 mg/mmol in women) or an eGFR less than 60 mL/min/1.73 m2.
- Delay the progression of DKD by aiming for glycaemic (general HbA1c target: less than 53 mmol/mol) and blood pressure (general target: less than 130/80 mmHg) control.
- Use single-agent renin–angiotensin system inhibitors at maximally tolerated doses and, when appropriate, sodium-glucose cotransporter-2 inhibitors and finerenone to slow the progression of DKD.
- Treat cardiovascular risk factors aggressively in patients with DKD.
The incidence of diabetes is increasing worldwide, secondary to the marked increase in the incidence of type 2 diabetes. Diabetes is also an established and major risk factor for the development and progression of chronic kidney disease (CKD). As a result, diabetes is now the leading cause of end-stage kidney disease in Western countries. Traditionally, diabetic kidney disease (DKD) has been referred to as ‘diabetic nephropathy’, but this is a term that should be reserved for people with progressive albuminuria. There is now growing appreciation that renal impairment can develop in people with diabetes in the absence of increasing albuminuria and in the presence of other nondiabetes-related causes of CKD. Therefore, the term DKD is now preferred to describe CKD in people with diabetes.1
DKD occurs in 25 to 40% of people with type 1 or type 2 diabetes who also have risk factors, including hyperglycaemia, hypertension, a history or current habit of smoking, poor plasma lipid control and a genetic predisposition.2 People who have DKD have increased rates of morbidity and mortality, mainly because of the high risk of cardiovascular (CV) events.3,4 In parallel, renal failure also predisposes to poorer glycaemic control (both hyper- and hypoglycaemia) because of an altered renal physiology, which disrupts normal glucose and insulin metabolism. Therefore, in people with diabetes, the early identification of DKD and implementation of effective treatments are imperative to minimise the progression of CKD and its associated comorbidities.
However, the good news is that novel effective treatments to help prevent the progression of DKD in people with type 2 diabetes have been developed over the past decade. Ample evidence indicates that optimising metabolic health and using renin-angiotensin system inhibitors and sodium-glucose cotransporter-2 (SGLT-2) inhibitors can delay the progression of DKD. The novel mineralocorticoid receptor antagonist finerenone is also emerging as a promising approach to slowing loss of renal function in people with type 2 diabetes. This article focuses primarily on DKD management in people with type 2 diabetes.
Screening for diabetic kidney disease
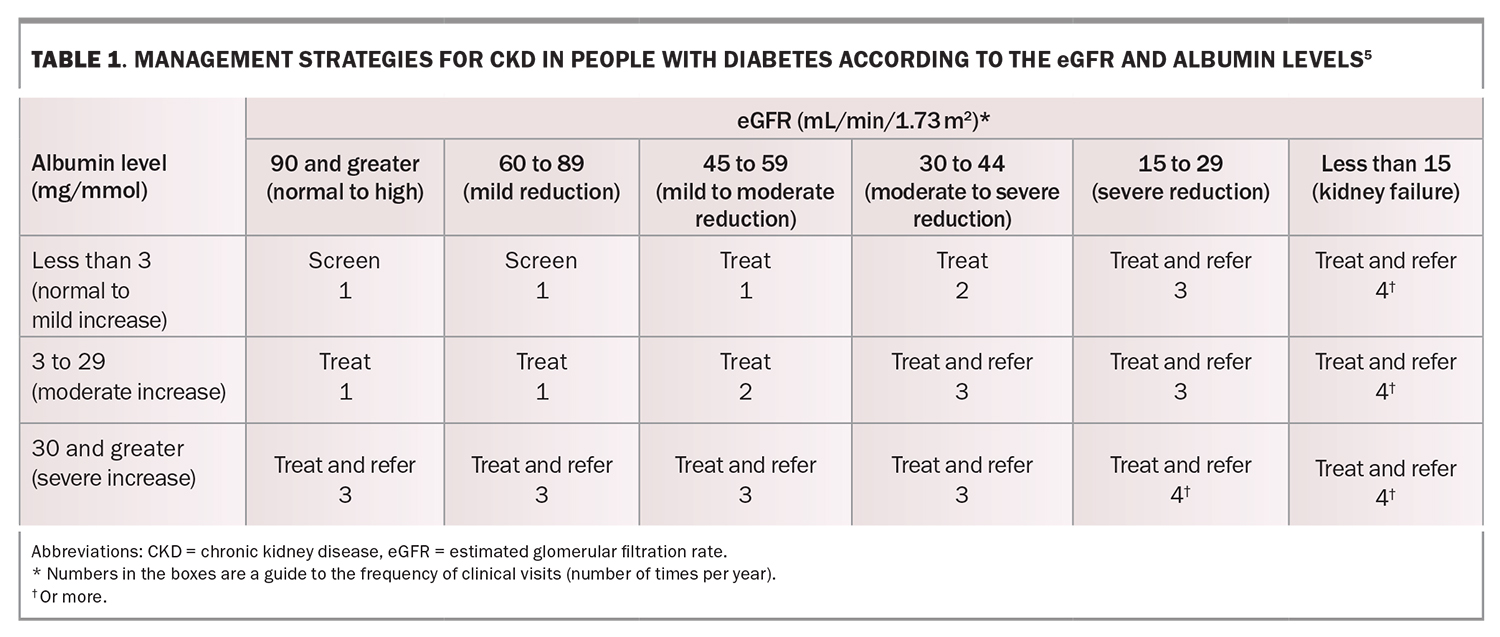
Early-stage DKD is usually a silent disease, with minimal clinical manifestations until the patient has progressed to an advanced stage of kidney disease. Therefore, it is essential to screen for markers of kidney disease in people with diabetes as part of routine clinical care (Table 1).5
A spot urine sample should be collected annually to measure the albumin to creatinine ratio. A diagnosis of DKD should only be made if repeat testing confirms an elevation in this ratio (greater than 2.5 mg/mmol in men or greater than 3.5 mg/mmol in women). The urine albumin to creatinine ratio fluctuates by 30 to 40% on the basis of several factors, such as the presence of a fever, dehydration, vigorous physical activity or urinary tract infection. Therefore, if an abnormal albumin to creatinine ratio is found, perform one to two more tests over the subsequent three months to ensure persistent albuminuria.
Serum creatinine levels should be measured to calculate the estimated glomerular filtration rate (eGFR). A persistent eGFR less than 60 mL/min/1.73 m2 indicates possible DKD, even in the absence of albuminuria if nondiabetes-related causes of CKD are excluded.
In type 2 diabetes, the timing of onset is often difficult to determine; therefore, screening should begin at the time of diagnosis. In type 1 diabetes, the general approach is to start screening for DKD five years after diagnosis.6 However, earlier screening is suggested in people with poor metabolic control or other risk factors.
Screening for nondiabetic kidney disease
Careful consideration should be given to nondiabetic causes of CKD, particularly in patients with rapidly increasing albuminuria or decreasing eGFR. Factors that should alert clinicians to possible nondiabetic aetiologies include an absence of retinopathy, duration of diabetes less than five years, acute renal injury pattern of renal dysfunction rather than gradual progression, presence of haematuria or other systemic disease and presence of nephrotic syndrome (albuminuria greater than 3 g/day, low serum albumin level, oedema and symptoms such as frothy urine). The absence of retinopathy and a short duration of diabetes are the strongest predictors of nondiabetes-related kidney disease, although DKD may still occur in some people without retinopathy.
A rapidly decreasing eGFR or rapidly increasing albuminuria should prompt referral of the patient to a nephrologist. A general cut-off of the eGFR of less than 30 mL/min/1.73 m2 can also be used as a guide for referral, but ideally, the severity of albuminuria should also be taken into account, which may warrant the involvement of a nephrologist before arriving at this eGFR cut-off.5 Referral of the patient to a nephrologist is important, as complications such as volume overload, anaemia, electrolyte imbalances and CKD-related mineral and bone disorders can become prominent management issues when the eGFR declines to below the cut-off. Timely initiation of iron replacement, epoetin and phosphate-binding treatments are important considerations. Furthermore, early preparation for pre-emptive kidney or possible combined kidney and pancreas transplantation for type 1 diabetes is beneficial, as is early preparation for kidney replacement therapy.
Preventing the progression of diabetic kidney disease
Multimorbidity is common in people with DKD and, therefore, there is an increasing emphasis on comprehensive, holistic medical care to improve overall patient outcomes.5 This approach incorporates treatment directed to optimise lifestyle factors, targeted pharmacological therapy to preserve end-organ function and additional therapies to address risk factors such as glycaemia, hypertension and dyslipidaemia.
Management of lifestyle factors
The newest guidelines from the American Diabetes Association and Kidney Disease Improving Global Outcomes (KDIGO) highlight the role of patient education in nutritional management from dietitians for optimal diabetes management. The recommendation is for patients to consume balanced diets high in vegetables, fruits and whole grains but low in refined carbohydrates and sugar-containing sweetened beverages.7,8
Although there is some evidence that low protein diets can slow the progression of DKD, this approach is rarely used in clinical practice. People with advanced-stage kidney disease on maintenance dialysis are advised to have a higher protein intake, considering that this population is often malnourished or in a catabolic state. Moderate to intense physical activity for more than 150 min/week and the avoidance of sedentary activity is also recommended.7,8 Tobacco should not be consumed by any person with diabetes. Alcohol consumption should be limited.6
Glycaemia management
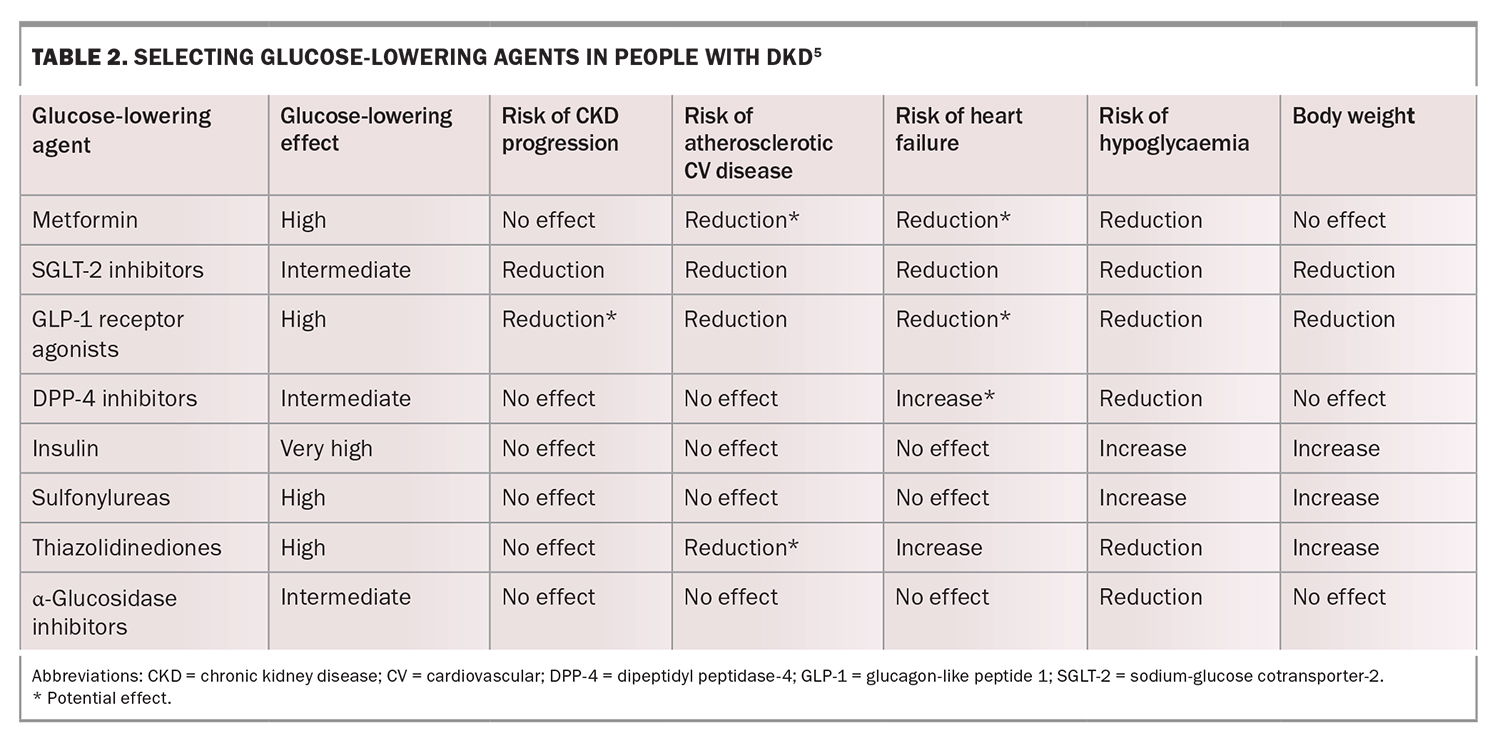
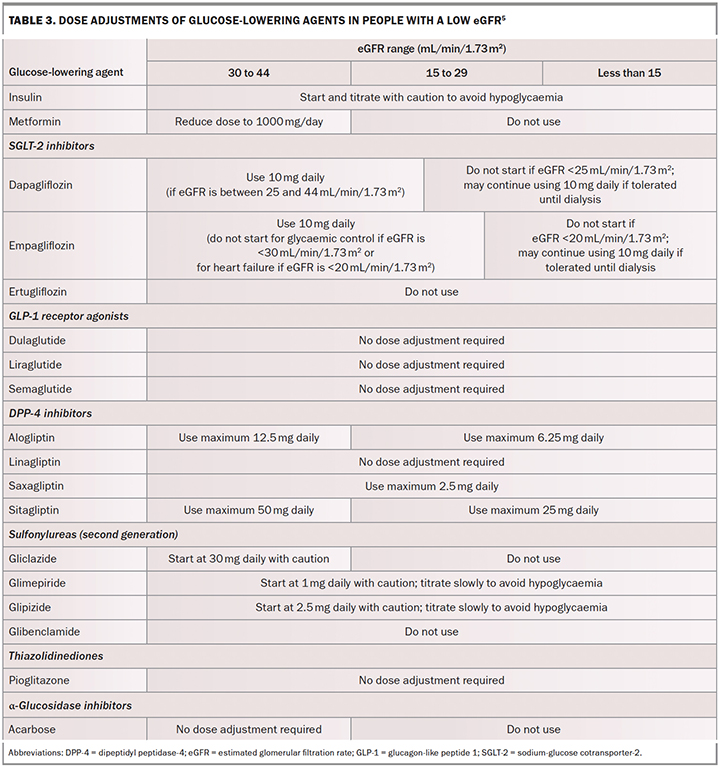
Intensive glycaemic management (glycated haemoglobin [HbA1c] less than 53 mmol/mol) delays the development and progression of albuminuria and slows the rate of eGFR decline and progression to end-stage kidney disease. Early initiation of metformin treatment plus an SGLT-2 inhibitor is recommended in most people with type 2 diabetes, followed by the addition of glucose-lowering agents as needed to achieve individualised glycaemic targets (Table 2).5 Dose adjustment and medication choice based on the eGFR (Table 3) is important to take into consideration.5
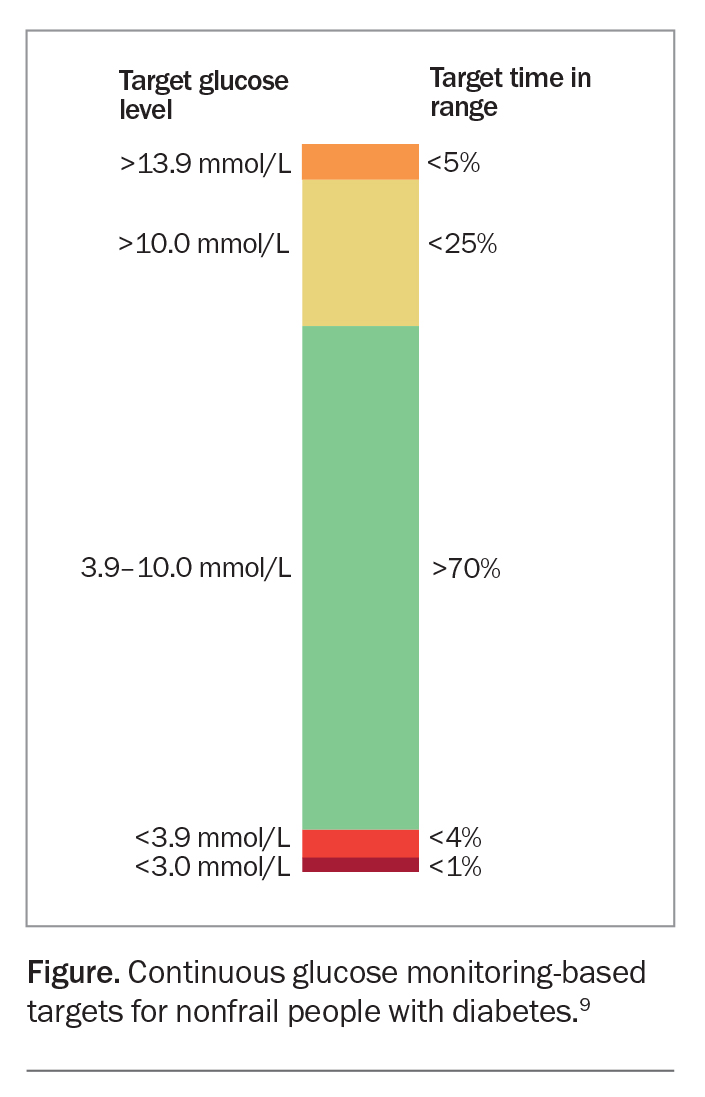
Diabetes technologies, including continuous glucose monitors, are increasingly becoming a part of routine clinical management, particularly for type 1 diabetes, for which they are subsidised under the National Diabetes Services Scheme. Continuous glucose monitoring technology is a powerful tool to identify and correct glycaemic derangements, prevent hypoglycaemia, direct medication management and guide medical nutritional therapy and physical activity recommendations. Continuous glucose monitoring technology with and without insulin pump therapy is an emerging attractive approach to help insulin-treated people with DKD optimise their glycaemic control.
Recent recommendations from the Joint British Diabetes Societies suggest the use of continuous glucose monitors, where available, in patients on dialysis as the best way to monitor glucose control (Figure).9,10 When continuous glucose monitoring technology can be used, there is an emphasis on looking at the time in range, with the range defined as 3.9 to 10.0 mmol/L, rather than using an HbA1c target. The target time in range is greater than 70% for most people with diabetes. This should be achieved while minimising the time in range for hypoglycaemia, aiming for less than 4% in a range of less than 3.9 mmol/L.9 At the time of writing, there are no specific targets for people with DKD.
Blood pressure management
A crucial goal for the prevention of CKD progression, CV disease and heart failure is managing blood pressure. For people with diabetes, hypertension and a high risk of atherosclerotic CV disease (10-year risk greater than 15%), a blood pressure target of less than 130/80 mmHg is suggested. Previously, for those with diabetes, hypertension and a low risk of atherosclerotic CV disease (10-year risk less than 15%), a blood pressure target of less than 140/90 mmHg was the recommendation.8 The general blood pressure target for people with diabetes has now been revised to 130/80 mmHg by the American Diabetes Association, and the systolic blood pressure target recommended by KDIGO for patients with CKD not on dialysis is less than 120 mmHg.11 Therefore, lowering systolic blood pressure levels to less than 130 mmHg in most people with DKD and to less than 120 mmHg in high-risk patients may be a reasonable approach. The blood pressure targets should be individualised and account for possible adverse outcomes, such as postural hypotension, which is particularly relevant in people with concurrent autonomic neuropathy.
Renin–angiotensin system inhibitors are the preferred initial pharmacological antihypertensive agents. It is important that these medications are titrated to maximally tolerated approved doses. They have been shown to decrease the risk of CKD, as well as slowing the progression to end-stage kidney disease in people with a reduced eGFR and macroalbuminuria. Calcium channel blockers are suggested as a second-line agent, followed by a diuretic as a third-line antihypertensive agent. General advice to patients to reduce salt intake by substituting with nonsalt-containing food flavourings (e.g. pepper, garlic, lemon and ginger) are also helpful lifestyle measures to lower blood pressure.
A new nonsteroidal, mineralocorticoid receptor agonist, finerenone, is available in Australia. Although its blood pressure-lowering effects appear to be less than spironalactone and eplerenone (both steroidal mineralocorticoid receptor antagonists), this drug has impressive benefits in the setting of DKD.
Management of lipid levels
Lipid-lowering agents, particularly statins, are the cornerstone of both primary and secondary prevention of atherosclerotic CV disease, which has a markedly high risk of developing in people with DKD. There are no specific lipid targets for patients with diabetes and CKD, but it is recommended to initiate statin therapy in most of these patients who are not on dialysis.12,13 By virtue of having both diabetes and CKD, this population is considered to be at high risk for CV disease. Therefore, the following targets are recommended:14
- LDL-cholesterol less than 2.0 mmol/L (or less than 1.8 mmol/L if CV disease is established)
- HDL-cholesterol greater than 1.0 mmol/L
- triglycerides: less than 2.0 mmol/L.
Proprotein convertase subtilisin/kexin type 9 inhibitors can also lower LDL-cholesterol levels and improve CV outcomes when added to statin therapy for secondary prevention.15,16 Some evidence indicates that fenofibrate, which is effective in treating hypertriglyceridaemia, attenuates albuminuria and eGFR decline in type 2 diabetes.17
Recent advances in pharmacological treatments
Sodium-glucose cotransporter-2 inhibitors
SGLT-2 inhibitors (empagliflozin and dapagliflozin) are recommended in most people with type 2 diabetes and CKD, given the strong evidence that SGLT-2 inhibitors reduce CKD progression, heart failure and the risk of atherosclerotic CV disease in people with type 2 diabetes and CKD. These effects are independent of HbA1c levels or the need for additional glucose lowering.5,18
Empagliflozin is not recommended for use in patients with an eGFR of less than 20 mL/min/1.73 m2 and dapagliflozin is not recommended for patients with an eGFR of less than 25 mL/min/1.73 m2. Although the renoprotective effects of SGLT-2 inhibitors are maintained at these eGFRs, the glucose-lowering effects of SGLT-2 inhibitors are significantly impaired at low eGFRs. The addition of other glucose-lowering therapies may therefore be required in people with low eGFRs.
Dapagliflozin is PBS listed for CKD and for heart failure with reduced ejection fraction (HFrEF), independent of diabetes status. Empagliflozin is also PBS listed for HFrEF, independent of diabetes status, but is not currently TGA approved for CKD. Both medications are TGA approved for the treatment of heart failure with preserved ejection fraction (HFpEF), but at the time of writing, neither is PBS subsidised for this indication (refer to the PBS schedule and TGA website for full details). Although the atherosclerotic CV disease risk reduction and CKD progression benefits of SGLT-2 inhibitors may be found to be a class effect in future, the evidence for ertugliflozin (a third SGLT-2 inhibitor recently deleted from the market in Australia) is less robust with further studies ongoing. Importantly, despite the evidence for use of SGLT-2 inhibitors for DKD treatment, they should not be used in patients with type 1 diabetes because of the risk of euglycaemic diabetic ketoacidosis. SGLT-2 inhibitors can be associated with diabetic ketoacidosis in patients with type 2 diabetes, particularly during times of fasting or illness. Therefore, guidelines have been developed to address appropriate SGLT-2 inhibitor withdrawal for these cases.19
Glucagon-like peptide-1 receptor agonists
Many of the large-scale CV outcome trials of glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes have included kidney disease outcomes as secondary outcomes. These studies have indicated a reduction in albuminuria or lower risk of new or worsening nephropathy and slowing of the expected eGFR decline.20-22 However, a reduction in the progression to end-stage kidney disease is yet to be demonstrated.
An ongoing trial of the GLP-1 receptor agonist semaglutide for type 2 diabetes and CKD is ongoing (clinical trial registration no. NCT03819153). In people with type 2 diabetes and advanced-stage CKD who have obesity and exceed body mass index limits required for kidney transplantation, the use of GLP-1 receptor agonists has been suggested to aid with weight loss, which may then facilitate eligibility for transplantation.
Furthermore, the TGA-approved dual glucose-dependent insulinotropic polypeptide/GLP-1 receptor agonist tirzepatide has some evidence of renal benefit, based on a post-hoc analysis of the SURPASS-4 trial findings.23 Dedicated renal outcome trials of tirzepatide are warranted.
Nonsteroidal mineralocorticoid receptor antagonist
Finerenone has recently been investigated in people with DKD (defined as an albumin to creatinine ratio of at least 3 mg/mmol and eGFR of 25 to 75 mL/min/1.73 m2) who were treated with renin–angiotensin system inhibitors. This medication was shown to reduce the risk of CKD progression and renal failure, as well as dialysis initiation, in the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease trial, which involved participants with type 2 diabetes who had albuminuria and advanced CKD.24 Furthermore, the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease trial, which involved participants with stage 2 to 4 CKD and moderately elevated albuminuria or stage 1 or 2 CKD and severely increased albuminuria, showed a reduction in CV morbidity, most prominently through a reduction in heart failure-related hospitalisations, with finerenone therapy.25 A pooled analysis of the findings of these two trials further showed the safety and efficacy of finerenone in a large cohort of patients with type 2 diabetes and CKD to reduce important renal and CV outcomes.26 Hyperkalaemia was an adverse effect in a small proportion of participants; regular monitoring of potassium levels is recommended when using this medication. Finerenone is TGA approved to delay progressive decline of kidney function in adults with CKD associated with type 2 diabetes (with albuminuria). It is also listed on the PBS for patients who fulfill the following key clinical criteria:
- an eGFR of 25 mL/min/1.73 m2 or greater
- an ACR of 22.6 mg/mmol or greater
- must be on a stable dose of either an ACE inhibitor or ARB
- must not have established heart failure with reduced ejection fraction
- must be on an SGLT-2 inhibitor unless medically contraindicated or intolerant (please refer to the full PBS clinical criteria at: https://www.pbs.gov.au/pbs/home).
Combination therapy
Some evidence suggests that combination therapy with SGLT-2 inhibitors and mineralocorticoid receptor antagonists in patients with type 2 diabetes and CKD reduces the risk of CV events and the mineralocorticoid receptor antagonist-associated risk of hyperkalaemia without a significant interaction between the two drugs.27 However, dedicated trials involving both SGLT-2 inhibitors and mineralocorticoid receptor antagonists are required to demonstrate the true benefits of combining these two classes of medications.
Of note, even before the introduction of the newer classes of medications, multifactorial, target-driven therapies addressing glycaemic, blood pressure and lipid control that incorporated renin–angiotensin system blockade, the use of statins and attention to lifestyle factors significantly reduced the progression to end-stage kidney disease by about 50% in people with type 2 diabetes, hypertension and microalbuminuria.28
Sick day management
GPs can play an important role in reinforcing the principles of sick day management, especially those related to medication use, in people with DKD. The acronym ‘SAD MANS’ is a useful mnemonic to remember medications that may have reduced clearance and an increased risk of adverse effects in people with CKD (Box). Some medications need dose adjustments or withdrawing, especially in the setting of acute illness that can result in dehydration and acute renal failure.
Most guidelines support the use of metformin in people with an eGFR of 30 mL/min/1.73 m2 or greater (although not at maximum recommended doses) and that there is a growing appreciation of temporarily withholding SGLT-2 inhibitors when patients are unwell or need to fast for a prolonged period of time to prevent the development of euglycaemic diabetic ketoacidosis.
Conclusion
Screening people with diabetes for early markers of DKD and initiating measures to slow the progression of kidney disease are part of routine clinical practice. In addition, it is necessary to measure, assess and manage CV risk factors aggressively. Attention to glycaemic, blood pressure and lipid control, as well as improving lifestyle factors (e.g. consuming a healthy diet, avoiding weight gain and undertaking regular physical activity) remain the cornerstone of management in people with DKD. The use of renin–angiotensin system inhibitors, SGLT-2 inhibitors and novel agents, such as finerenone, have also been shown to slow the progression of DKD to end-stage kidney disease and provide CV protection. There is emerging evidence for GLP-1 receptor agonists as renoprotective agents, but their role in slowing progression to end-stage kidney disease is yet to be established. Consideration of sick day management, patient education, the adjustment of glucose-lowering medications as the eGFR declines and screening for other microvascular diabetes-related complications (e.g. retinopathy and foot disease), together with a timely referral to a nephrology service, are important considerations in the optimal care of people with DKD. MT
COMPETING INTERESTS: Dr Lu and Professor O'Neal: None. Professor Ekinci has received research funding for her institution from Novo Nordisk, Eli Lilly, Sanofi, Boehringer and Versanis, Endogenex; consulting fees from Eli Lilly, Boehringer and Bayer (funds received are donated to The University of Melbourne for diabetes research); payment honoraria from Eli Lilly, Boehringer and Amgen (funds received are donated to The University of Melbourne for diabetes research); and support to attend national diabetes meetings from Eli Lilly. Professor MacIsaac was a recipient of St Vincent’s Hospital Research Endowment Fund 2021–2023 and the Australian Government’s Medical Research Future Fund (established by MTPConnect’s Targeted Transition Research Accelerator initiative); received study funding from Medtronic Pty Ltd, GlySens Pty Ltd and Insulet Pty Ltd. He was an Advisory Board Member for Astra Zeneca (2021, 2023), Novo Nordisk (2021), Boehingher Ingelheim (2022), Bayer (2021–2022) and Servier (2021); and received speaker’s fees from Boehingher Ingelheim (2021, 2023), Novo Nordisk (2021–2022), Eli Lilly (2022) and Servier (2021).
References
1. MacIsaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis 2014; 63: S39-S62.
2. Hall PM. Prevention of progression in diabetic nephropathy. Diabetes Spectrum 2006; 19: 18-24.
3. Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc 2012; 1: 8-15.
4. Groop P-H, Thomas MC, Moran JL, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009; 58: 1651-1658.
5. de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022; 45: 3075-3090.
6. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005; 28: 164-176.
7. American Diabetes Association. Introduction: standards of medical care in diabetes—2022. Diabetes Care 2022; 45: S1-S2.
8. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int, 2022; 102: S1-S127.
9. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019; 42: 1593-1603.
10. Frankel AH, Wahba M, Ashworth V, et al. Management of adults with diabetes on dialysis: Summary of recommendations of the Joint British Diabetes Societies guidelines 2022. Diabet Med 2023; 40: e15027.
11. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021; 99: S1-S87.
12. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 2014; 85: 1303-1309.
13. Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J 2010; 160: 785-794.e10.
14. Royal Australian College of General Practitioners. Management of type 2 diabetes: a handbook for general practice. East Melbourne: Victoria; 2020.
15. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379: 2097-2107.
16. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713-1722.
17. Keech A, Drury P, Davis TM, et al. Protection against nephropathy with fenofibrate in type 2 diabetes mellitus: the FIELD study. Am Heart Assoc 2009; 120: S419-S420.
18. American Diabetes Association Professional Practice Committee. 11. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care 2022; 45: S175-S184.
19. Periprocedural diabetic ketoacidosis (DKA) with SGLT2 inhibitor use in people with diabetes. Sydney: Australian Diabetes Society; 2022. Available online at: https://www.diabetessociety.com.au/guideline/periprocedural-diabetic-ketoacidosis-dka-with-sglt2-inhibitor-use-in-people-with-diabetes-july-2022/ (accessed June 2023).
20. Marso SP, Holst AG, Vilsbøll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017; 376: 891-892.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394: 121-130.
22. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
23. Heerspink HJL, Sattar N, Pavo I, et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol 2022; 10: 774-785.
24. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219-2229.
25. Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385: 2252-2263.
26. Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022; 43: 474-484.
27. Tsukamoto S, Morita R, Yamada T, et al. Cardiovascular and kidney outcomes of combination therapy with sodium-glucose cotransporter-2 inhibitors and mineralocorticoid receptor antagonists in patients with type 2 diabetes and chronic kidney disease: A systematic review and network meta-analysis. Diabetes Res Clin Pract 2022; 194: 110161.
28. Oellgaard J, Gæde P, Rossing P, Persson F, Parving HH, Pedersen O. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int 2017; 91: 982-988. Erratum in: Kidney Int 2017; 91: 1257.