Diabetic kidney disease: a new era in therapeutic management
Diabetes is associated with chronic kidney disease (CKD) and is the major cause of renal failure in Australia. New therapies are emerging to reduce the adverse cardiovascular outcomes and progression of CKD in type 2 diabetes. These therapies, including renin-angiotensin system inhibitors, sodium-glucose cotransporter-2 inhibitors, the nonsteroidal mineralocorticoid receptor antagonist finerenone and glucagon-like peptide-1 receptor agonists, should be considered for all patients with type 2 diabetes for renoprotection.
Correction
A correction for this article appears in the March 2024 issue of Medicine Today (see link here). The online version and the full text PDF of this article have been corrected.
Note
This article has been updated for National Diabetes Week 2024. The updated article is available here.
- Directed therapies for diabetic kidney disease (DKD) can lower chronic kidney disease progression and cardiovascular events.
- All patients with DKD and proteinuria should be on an ACE inhibitor or angiotensin receptor blockers (ARB) at the maximally tolerated dose.
- Sodium-glucose cotransporter-2 (SGLT-2) inhibitors should be considered for all patients with type 2 diabetes requiring glycaemic control or to further reduce proteinuria after ACE inhibitor or ARB therapy.
- Finerenone should be prescribed if there is persistent proteinuria despite use of an ACE inhibitor or ARB and SGLT-2 inhibitor therapy.
- Glucagon-like peptide-1 receptor agonists should be considered for patients requiring glycaemic control after SGLT-2 inhibitor therapy.
- If there is intolerability of treatment, patients should be referred for specialist input.
In 2021, about one in 20 individuals in Australia had diagnosed diabetes mellitus.1 Diabetes contributes significantly to the burden of chronic kidney disease (CKD) and is the leading cause of kidney failure.2 It is also a major risk factor for major atherosclerotic cardiovascular events (MACE) and death, which are significantly amplified in the presence of CKD. Novel therapies are now available to manage diabetic kidney disease (DKD) which slow the progression of CKD and reduce MACE. This article discusses current therapies for managing CKD in patients with type 2 diabetes and when they should be prescribed.
ACE inhibitors and ARBs
ACE inhibitors and angiotensin receptor blockers (ARBs) are the cornerstone of treatment for DKD. They have a profound effect on lowering proteinuria by lowering systemic blood pressure while having a direct action on the kidney microcirculation and reducing glomerular hypertension and hyperfiltration. This has a marked effect on lowering the rate of CKD progression, demonstrated over 20 years ago in the RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan) trial and IDNT (Irbesartan Diabetic Nephropathy Trial).3,4
All patients with diabetes (including those with type 1 diabetes) and hypertension or albuminuria should be considered for ACE inhibitor or ARB therapy. In patients who are normotensive, an ACE inhibitor or ARB should be started at a low dose and progressively uptitrated to avoid precipitating symptomatic hypotension. There is a direct correlation with dosing and the degree of proteinuria reduction. All patients should be on the maximally tolerated dose. These medications act in a class effect with no single ACE inhibitor or ARB being more efficacious.
The dose should be titrated at four-week intervals.5 This is the typical time to see the peak effect on blood pressure and elevations in serum creatinine and potassium levels. A rise in creatinine level up to 30% from baseline is an anticipated finding with drug initiation or dose modification and is due to the reduction in intraglomerular pressure; however, this does not warrant withdrawal of the medication. Studies have shown that this initial drop in glomerular filtration rate (GFR) stabilises within two months and leads to long-term preservation of kidney function.5
Patients should be carefully monitored for the presence of hyperkalaemia. A serum potassium level above 5.5 mmol/L should prompt a clinical review to consider the possibility of concurrent culprit medications or excessive dietary potassium intake. The use of potassium binders should be considered to facilitate ongoing treatment. ACE inhibitors and ARBs are contraindicated in pregnancy; therefore, women of reproductive age who are taking these medications should be advised to use appropriate contraception. These drugs should be discontinued in patients planning pregnancy or if an unexpected pregnancy is confirmed.
SGLT-2 inhibitors
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of drug that promotes glucose excretion in the urine by inhibiting its reabsorption in the proximal tubule. Initially designed to improve glycaemic control, they have revolutionised treatment of CKD and have conclusively shown to reduce the rate of CKD progression and MACE.6 This benefit is irrespective of weight loss, blood pressure reduction or improvement in glycaemic index associated with these drugs.
The nephroprotective benefits of SGLT-2 inhibitors are attributed to multiple factors but, primarily, to a reduction in intraglomerular hypertension. Ordinarily, sodium is reabsorbed alongside glucose in the proximal tubule. Inhibiting the reabsorption of glucose leads to sodium wasting. This increased sodium excretion is detected by the macula densa, which acts to minimise further salt wasting by causing afferent arteriole vasoconstriction. This has the effect of lowering intraglomerular pressures and reducing single nephron GFR and subsequent proteinuria. Additional mechanisms acting outside of the kidney account for the impressive cardiovascular benefits associated with these drugs.
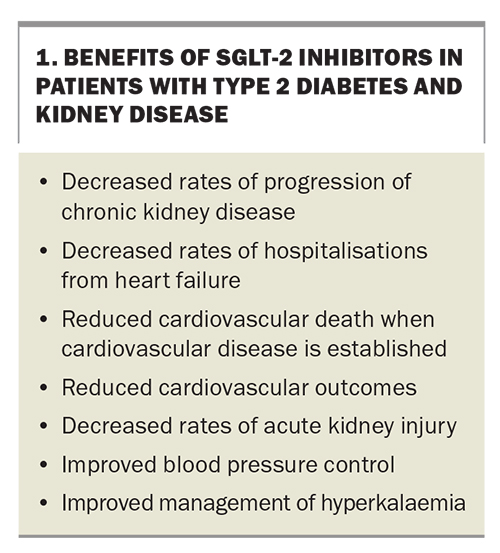
SGLT-2 inhibitors offer numerous benefits to patients with type 2 diabetes and kidney disease (Box 1). They should be prescribed for all eligible patients with type 2 diabetes only once the a maximally tolerated dose of a chosen ACE inhibitor or ARB, with or without metformin, has been achieved. Most patients with proteinuric kidney disease, irrespective of having type 2 diabetes, should also be considered candidates for SGLT-2 inhibitor therapy. Given the safety of these drugs has not been established for type 1 diabetes and their association with euglycaemic ketoacidosis, they should be avoided in this population.
The available SGLT-2 inhibitors in Australia are empagliflozin and dapagliflozin. A third SGLT-2 inhibitor, ertugliflozin, was deleted from the Australian market in mid-2023. Only dapagliflozin is PBS approved for proteinuric CKD, independent of diabetes status. Empagliflozin and dapagliflozin are TGA approved for heart failure independent of ejection fraction or diabetes status and are PBS listed for heart failure with reduced ejection fraction (HFrEF, with left ventricular ejection fraction ≤40%). Both have proven benefit in heart failure with preserved ejection (HFpEF, with left ventricular ejection fraction >40%):7,8 empagliflozin is PBS listed for HFpEF, dapagliflozin is not currently TGA approved for this indication. Refer to the PBS schedule and TGA website for full details.
The benefits of these drugs are likely class related. However, a key consideration in their use is the estimated GFR (eGFR) cut off associated with each drug. Dapagliflozin currently has the lowest eGFR cut off for the indication of CKD at above 25 mL/min/1.73 m2. Empagliflozin has an eGFR cut off of above 20 mL/min/1.73 m2, although this is for the indication of heart failure. The urine albumin to creatinine ratio should be at least 22.6 mg/mmol at initiation. However, once commenced for CKD, the SGLT-2 inhibitor should be continued until kidney replacement therapy (i.e. dialysis or transplantation) occurs.
SGLT-2 inhibitors are largely well tolerated. Notable adverse effects include a higher association with genital mycotic infections and urinary tract infections. These can be managed with education regarding good hygiene practices and the use of topical antifungal agents. Initial studies suggested a higher incidence of lower limb amputation; however, this has not been a consistent finding within a meta-analysis.9 SGLT-2 inhibitor monotherapy is associated with a low risk of hypoglycaemia, as glycosuria is minimised when serum glucose levels normalise. However, the risk increases with the addition of therapies, notably sulfonylureas and insulin, which are associated with hypoglycaemia. If appropriately considering an SGLT-2 inhibitor for cardiovascular or renal protection for a patient with well-controlled blood glucose levels, a dose reduction of their existing therapies should be considered.
All patients prescribed an SGLT-2 inhibitor should be cautioned on the possibility of euglycaemic ketoacidosis, a rare complication that may occur if patients are fasting or have an intercurrent illness. The drug should be withheld for at least two days before surgery or during periods of acute illness. For patients taking concurrent diuretics, it may be appropriate to reduce the dose of these medications given the diuretic effect associated with SGLT-2 inhibitors and the risk of precipitating hypovolaemia.
An increase in serum creatinine level of up to 30% is an expected finding and should not lead to drug withdrawal.5 This phenomenon is a result of reducing intraglomerular hypertension and is reversible if the medication is ceased.
Given the significant benefits associated with these medications, adverse side effects that lead to drug discontinuation warrant the consideration of a specialist consultation before withdrawal of treatment.
Nonsteroid MRAs
Finerenone is an oral nonsteroidal mineralocorticoid receptor antagonist (MRA) is indicated for patients with DKD. Unlike spironolactone, which is a steroidal MRA, finerenone more selectively inhibits the mineralocorticoid receptor and has limited antiandrogenic and oestrogenic effects. Overactivation of the mineralocorticoid receptor has been implicated in the progression of CKD and cardiovascular disease through the promotion of inflammation and fibrosis.8 Downregulation of the mineralocorticoid receptor has also been shown to lower proteinuria, a strong predictor for progression of CKD.
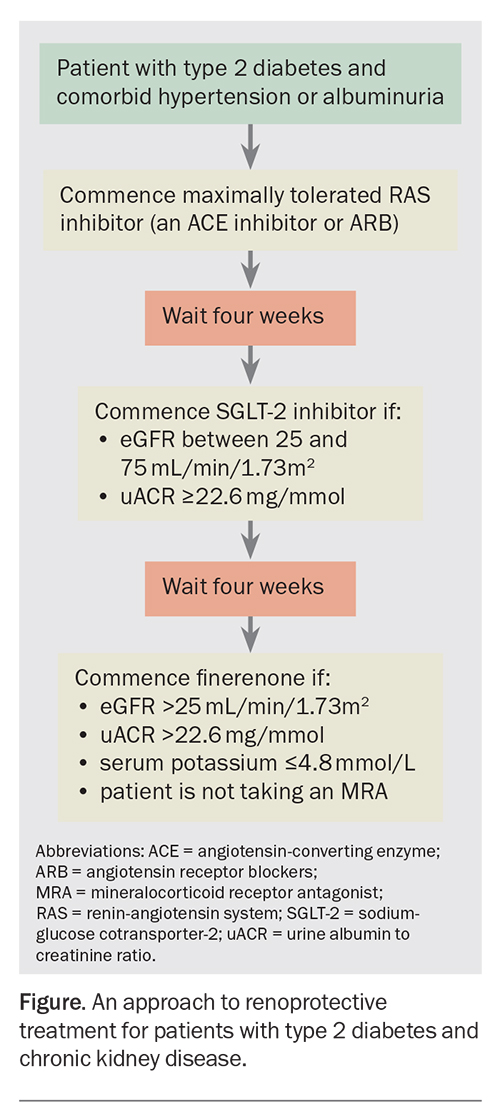
Finerenone is the most recently PBS approved therapy for DKD that reduces progression of CKD and cardiovascular events.10 Notable PBS criteria for prescribing finerenone include concurrent therapy with a renin-angiotensin system (RAS) inhibitor (an ACE inhibitor or ARB) for at least four weeks, followed by combination therapy with an SGLT-2 inhibitor and the avoidance of coadministration of other MRAs such as spironolactone. If the urine albumin to creatinine ratio remains above 22.6 mg/mmol despite these therapies, finerenone can be prescribed. Finerenone should not be commenced in patients with a serum potassium level above 5.0 mmol/L and should be avoided in women who are pregnant or planning pregnancy as well as in those who are breastfeeding due to a lack of safety data. An approach to renoprotective treatment for patients with type 2 diabetes and CKD, including prescription requirements for finerenone, are outlined in the Figure.5
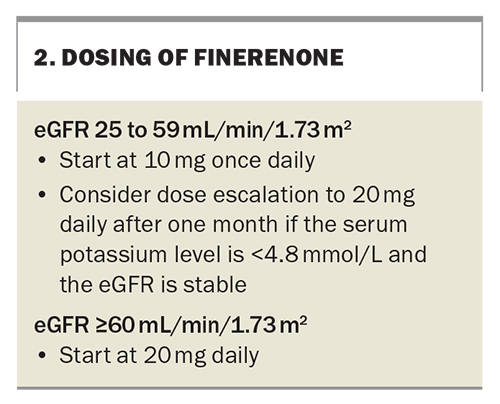
The dosing of finerenone is dependent on renal function (Box 2). Patients with an eGFR between 25 and 59 mL/min/1.73 m2 should be started on 10 mg finerenone daily and the dose uptitrated. For those with an eGFR 60 mL/min/1.73 m2 or higher, treatment should start at 20 mg daily. Finerenone is contraindicated in people with an eGFR below 25 mL/min/1.73 m2.
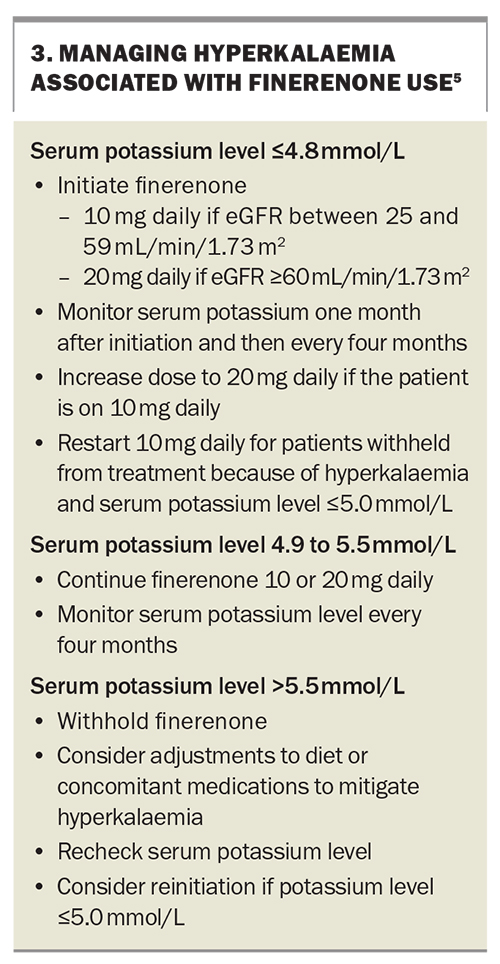
Following commencement, patients should be monitored for hyperkalaemia (Box 3). This should initially occur at one month and subsequently every four months. A dose reduction is required for patients with serum potassium levels between 4.9 and 5.5 mmol/L and it should be withheld for those with a potassium level above 5.5 mmol/L. Similar to other medications known to be protective in CKD, finerenone can cause a slight elevation in the serum creatinine level on commencement. A rise in the serum creatinine level above 25% should lead to consultation with a nephrologist.
The most common side effect associated with finerenone is hyperkalaemia. Most incidents of hyperkalaemia can be managed with treatment pauses of 72 hours, given its short drug half-life, or the use of oral potassium binders. In particular, patiromer is PBS listed for patients with stage 3 and 4 CKD with confirmed hyperkalaemia (serum potassium level above 6 mmol/L). See PBS website for full details (www.pbs.gov.au). The use of potassium binders may facilitate the use of finerenone in selected patients. Unlike other MRAs, finerenone does not significantly impact blood pressure and, given its nonsteroidal nature, is not associated with gynaecomastia.
GLP-1 receptor agonists
Glucagon-like peptide-1 (GLP-1) receptor agonists are used to improve glycaemic control in people with type 2 diabetes. GLP-1 is a hormone secreted from the intestine after the ingestion of glucose. It acts on the pancreas to stimulate the glucose-dependent release of insulin and inhibit glucagon release. This has the net effect of improving glycaemic index. It additionally acts to delay gastric emptying, reducing appetite and leading to weight loss.
The role of GLP-1 receptor agonists in the management of type 2 diabetes has been established, with compelling evidence showing marked cardiovascular benefits associated with treatment. A 2021 systemic review of eight trials demonstrated that GLP-1 receptor agonist use was associated with a reduction in cardiovascular death, stroke, myocardial infarction, hospitalisation for heart failure and all-cause mortality in patients with type 2 diabetes.11 Although benefits were seen in DKD, the trials were not selected to assess benefits in CKD.
However, the recent FLOW study primarily assessed for a kidney outcome and demonstrated a reduction in the progression of CKD and MACE (Clinical trial ID NCT03819153). The study included patients with type 2 diabetes who had an eGFR between 50 and 75 mL/min/1.73 m2 and urine albumin to creatinine ratio above 33 and below 565 mg/mmol, or an eGFR between 25 and 50 mL/min/1.73 m2 and urine albumin to creatinine ratio above 11.3 and below 565 mg/mmol. Overwhelming efficacy led to the trial being stopped early by the data safety monitoring committee. The key findings of the FLOW trial are expected later in 2024.
The major adverse effects associated with GLP-1 receptor agonist use are nausea and vomiting. These can be minimised by starting on the lowest dose with progressive uptitration as required for improved glycaemic control.
Conclusion
DKD is the leading cause of renal failure in Australia and a significant risk factor for cardiovascular disease. Until recent years, treatment options for managing this disease were limited. Current therapies represent an exciting opportunity to not only minimise the rate of CKD progression, but also reduce cardiovascular events. All patients with diabetes should be screened for DKD and, where possible, should be considered candidates for therapy. Specialist input should always be sought if there are any concerns with drug tolerability. Practice points for managing DKD are summarised in Box 4. MT
COMPETING INTERESTS: Dr Kolovos: None. Professor Pollock has received fees for consultation and lectures from Astra Zeneca, Bayer and Boehringer Ingelheim; and support for attending meetings from Astra Zeneca and Boehringer Ingelheim.
References
1. Australian Institute of Health and Welfare (AIHW). Diabetes: Australian facts. AIHW; Canberra, 2022. Available online at: https://www.aihw.gov.au/reports/diabetes/diabetes/contents/summary (accessed February 2024).
2. Australia and New Zealand dialysis and transplant registry. Annual Report 2022 Chapter 1: Summarising the number of incident patients with kidney failure with replacement therapy in Australia and New Zealand, the rate per million population and the demographic and clinical characteristics of incident patients ANZDATA; Adelaide, 2022. Available online at: https://www.anzdata.org.au/wp-content/uploads/2023/02/c01_incidence_2021_ar_2022_v1.0_FINAL.pdf (accessed February 2024).
3. Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New Engl J Med 2001; 345: 851-560.
4. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New Engl J Med 2001; 345: 861-869.
5. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney International 2022; 102. Available online at: https://kdigo.org/wp-content/uploads/2022/10/KDIGO-2022-Clinical-Practice-Guideline-for-Diabetes-Management-in-CKD.pdf (accessed February 2024).
6. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023; 388: 117-127.
7. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022; 387: 1089-1098.
8. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385: 1451-1461.
9. Huang CY, Lee JK. Sodium‐glucose co‐transporter‐2 inhibitors and major adverse limb events: a trial‐level meta‐analysis including 51 713 individuals. Diabetes Obes Metab 2022; 22: 2348-2355.
10. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219-2229.
11. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021; 9: 653-662.