Psoriasis – presentation, types and management
Psoriasis is more than just a skin condition: it occurs as a result of a combination of genetic, immune and environmental factors. Psoriasis ranges from mild to severe, and acute to chronic. Chronic psoriasis is associated with numerous comorbidities, including psoriatic arthritis, inflammatory bowel disease, metabolic syndromes, and issues related to cardiovascular, respiratory and mental health. Optimal management includes correct early diagnosis and treatment that considers chronic comorbidities.
Correction
A correction for this article appears in the March 2024 issue of Medicine Today (see link here). The online version and the full text PDF of this article have been corrected.
- Psoriasis is considered an autoimmune disease, occurring as a result of genetic and environmental factors, and is associated with an increased morbidity and mortality.
- Psoriasis can vary in its onset, appearance and severity, and careful assessment and investigation are needed to differentiate subtypes from similar dermatological conditions.
- Psoriatic arthritis (PsA) is a common comorbidity and should be assessed in any patient with psoriasis.
- Patients and doctors need to be aware of the range of comorbidities associated with chronic psoriasis, including PsA, metabolic syndrome, obesity and diabetes.
- A range of systemic and biologic therapies are available for the treatment of psoriasis, many of which are PBS listed.
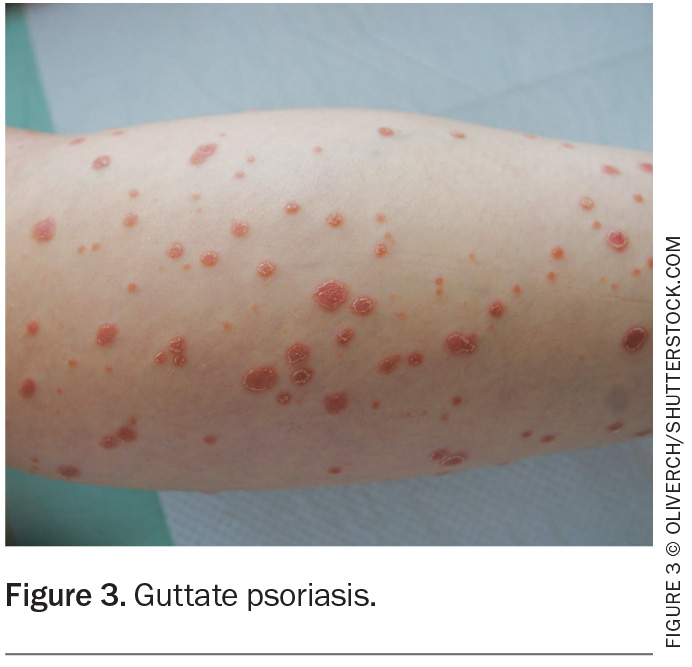
Psoriasis is a heterogeneous immune-mediated skin condition with a range of skin and variable extracutaneous manifestations and associations. Psoriasis affects 1 to 3% of the population. It has a bimodal distribution of age of onset that peaks from 16 to 22 years and 57 to 60 years.1 The most common presentation is chronic plaque type psoriasis (CPP). Other main types include guttate, inverse, pustular and erythrodermic (Table 1).
Pathophysiology of psoriasis
Psoriasis is the result of a complex interplay between an individual’s genetics, their immune system and the environment. Both the innate and adaptive immune systems are involved, including dendritic cells, lymphocytes, interleukins (IL)-23 and -17, tumor necrosis factor (TNF)-alpha and interferon gamma. IL-23 activates T-helper 17 cells, which excrete IL-17A. Elevation of IL-17A levels also occurs through other pathways, involving mast cells, innate lymphocytes and neutrophils. IL-17A excretion results in keratinocyte activation and proliferation with epidermal hyperplasia.
About 30% of patients with psoriasis have a first-degree relative who is affected. There is no one gene implicated in psoriasis. Multiple chromosomal loci have been identified, including psoriasis susceptibility regions (PSORS)1-9, which have been found in patients with psoriasis and other conditions such as Crohn’s disease. The allele HLA-C*06:02 is located on the PSORS1 locus and associated with early onset psoriasis, but not with late onset or psoriatic arthritis (PsA).2
Classification of psoriasis
Chronic plaque psoriasis
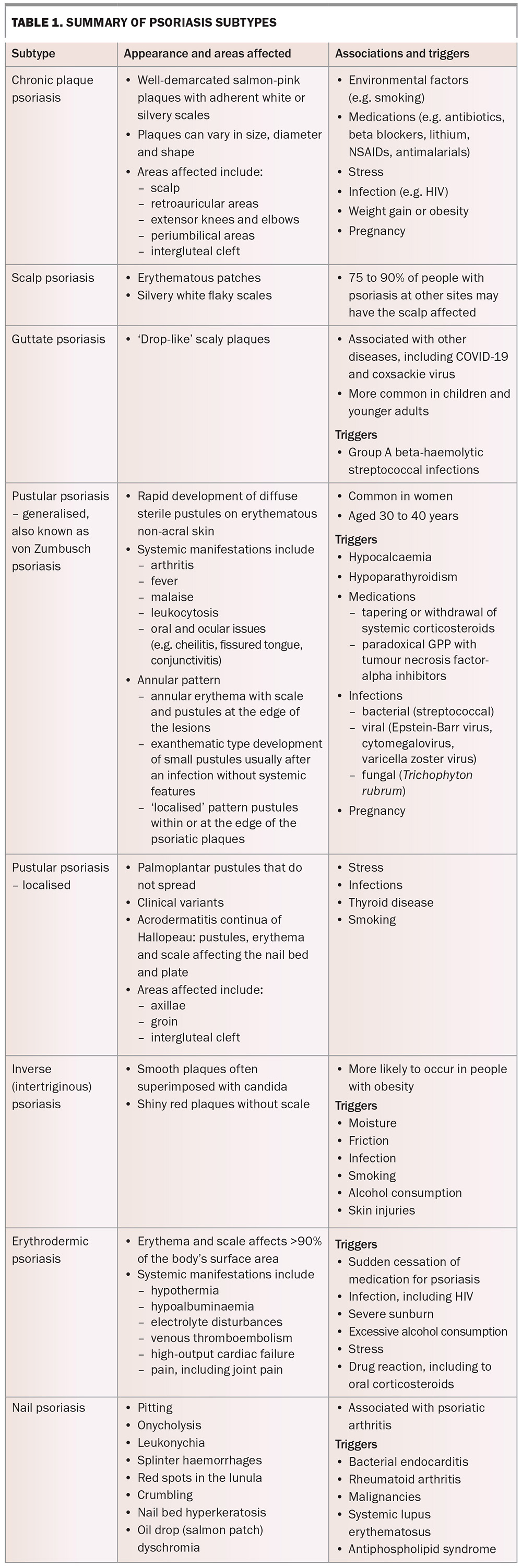
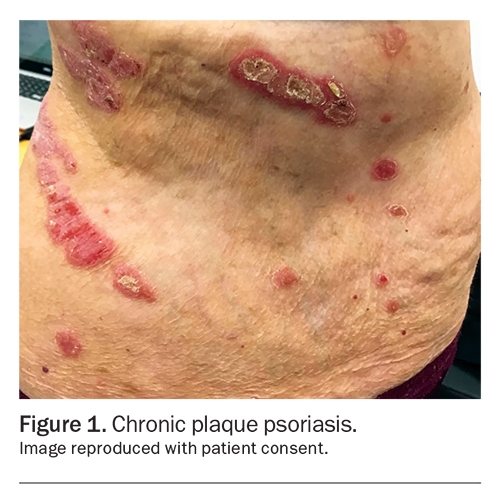
CPP or psoriasis vulgaris is the most common type of psoriasis, accounting for 90% of all cases. It is characterised by well-demarcated salmon-pink plaques with adherent white or silvery scales (Figure 1). The plaques can vary in size, diameter and shape. Typical locations affected include the scalp, retroauricular and periumbilical areas, extensor surface of elbows and knees, and the intergluteal cleft.1 CPP can be triggered by environmental factors (e.g. smoking), medications (e.g. antibiotics, beta blockers, lithium, NSAIDs, antimalarials), stress, infection (including HIV), weight gain, obesity and pregnancy.2 Scalp psoriasis affects about 75 to 90% of patients with psoriasis (Figure 2).3
Psoriasis is associated with the Koebner phenomenon, whereby new areas of psoriasis occur at sites of injury, such as after a fall, scratch, tattoo or surgery. Psoriasis is on a spectrum with sebopsoriasis or seborhoeic dermatitis, the latter presenting with greasy scaly erythematous plaques on the glabella, eyebrows, nasolabial folds, postauricular area and the chest.
Guttate psoriasis
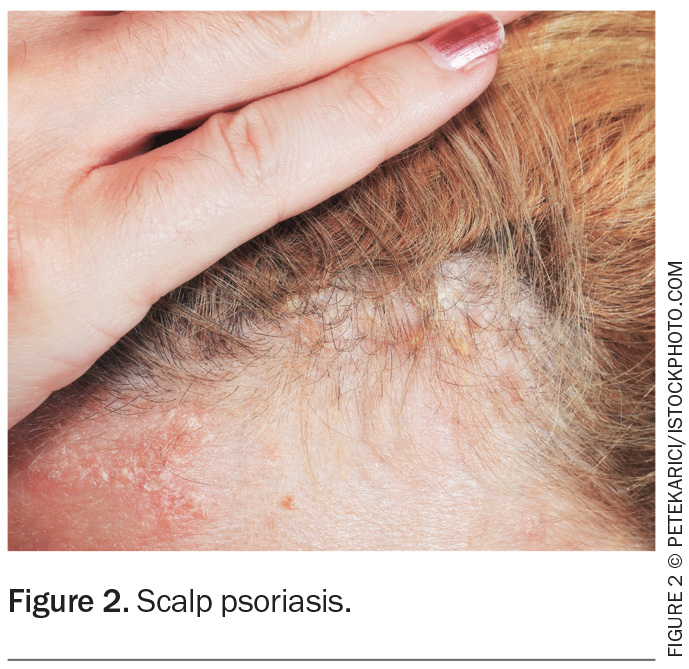
Guttate psoriasis is a variant of psoriasis that is triggered by group A beta-haemolytic streptococcal infections. It is more common in younger adults and children and is characterised by ‘drop-like’ scaly plaques (Figure 3). It typically occurs one to two weeks after a streptococcal infection (e.g. pharyngitis or perianal streptococcus) but is also associated with other diseases, including COVID-19 and coxsackie virus. Guttate psoriasis usually resolves within four months, although a third of patients develop CPP.1
Pustular psoriasis
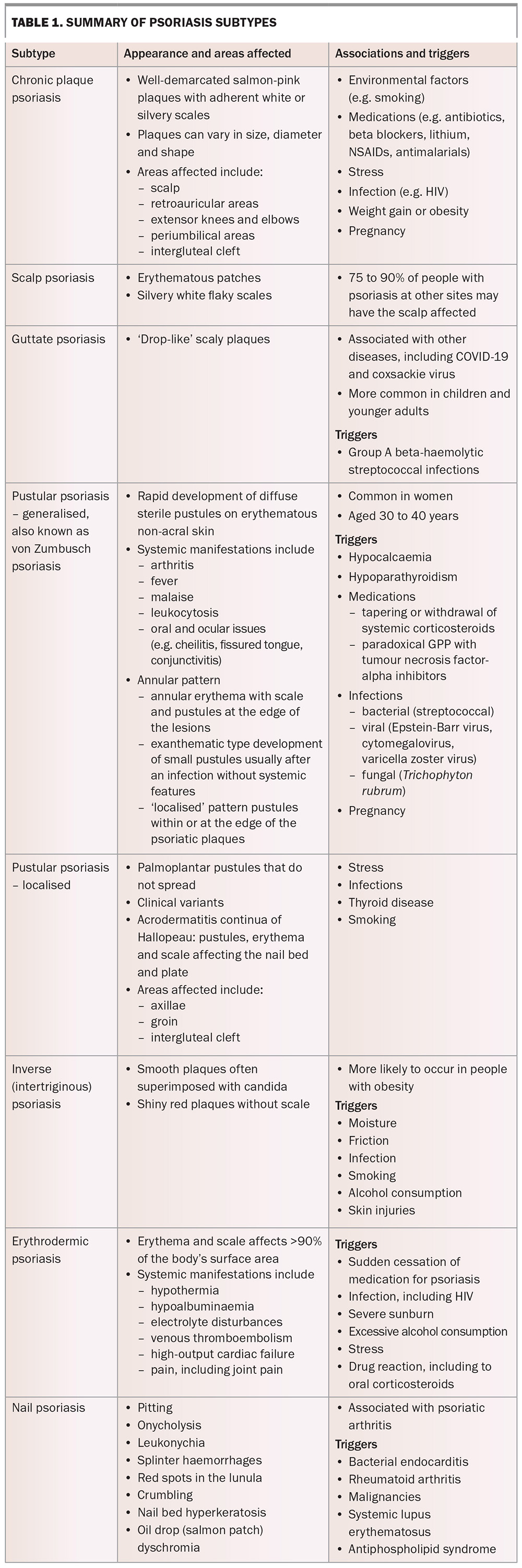
Pustular psoriasis can be categorised as either localised or generalised. Acute generalised pustular psoriasis (GPP) (von Zumbusch psoriasis) is a rare, potentially life-threatening disease characterised by the rapid development of diffuse sterile pustules on erythematous non-acral skin. It is more common in women and typically occurs in the 30-to-40-year age group. Systemic manifestations include arthritis, fever, malaise and leukocytosis, and some patients develop oral and ocular issues (e.g. cheilitis, fissured tongue, conjunctivitis). Some patients have pre-existing CPP. Triggers for acute GPP include hypocalcaemia, hypoparathyroidism, medications, infections and pregnancy (Table 1).4
Other clinical variants of GPP include annular pattern (annular erythema with scale and pustules at the edge of the lesions), exanthematic type (development of small pustules usually after an infection without systemic features); and ‘localised’ pattern where there are pustules within or at the edge of the psoriatic plaques (Figure 4).
The differential diagnoses of GPP include: acute generalised exanthematous pustulosis (AGEP), which is often a clinical manifestation of a widespread drug reaction; varicella zoster infection; tinea corporis; or IgA pemphigus. Investigations to exclude differential diagnoses and confirm the diagnosis include:
- fungal scraping for microscopy and culture
- serology testing for hypocalcaemia, hypothyroidism and hypoparathyroidism
- skin biopsy
- bacterial, viral and fungal swabs.
Genetic testing has recently become available for the IL36RN gene. Variants in IL36RN are associated with more widespread GPP and earlier onset of disease without a history of CPP.5
The Generalised Pustular Psoriasis Physician Global Assessment (GPPGA) and Generalised Pustular Psoriasis Area and Severity Index (GPPASI) can be used alongside the other disease measures and patient-reported outcomes (e.g. Psoriasis Area Severity Index or Dermatology Life Quality Index) to specifically assess GPP severity; however, they need to be further validated.6,7
Localised pustular psoriasis is usually characterised by palmoplantar pustules (also known as palmoplantar pustulosis) that do not spread elsewhere. Stress, infections, thyroid disease and smoking may trigger or exacerbate this condition. Acrodermatitis continua of Hallopeau is a rare localised form of pustular psoriasis, with pustules, erythema and scale affecting the nail bed and plate.
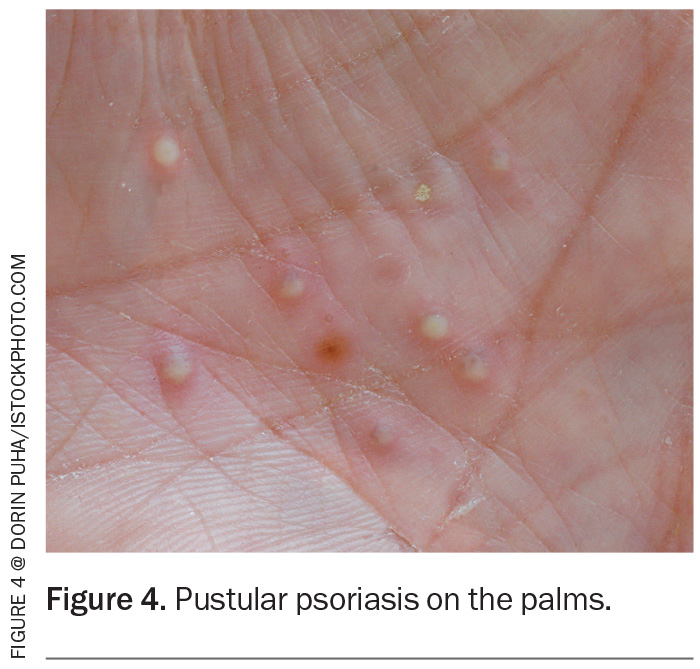
Inverse psoriasis
Flexural psoriasis, also known as inverse psoriasis, affects the folds such as the axillae, groin and intergluteal cleft (Figure 5). Plaques can be smooth and superimposed with candida. It can present with shiny red plaques without scale and is often misdiagnosed as dermatitis, tinea or candida.
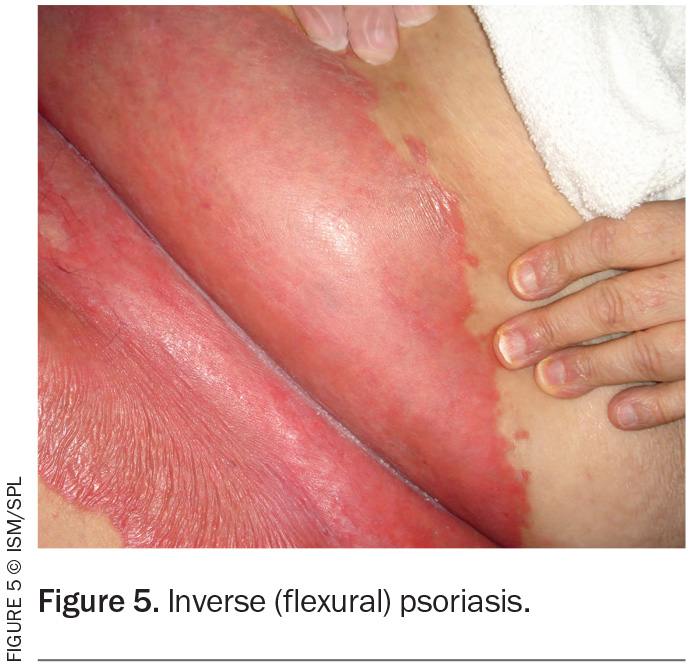
Erythrodermic psoriasis
Erythrodermic psoriasis is a potentially life-threatening condition where more than 90% of the body’s surface area is affected with erythema and scale (Figure 6). It can be associated with systemic complications including hypothermia, hypoalbuminaemia, electrolyte disturbances, venous thromboembolism, high-output cardiac failure and pain. Hospital admission is typically required for temperature regulation, intravenous fluids and directed skin management.
Other locations
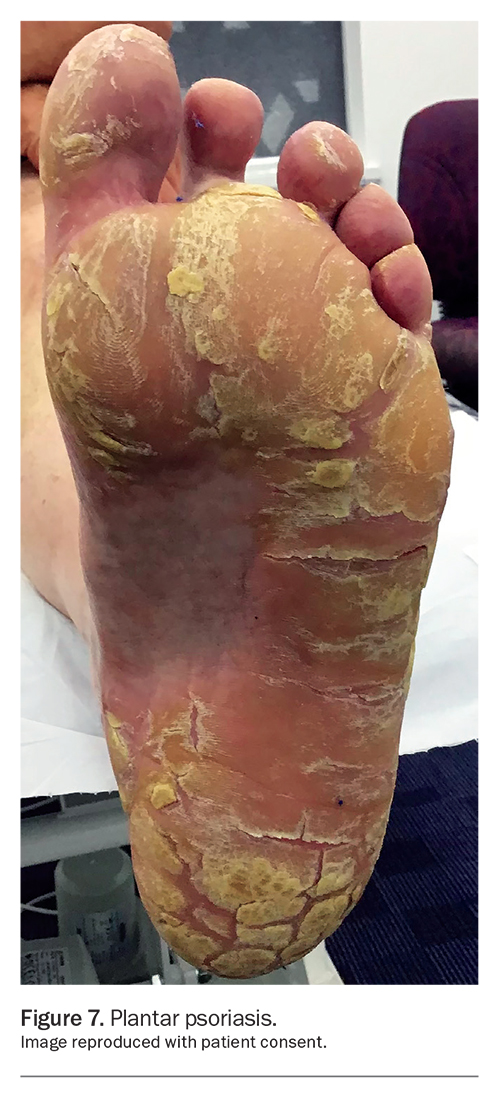
Psoriasis can be categorised according to the affected location, such as genital psoriasis, palmoplantar psoriasis (Figure 7), scalp psoriasis or flexural psoriasis. About 63% of patients with psoriasis have genital involvement at some stage, which can be a cause of embarrassment, emotional distress and physiological abnormalities. Consequently, these can impact on relationships and quality of life (QOL).8 The thinness of the skin, its predisposition to irritation and the potential side effects of medication should be taken into consideration when treating genital psoriasis. Patients often do not disclose genital involvement and its impact, and may be self-treating the area with antifungals. Therefore, it is important to specifically ask about genital involvement and examine the area if appropriate.
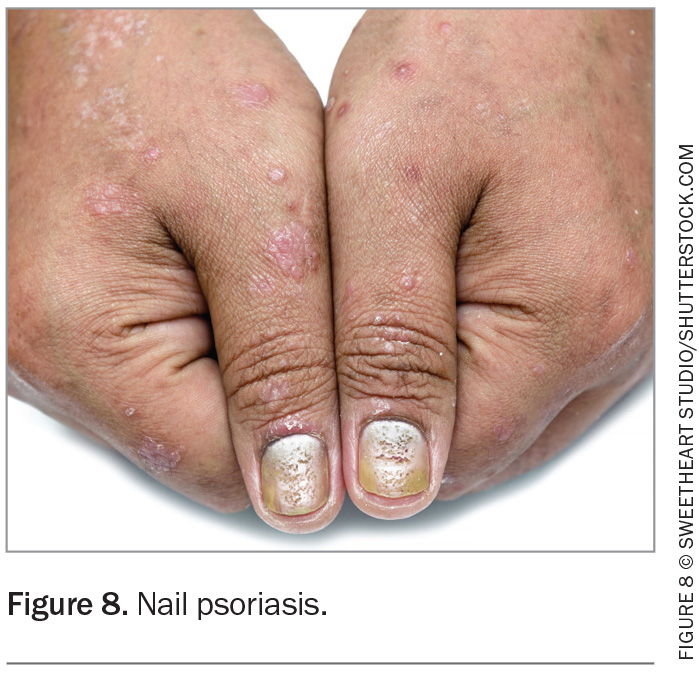
The features of nail psoriasis include pitting, onycholysis, leukonychia, splinter haemorrhages, red spots in the lunula, crumbling, nail bed hyperkeratosis and oil drop (salmon patch) dyschromia (Figure 8). The Nail Psoriasis Severity Index (NAPSI) is a tool used in specialised centres to assess the effect of psoriasis on the nail matrix and plate. The nail is divided into four areas and scores are given depending on the psoriatic involvement of each quadrant. Nail psoriasis is associated with PsA, so it is imperative to examine the nails when seeing a patient with psoriasis.
Comorbidities of psoriasis
Many diseases are associated with CPP, including PsA, metabolic syndrome, obesity, diabetes, cardiovascular and respiratory disease, inflammatory bowel disease, and mental health disorders. A full list of comorbidities are presented in the Box. The risk of developing metabolic syndrome increases with the severity of psoriasis.
When treating psoriasis, it is important to consider it as a chronic systemic disease, rather than a pure skin issue. PsA occurs in up to 42% of patients with psoriasis and is characterised by morning stiffness that lasts 30 minutes. CPP can precede PsA by more than 10 years, which provides ample opportunities to detect joint disease early, before permanent disfigurement or arthritis mutilans.9 Simple screening questionnaires, such as the psoriasis epidemiology screening tool (PEST), psoriatic arthritis screening and evaluation (PASE) questionnaire and Early Psoriatic Arthritis Screening questionnaire (EARP) can be used to identify patients who require referral to a rheumatologist.10 Alternatively, it is worth asking every patient who has psoriasis if they have symptoms of PsA or have had swelling of the fingers or toes (indicating dactylitis) or heel pain for enthesitis.
Guttate psoriasis is typically associated with a streptococcal pharyngitis, although perianal streptococcal infections triggering guttate psoriasis have been reported.11 There is no evidence to date that anti-streptococcal therapies reduce the cutaneous manifestations.
Pustular psoriasis is associated with pregnancy, medication use (including a paradoxical reaction to TNF-inhibitors) and the withdrawal of oral or systemic corticosteroids. Palmoplantar pustulosis is associated with smoking and clinically improves with its cessation.
Obesity is an independent risk factor for developing psoriasis and PsA. Psoriasis worsens with weight gain and improves with weight loss. A higher body mass index is associated with a lack of improvement of psoriasis with treatment, as obesity curtails the effect of systemic agents and biologics.12 As psoriasis becomes more severe, the risk of type 2 diabetes increases, and patients with type 2 diabetes who have psoriasis are less likely to respond to biologic agents than those without type 2 diabetes.
Patients with psoriasis are 2.9 times more likely to develop Crohn’s disease than the general population. Patients with severe psoriasis are almost three times more likely to develop heart failure and 1.8 times more likely to get atherosclerosis. Cardiovascular disease is one of the most common causes of death in people with severe psoriasis.
Genetic and environmental factors (e.g. infections, medications, pregnancy) induce immune dysregulation, resulting in proinflammatory cytokine development and messenger system activation with subsequent cytokine differentiation and chronic inflammation. Psoriasis is a chronic inflammatory state. The same cytokines that are elevated in psoriasis are present in and associated with other comorbidities. The ‘psoriatic march’ is the notion of a causal link between psoriasis and cardiovascular disease. Systemic inflammation results in insulin resistance leading to endothelial dysfunction, then atherosclerosis and, eventually, myocardial infarcts and cerebrovascular disorders. Recognising psoriasis as a systemic disorder can help identify associated comorbidities, improve treatment and reverse the impact of psoriasis on quality of life. Changing the approach to psoriasis management and introducing systemic agents early may reduce widespread inflammation, resulting in reduced morbidity and mortality.
The cytokines TNF-alpha and IL-17 are present in people with psoriasis, as well as in those with cardiovascular disease, diabetes, PsA and inflammatory bowel disease. Elevated levels of TNF-alpha are also found in those with obesity. Biologic agents targeting these two cytokines in cases of severe psoriasis may reduce systemic inflammation, possibly reducing the severity of these comorbidities.13
Investigations for psoriasis
Many skin conditions have a similar morphology to psoriasis. These include eczema (atopic dermatitis, irritant or allergic contact dermatitis), tinea (e.g. tinea corporis, manuum or capitis), candidiasis, cutaneous lymphoma (e.g. mycosis fungoides), lupus, dermatomyositis, lichen planus, pityriasis rosea, pityriasis rubra pilaris, syphilis and papulosquamous drug eruptions.
Psoriasis is typically a clinical diagnosis, although investigations (e.g. fungal scraping, bacterial and viral swabs, blood tests and skin biopsies) may be performed to rule out other diagnoses or confirm the diagnosis. The classic histological features of psoriasis are hyperkeratosis, acanthosis, hypogranulosis (loss of the granular layer), dilated vessels in the papillary dermis, elongated rete ridges and thinning of the suprapapillary plates.
Measure severity
Because psoriasis can significantly impact QOL, objective and subjective severity scores have been developed to compare the severity and monitor the effects of treatment. There can be discrepancy between the clinical severity and impact on QOL, with significant impact from minimal cutaneous disease. The Dermatology Life Quality Index (DLQI) is a general tool used to assess the effect of a skin disease on the activities of daily living, work or school, and how it makes the patient feel. The score ranges from 0 to 30, with a higher score indicating a greater impact on QOL. Psoriasis is associated with a poor QOL similar to that of cancer and myocardial infarction.3
Psoriasis Area and Severity Index (PASI) is an objective tool that measures disease severity and extent, with scores ranging between 0 and 72, and is often used to evaluate the change in psoriasis severity after a treatment. It is part of the PBS criteria to assess eligibility for a biologic medication. The final score is based on regional subscores from the head and neck, upper limbs, trunk and lower limbs. Erythema, induration and scale are evaluated in each region and assigned a score between 0 and 4, which is multiplied according to the body surface area affected for that region. In Australia, patients with a PASI score above 15 are deemed to have severe psoriasis. The PASI response is often used in clinical trials to report improvement from baseline PASI scores after treatment.
Other patient reported outcomes such as Psoriasis Symptom Scale (PSS) and Pain Visual Analogue Scale (pain VAS) are available. The Physician Global Assessment (PGA) in psoriasis is a general measure of disease.
Treatments for psoriasis
The goal of treatment is to alleviate symptoms and improve appearance and QOL. 70 to 80% of patients with psoriasis have mild disease that can be treated with topical therapies.3 Education about the disease, its comorbidities and effect on self-esteem and QOL should be addressed on a patient and community level. Lifestyle changes, such as modification of metabolic risk factors (including weight loss), smoking cessation and reducing alcohol intake, are essential and should not be underestimated. Mental health support should also be provided.
Topical therapies
Topical corticosteroids (TCSs) are used as first-line therapy. The location and extent of skin psoriasis determines the TCS used. Scalp disease is often treated with lotion, such as mometasone, while truncal skin is treated with more potent creams or ointments. A weak TCS or topical calcineurin inhibitors are used for facial psoriasis. Calcipotriol, a vitamin D analogue, is used in a combination betamethasone diproprionate/calcipotriol ointment or foam, which is effective for skin and scalp psoriasis. Occlusion of topical therapies with a dressing can increase their efficacy.
Other topical preparations include topical salicylic acid (a keratolytic agent), dithranol or tar. Intralesional triamcinolone acetonide or betamethasone acetate injections into resistant plaques are often successful.
Scalp psoriasis can be very difficult to treat and is associated with pain, pruritus, impaired QOL and alopecia. Topical therapies can be frustrating and cause cosmetic concerns, especially the use of tar-based products, which stain and have an unpleasant odour.
Systemic therapies
Systemic therapies are traditionally used for widespread disease, such as for patients with more than 10% body surface area affected or in those who do not respond or cannot tolerate topical treatments.
Phototherapy or ultraviolet (UV) therapy has been used to treat psoriasis for years. This includes psoralens and UVA as already defined UV, narrow band UVB (NB-UVB) and broad band UVB (BB-UVB). NB-UVB is usually performed at a dermatology practice three times a week and the UV dose is increased according to a protocol based on the minimal erythema dose or the patient’s Fitzpatrick skin phototype. The potential complications of phototherapy include short-term effects, such as burning, erythema, pruritus, tanning and photokeratitis, and longer-term photoageing.
Other systemic agents include oral therapies. These can be categorised into immunosuppressives, and include ciclosporin or methotrexate, or nonimmunosuppressive agents such as acitretin and apremilast. Methotrexate is usually administered orally; however, subcutaneous preparations, which have less gastrointestinal side effects and possibly better efficacy, are available for those who cannot tolerate the oral form.2 Methotrexate is associated with nausea, abdominal discomfort, teratogenicity, bone marrow suppression and hepatotoxicity, which need to be monitored regularly. A dose of 15 to 20 mg per week with folic acid resulted in a PASI 75 response (greater than 75% reduction in PASI) rate of 36% by week 16.2
Ciclosporin is an oral calcineurin inhibitor with a rapid onset of action. The duration of treatment is limited, sometimes to a year, due to nephrotoxicity. Other side effects include hypertension, gastrointestinal side effects, hypomagnaesaemia, gingival hyperplasia and drug interactions.
Acitretin is a retinoid used to reduce the hyperkeratosis and is effective in palmoplantar psoriasis. Acitretin is a teratogen and should be avoided in women of childbearing age. The side effects include xerosis, cheilitis, photosensitivity and hyperlipidaemia.
Apremilast is a phosphodiesterase-4 inhibitor given at a dose of 30 mg twice a day which reduces the proinflammatory cytokines. It is safe in patients with liver disease, but the dose needs to be reduced in patients with renal failure. Nausea, weight loss and diarrhoea are common side effects.
Biologic therapies
Biologic medications have been around for almost 20 years and have transformed the therapeutic landscape. They are available on the PBS for psoriatic conditions for patients who fulfill the following criteria:
- are older than 18 years of age
- have a PASI score above 15
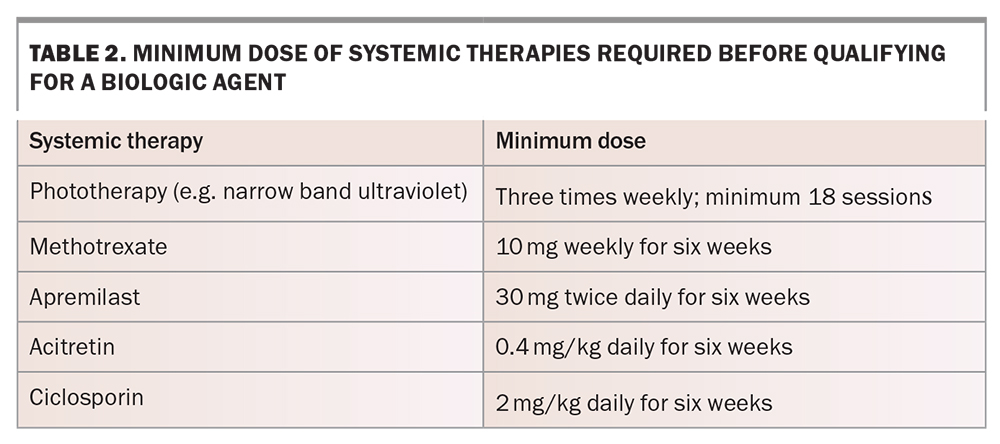
- have failed to respond after six weeks of treatment, or have a contraindication or significant adverse event, to two out of the five traditional systemic agents (Table 2).
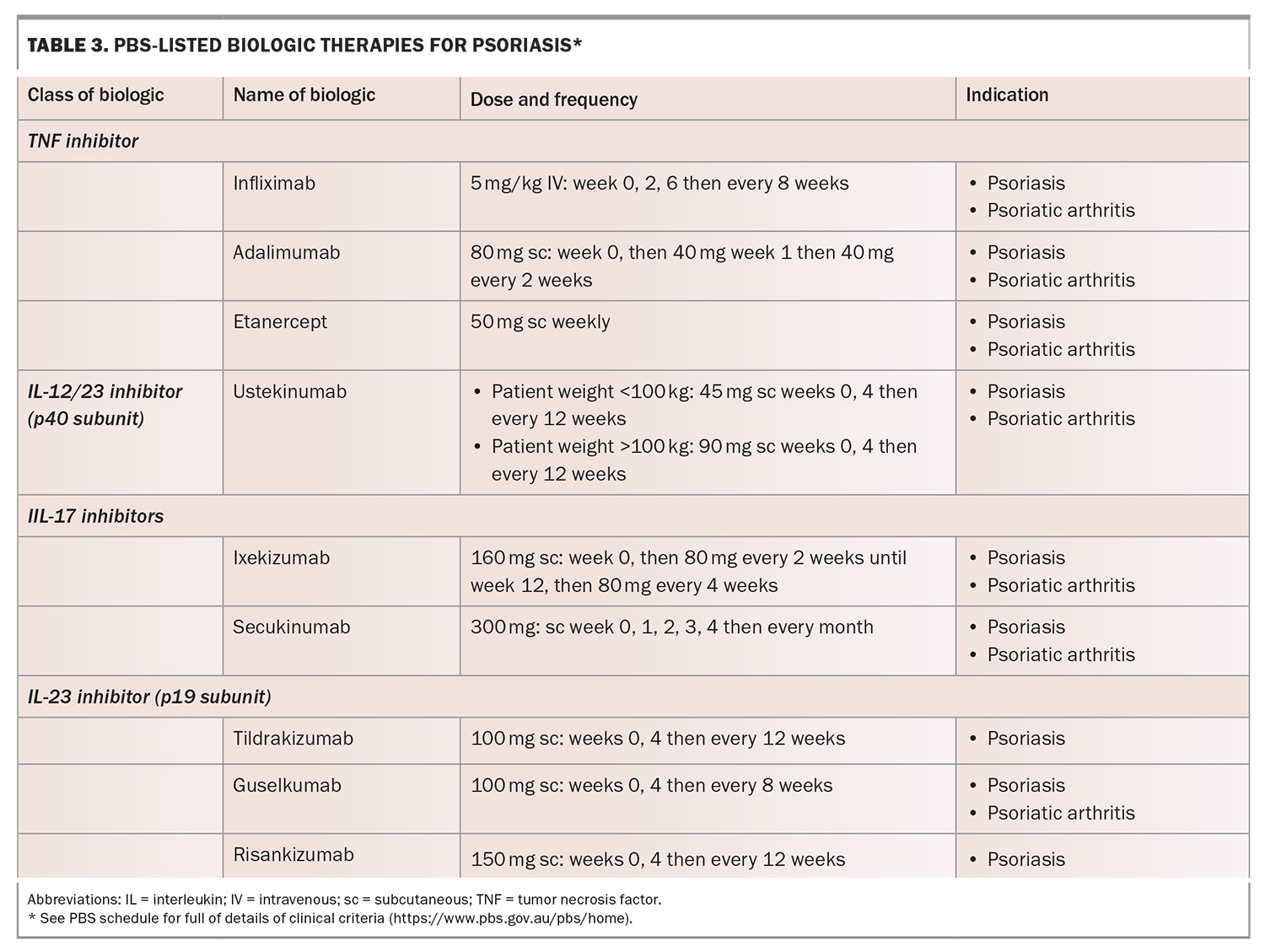
The first-generation biologics are the TNF-inhibitors adalimumab, etanercept and infliximab, followed by the IL-12/23 inhibitor ustekinumab. These biologics aimed to improve skin by 75% (i.e. PASI 75 response). The newer biologics are the IL-17 inhibitors secukinumab, ixekizumab and bimekizumab, and the IL-23 inhibitors guselkumab, tildrakizumab and risankizumab. They are more effective than first-generation biologics, have a safer side-effect profile, can often be used in concomitant PsA and have a quick onset of action. In recent randomised controlled trials, 75% of patients treated with risankizumab achieved a PASI 90 response at week 16, while 90% of those treated with ixekizumab achieved PASI 75 response at week 12.14,15 Comparisons of the efficacy of biologics should not be made without head-to-head studies.
Patients taking a biologic agent must avoid live vaccines due to the risk of serious infections and death in their immunosuppressed state. IL-17 inhibitors are associated with a greater risk of developing candida and tinea infections and must be used with caution in patients with inflammatory bowel disease. IL-23 inhibitors are associated with upper respiratory tract infections. Biologic therapies must therefore be prescribed with caution in patients with comorbidities and only after a thorough medical history is taken. At-risk patients taking biologics should be monitored regularly for any adverse effects. PBS-listed biologic therapies for psoriasis are summarised in Table 3.
Small molecule therapies, such as Janus-kinase inhibitors, are also available for the treatment of psoriasis; however, they are currently not approved by the PBS in Australia for this indication. The advent of biosimilar agents could make therapies for psoriasis more affordable and easier to access.
Conclusion
Recent advances in our understanding of the pathophysiology of psoriasis and the development of new therapies have improved its management. Instead of purely treating the skin, clinicians need to consider psoriasis a systemic inflammatory condition. Early introduction of systemic therapies may improve the skin, prevent long-term recurrence and reduce development of comorbidities. MT
COMPETING INTERESTS: Dr Daniel has received honoraria for presentations from Lilly and Novartis.
References
1. Griffiths CE JN Barker. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263-271.
2. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet 2021; 397: 1301-1315.
3. Boehncke WH, Schön MP. Psoriasis. Lancet 2015; 386: 983-994.
4. Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol 2022; 23(Suppl 1): 21-29.
5. Reynolds K, Pithadia DJ, Lee EB, Clarey D, Liao W, Wu JJ. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations, diagnosis, and treatment. Cutis 2022; 110(Suppl 2): 19-25.
6. Burden AD, Choon SE, Gottlieb AB, Navarini AA, Warren RB. Clinical disease measures in generalized pustular psoriasis. Am J Clin Dermatol 2022; 23(Suppl 1): 39-50.
7. Burden AD, Bissonnette R, Lebwohl MG, et al. Psychometric validation of the generalized pustular psoriasis physician global assessment (GPPGA) and generalized pustular psoriasis area and severity index (GPPASI). J Eur Acad Dermatol Venereol 2023; 00: 1-9.
8. Hong JJ, Mosca ML, Hadeler EK, Brownstone ND, Bhutani T, Liao WJ. Genital and inverse/intertriginous psoriasis: an updated review of therapies and recommendations for practical management. Dermatol Ther (Heidelb) 2021; 11: 833-844.
9. Daniel BS. The multiple comorbidities of psoriasis: the importance of a holistic approach. Aust J Gen Pract 2020; 49: 433-437.
10. Iragorri N, Hazlewood G, Manns B, Danthurebandara V, Spackman E. Psoriatic arthritis screening: a systematic review and meta-analysis. Rheumatology (Oxford) 2019; 58: 692-707.
11. Garritsen FM, Kraag DE, de Graaf M. Guttate psoriasis triggered by perianal streptococcal infection. Clin Exp Dermatol 2017; 42: 536-538.
12. Anghel F, Nitusca D, Cristodor P. Body Mass index influence for the personalization of the monoclonal antibodies therapy for psoriasis. Life (Basel) 2021; 11: 1316.
13. Tsiogka A, Gregoriou S, Stratigos A, et al. The impact of treatment with IL-17/IL-23 inhibitors on subclinical atherosclerosis in patients with plaque psoriasis and/or psoriatic arthritis: a systematic review. Biomedicines 2023; 11: 318.
14. Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus Ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 2017; 376: 1551-1560.
15. Papp KA, Leonardi A, Blauvelt K, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double-blinded, controlled studies (UNCOVER-1, UNCOVER-2, UNCOVER-3). Brit J Derm 2018; 178: 674-681.