Multiple myeloma in general practice: a guide to diagnosis and management
This article is written on behalf of the Medical and Scientific Advisory Group, Myeloma Australia.
Multiple myeloma (MM) is a clinically aggressive blood cancer that requires prompt assessment and treatment to avoid irreversible organ damage. The finding of monoclonal protein on serum or urine testing is a hallmark feature of MM, and other laboratory investigations are readily available to help establish the diagnosis. A range of effective treatment options are available to control the disease in order to alleviate symptoms, delay organ damage and prolong survival.
- Multiple myeloma is a common blood cancer that is most frequently diagnosed in late adulthood.
- Timely diagnosis is important and relies on an appropriate index of suspicion supported by several key investigations that are readily available in the primary care setting.
- Patient outcomes are improving because of an expanding range of therapies that produce meaningful improvements in survival and quality of life.
- Care needs for patients with multiple myeloma can be complex and are best met with a multidisciplinary approach based on shared care between the GP and specialist haematologist.
Multiple myeloma (MM) is one of the most common blood cancers; however, it may be unfamiliar to many GPs because of its relatively low prevalence compared with other cancers. MM represents the most clinically aggressive form of a spectrum of plasma cell disorders, often presenting with multisystem manifestations and a median life expectancy in Australia of 5.5 years from the time of diagnosis.1 MM can be rapidly recognised and triaged in the primary care setting with several key investigations. Timely referral is important to avoid permanent organ damage and functional impairment, so a high index of suspicion is required when faced with characteristic clinical and laboratory findings. This article provides a guide to diagnosing and managing MM in general practice.
Diagnostic approach
The cardinal laboratory feature of MM is the presence of monoclonal protein (M protein) in serum or urine. M proteins may be detected during investigation of clinical symptoms, unexplained renal failure or anaemia, or incidental laboratory abnormalities, such as a raised erythrocyte sedimentation rate or total serum protein level. As not all M proteins signify MM, the term ‘monoclonal gammopathy’ is often used to refer to the presence of M proteins in the blood until a definitive diagnosis is made.
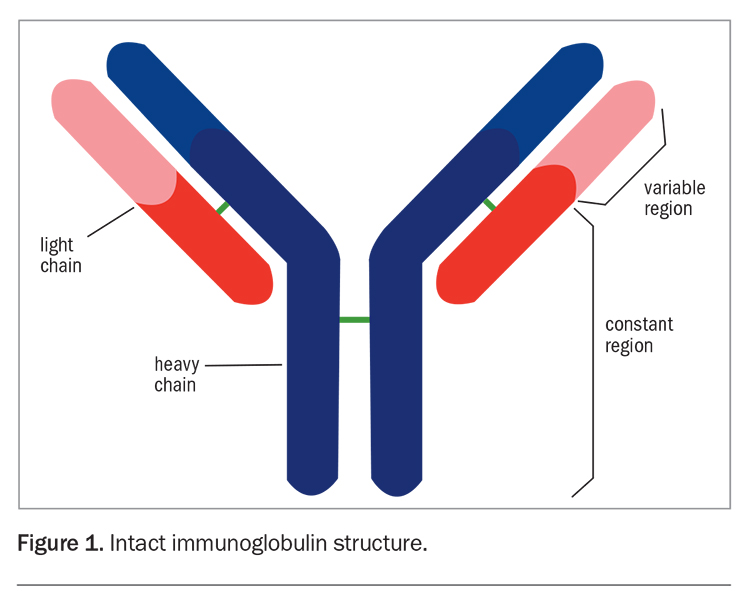
The M protein is most commonly an intact immunoglobulin (Ig) composed of both heavy (IgG, IgA, IgD, IgE or IgM) and light (kappa or lambda) chains (relative frequency: 80%) (Figure 1).2 Alternatively, there may be excessive and imbalanced production of a single light chain isotype without a heavy chain component (12%).2 Occasionally, two or more M proteins are detected and, rarely, no M band is detected. Light chains detectable in the urine are referred to as Bence-Jones proteins.
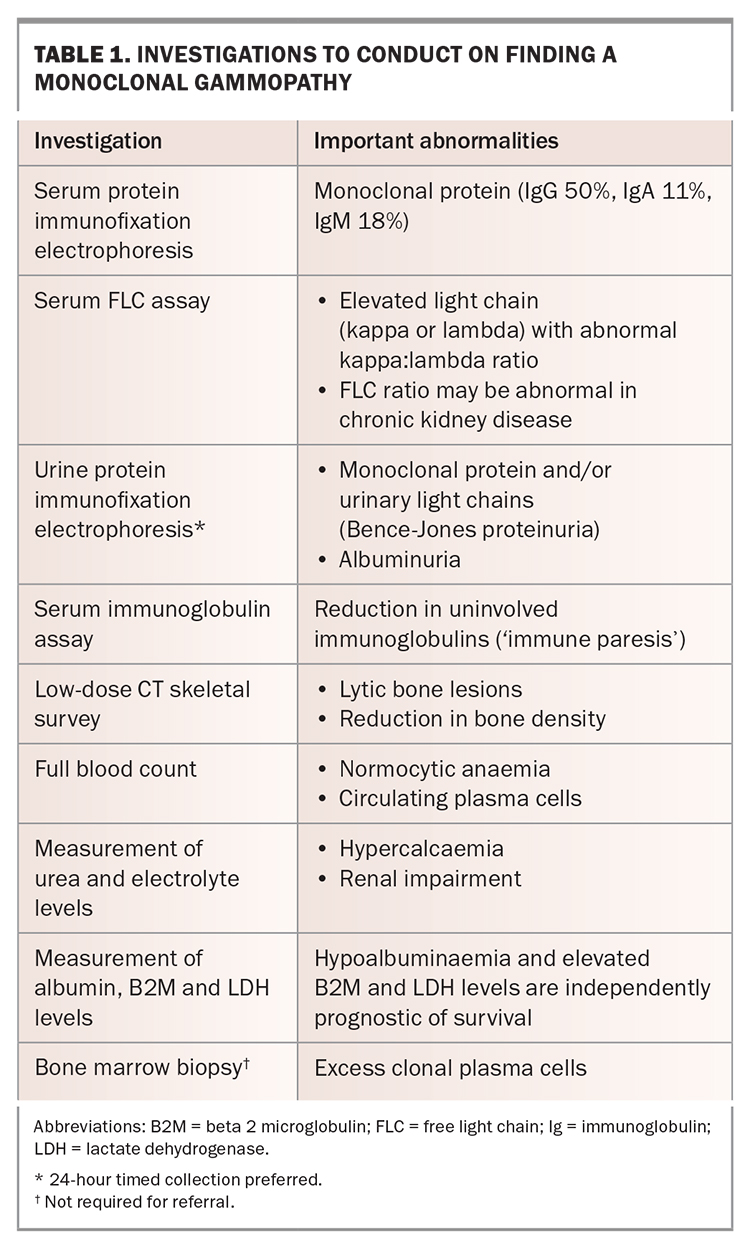
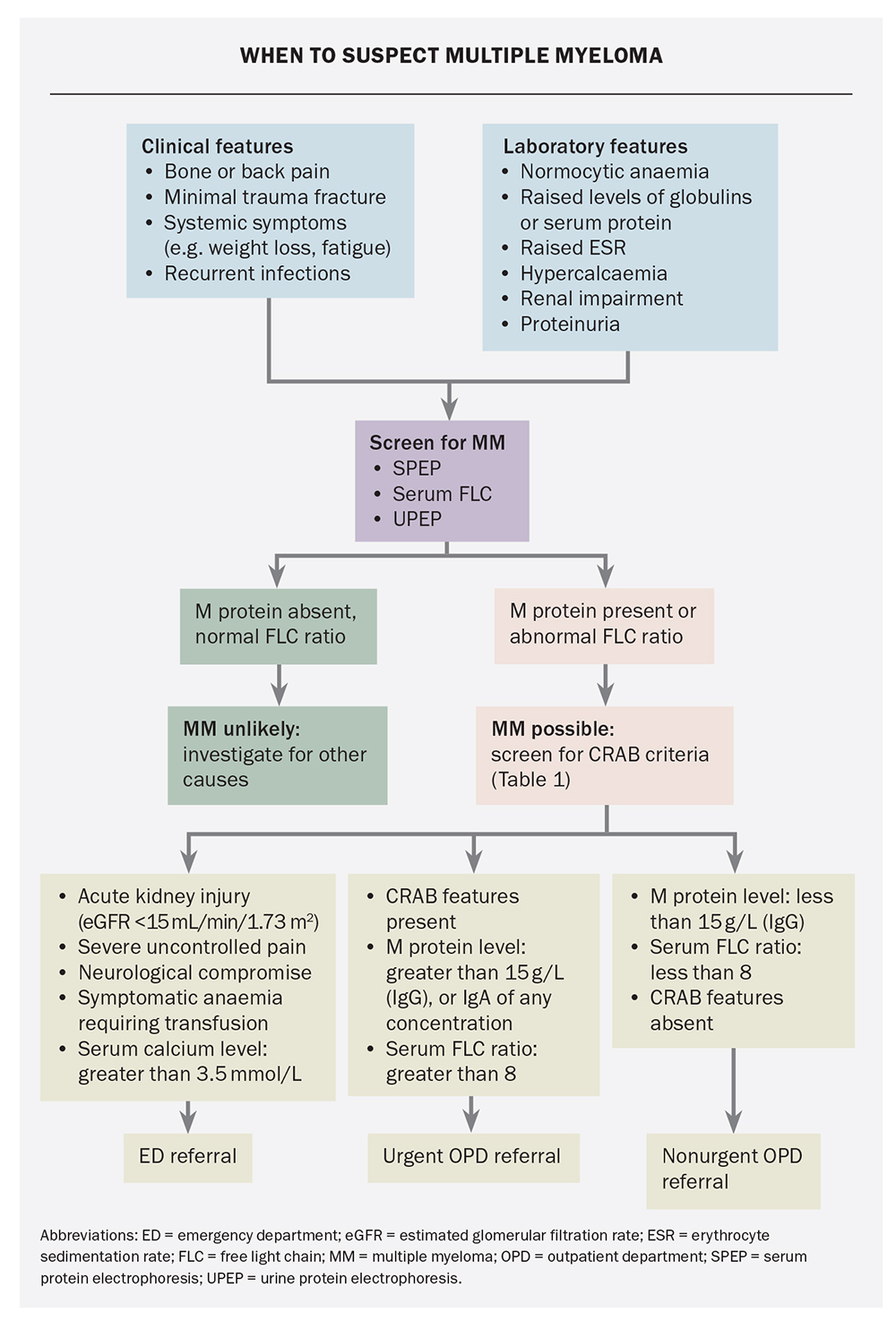
The finding of a monoclonal gammopathy should prompt further investigations to assess the underlying plasma cell clone and to screen for myeloma-defining events that, in turn, guide the urgency of referral (Table 1). The combination of serum and urine immunofixation electrophoresis with serum free light chain evaluation has a high negative predictive value for plasma cell disorders; hence, normal results should prompt investigations for other causes. In cases of focal bone pain or suspected fractures, imaging with plain radiography is appropriate as a first-line investigation; however, imaging of the complete skeleton using low-dose CT is required to exclude subclinical lesions at other sites.3
Disease definitions
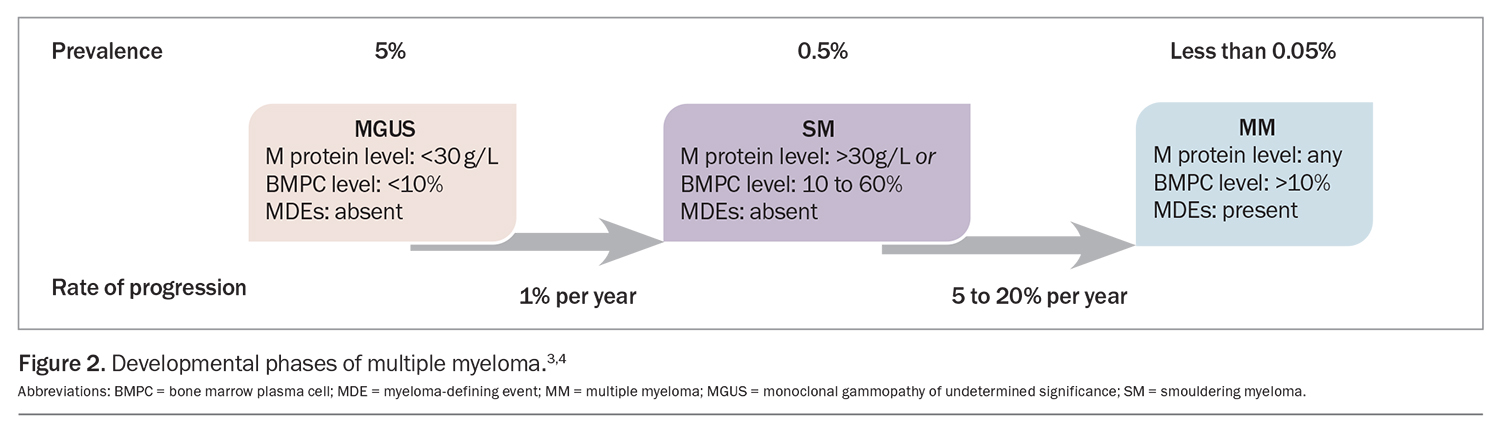
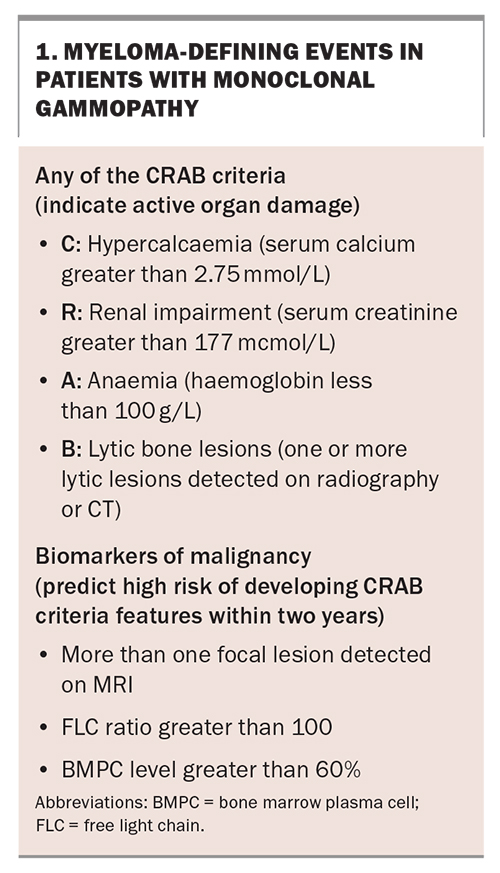
MM develops most commonly in the setting of non-IgM or light chain monoclonal gammopathy, which characteristically progresses through two precursor phases: monoclonal gammopathy of undetermined significance (MGUS) and smouldering myeloma (SM). Each phase is defined by the burden of clonal plasma cells as assessed by M protein and bone marrow plasma cell quantitation (Figure 2).4,5 Progression to MM is characterised by the occurrence of myeloma-defining events in patients with MGUS or SM (Box 1). The traditional ‘CRAB’ criteria (hypercalcaemia, renal impairment, anaemia and bone disease) are somewhat stringently defined by international guidelines with specific laboratory cutoffs; however, in the absence of alternative causes and especially with rapid tempo of progression, any degree of anaemia, hypercalcaemia or renal impairment should prompt screening for MM. Updates to the CRAB criteria were proposed in 2014, which incorporate additional findings on MRI and bone marrow biopsy that predict a high risk of progression to CRAB criteria-fulfilling disease, thus providing a clinical indication for treatment.4
In IgM monoclonal gammopathy, the M protein is produced by clonal lymphoid cells, rather than plasma cells. Although progression to MM may rarely occur in these patients, it is more common to see progression to B-cell lymphomas, such as Waldenström macroglobulinaemia, chronic lymphocytic leukaemia and follicular lymphoma.
MGUS of any subtype can occasionally cause manifestations of disease that are the result of particular physicochemical properties of the M protein that cause deposition or toxicity in various organs, with the heart, kidneys, nerves and skin being particularly affected. Patients may present with the insidious onset of heart failure, nephrotic syndrome, sensory neuropathy and skin changes. These syndromes can occur at minute serum concentrations of M protein and, in most cases, require tissue biopsy for accurate diagnosis. The term ‘monoclonal gammopathy of clinical significance’ was coined to encompass this group of rare conditions, the further discussion of which is beyond the scope of this article.
When to refer patients
A general approach to the rapid assessment of monoclonal gammopathy is presented in the Flowchart. Comprehensive guidance is also available through the national Optimal Care Pathways (https://www.canceraustralia.gov.au/
optimal-cancer-care-pathways). Emergent assessment and management are essential in cases of advanced organ dysfunction, threatened spinal cord compression or uncontrolled symptoms to avert complications, such as paraplegia, pathological fracture and dialysis dependency. Urgent outpatient referral is generally appropriate for ambulatory patients with high M protein levels or features consistent with the CRAB criteria without imminent organ compromise. Patients with small M proteins who are asymptomatic with normal organ function can be referred for nonurgent assessment. Telephone discussion with a local haematologist is strongly encouraged if the referring doctor is concerned about the optimal course of action.
Principles of treatment
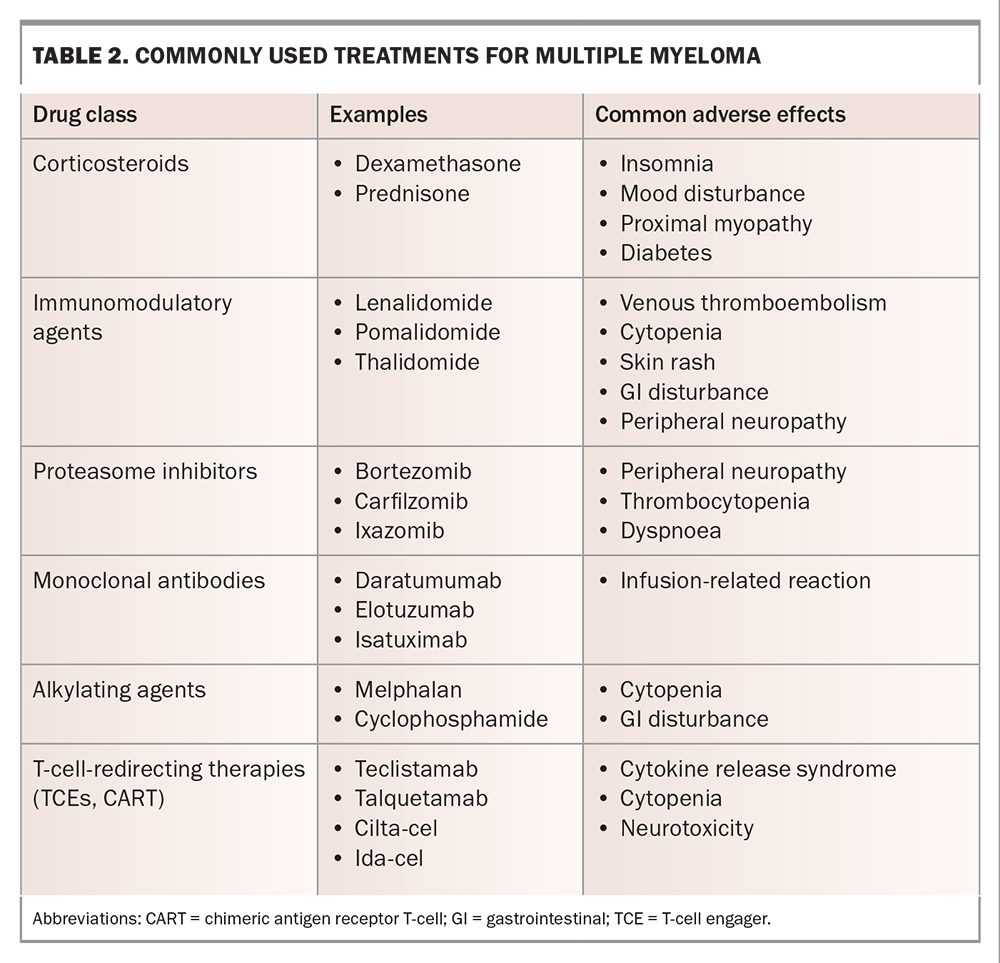
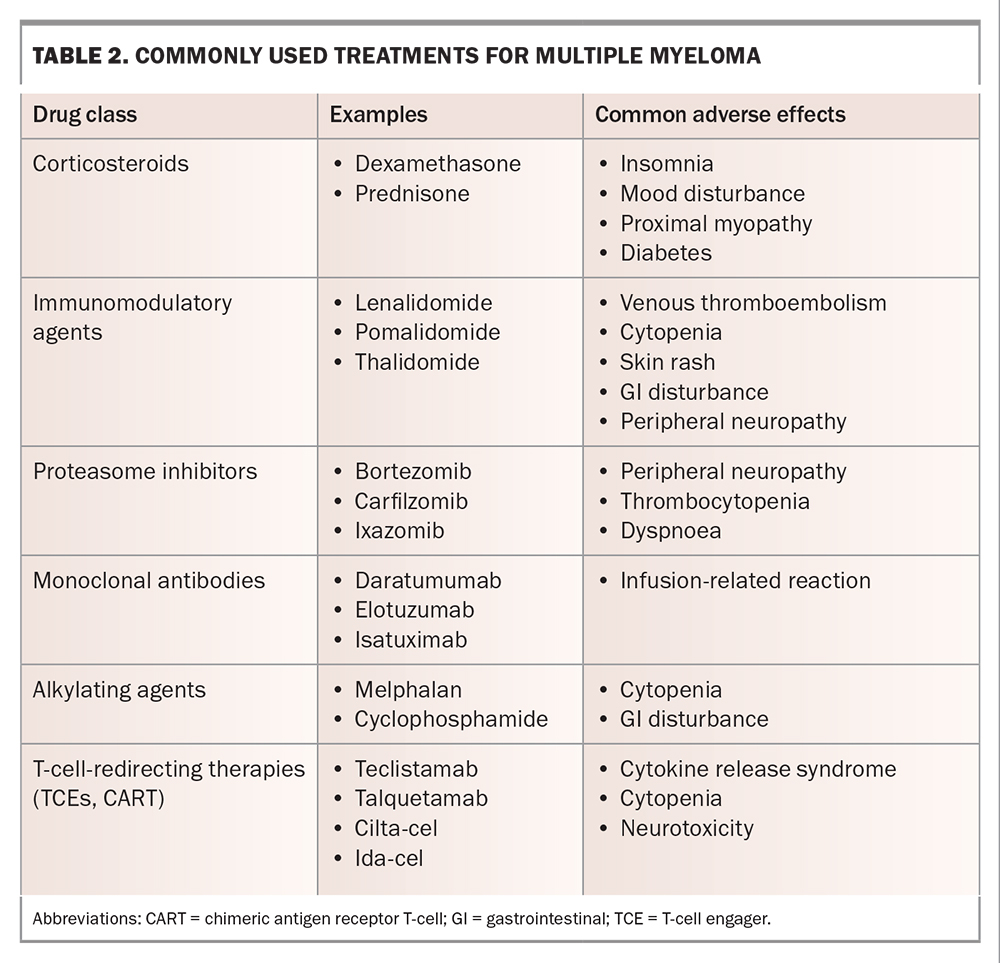
The goals of treatment for MM are to induce and maintain disease control sufficient to relieve symptoms and delay further myeloma-related organ damage. Relapse remains inevitable, and patients will generally cycle through several lines of treatment, with shortening remission durations and cumulative treatment toxicity, eventually leading to treatment cessation and palliative care. The most commonly used agents for MM treatment are listed in Table 2. Therapies tend to be used in combinations of two, three or even four agents concurrently, and there is an increasing trend towards continuous or ‘maintenance’ therapy in all lines of treatment, as this may be associated with longer remission duration and, in some cases, overall survival compared with defined-duration treatment.6
The current standard of care in Australia for newly diagnosed MM is ‘triplet’ induction therapy with subcutaneous bortezomib, oral lenalidomide and oral dexamethasone delivered in the outpatient setting for six to eight cycles.6 Patients with frailty or those who are very elderly may require dose attenuation or de-escalation to ‘doublet’ regimens to minimise toxicity. Patients who meet fitness and age criteria may be offered high-dose melphalan chemotherapy followed by autologous stem cell transplantation to enhance treatment response and prolong remission duration. Most patients will then continue on oral lenalidomide maintenance therapy until disease relapse or the development of intolerable side effects. Second-line therapies comprise combinations of the drugs listed in Table 2.
Emerging therapies
Exciting emerging agents that rely on immunological rather than cytotoxic mechanisms have led to outstanding responses in clinical trials and promise the imminent arrival of an entirely new class of therapies for MM. T-cell engagers are antibody drugs that bind simultaneously to T-cells and MM cells, bringing the two into close proximity and inducing T-cell-mediated tumour cell death.7 Chimeric antigen receptor T-cells are produced by genetically manipulating T-cells to express a MM cell-specific receptor, through which binding to MM cells causes T-cell activation and tumour cell death.8 Both therapies have been shown to produce high response rates in patients with advanced myeloma, with prolonged remissions compared with those following conventional care regimens. Novel toxicities associated with these agents include cytokine release syndrome, a systemic inflammatory state caused by T-cell cytokine storms, and immune effector cell-associated neurotoxicity syndrome, characterised by a range of usually transient neurological deficits, the mechanism of which remains unclear.
Supportive care and the role of the GP
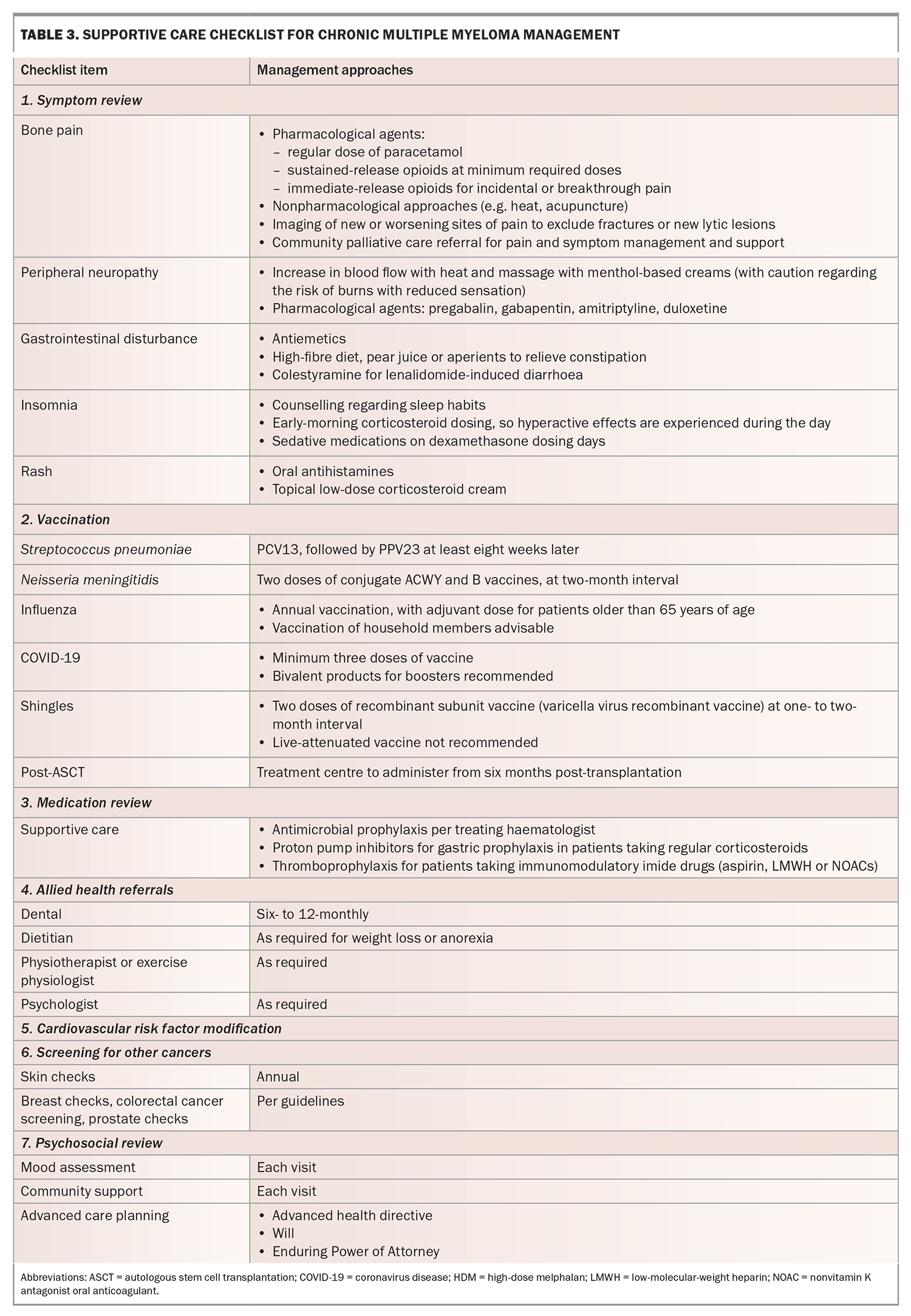
Supportive care is critically important for optimal outcomes in patients with MM, and GPs play an important role in this area. Medical and psychosocial issues frequently arise during treatment, and a multidisciplinary approach is recommended to support patient-centred care. In addition to ‘routine’ primary care, the following key aspects should be considered.
Infection prevention
Patients with MM are at an increased risk of contracting infections because of impaired immune function associated with both the disease and treatment. The most common cases involve bacterial and viral respiratory tract infections and reactivation of varicella zoster virus, which occurs during treatment in up to 20% of patients.9 All patients should be vaccinated for influenza, COVID-19, Streptococcus pneumoniae and Neisseria meningitidis. Antimicrobial prophylaxis varies according to the treatment regimen, although most patients receive prophylaxis against herpes zoster and Pneumocystis pneumonia as part of routine supportive care. Prompt treatment of intercurrent infection is encouraged when patients present to their GPs for assessment, and a low threshold for referral to a hospital for assessment and intravenous treatment should be adopted in patients presenting with clinical signs of moderate to severe infection.
Bone health
MM causes reduced bone density, and regular bisphosphonate use is a part of standard care in all patients. The frequency of administration is higher than that for osteoporosis, and it is recommended that patients undergo six-monthly to annual dental reviews to screen for osteonecrosis of the jaw.10 Calcium and vitamin D supplementation are generally encouraged in the presence of normocalcaemia.11
Chronic pain
Many patients experience both acute and chronic pain related to lytic bone disease, pathological fractures and treatment-related peripheral neuropathy. Pharmacological treatments are often required, including paracetamol, neuropathic agents and opioids. Authority approval for monthly quantities of controlled analgesic agents is preferable to avoid shortfalls in patient supply. NSAIDs are generally discouraged because of concerns about additive nephrotoxicity. Nonpharmacological modalities, including radiotherapy, heatpacks, acupuncture and massage, may be effective for chronic pain.
Mental health and advanced care planning
Patients with MM experience the poorest quality of life compared with patients with other cancers.12 Chronic symptoms, indefinite therapy duration and the threat of relapse can be psychologically burdensome, placing patients at risk of developing depression and carers at risk of experiencing burnout. The GP–patient relationship is a valuable source of support outside of the hospital setting and is often the optimal environment to broach discussions about end-of-life planning and goals of care. Valuable support can also be provided using a mental healthcare plan.
Chronic disease management plan
Many patients are unaware of their entitlement to subsidised allied health professional support. Cost is often a barrier to enlisting the expertise of exercise physiologists, dietitians and podiatrists, among others. The Chronic Disease Management Plan is an excellent way to provide equitable access to these services.13 A checklist to assist with formulating this plan is provided in Table 3.
Exercise
MM typically causes patients to experience overwhelming fatigue, as well as pain associated with bone lesions or fractures. These symptoms make it difficult for patients to find motivation to exercise or, in the case of bone pain, produce fear of causing more damage. Exercise can help reduce fatigue and maintain muscle mass and bone density, but it must be incorporated with guidance. Referral to exercise physiology under the Chronic Disease Management Plan is highly recommended for these patients.13
Resources for information about MM
The treatment landscape in Australia is largely dictated by PBS reimbursement restrictions; hence, patients and GPs obtaining information from overseas sources may be frustrated to find that certain therapies are not available in Australia. Some key local sources of information about the disease, treatments and supportive care are provided in Box 2.
Conclusion
The role of GPs in managing MM is twofold: to recognise the disease and refer patients appropriately for specialist consultation: and to provide co-ordinated, multidisciplinary supportive care throughout the disease course. Treatments for MM are often complex, individualised and rapidly changing; thus, close communication between the patient, specialist and GP is necessary to achieve the best possible outcomes. MT
COMPETING INTERESTS: Dr Weber has received a speaker honorarium from The Limbic and support from Novartis for virtual conference registration. Ms Beer is a member of the Janssen Global Myeloma Collaboration Council.
References
1. Bergin K, Wellard C, Moore E, et al; Australian and New Zealand Myeloma and Related Diseases Registry. The myeloma landscape in Australia and New Zealand: the first 8 years of the Myeloma and Related Diseases Registry (MRDR). Clin Lymphoma Myeloma Leuk 2021; 21: e510-e520.
2. Kristinsson SY, Rögnvaldsson S, Thorsteinsdottir S, et al. Screening for monoclonal gammopathy of undetermined significance: a population-based randomized clinical trial. First results from the Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study. Blood 2021; 138: 156.
3. Creeper K, Augustson B, Kusel K, et al. Imaging of patients with multiple myeloma and associated plasma cell disorders: consensus practice statement by the Medical Scientific Advisory Group to Myeloma Australia. Intern Med J 2021; 51: 1707-1712.
4. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15: e538-e548.
5. Rögnvaldsson S, Love TJ, Thorsteinsdottir S, et al. Iceland screens, treats, or prevents multiple myeloma (iStopMM): a population-based screening study for monoclonal gammopathy of undetermined significance and randomized controlled trial of follow-up strategies. Blood Cancer J 2021; 11: 94.
6. Clinical practice guideline: multiple myeloma. Burnely: Myeloma Australia; 2022. Available online at: https://myeloma.org.au/health-professional-resources/ (accessed Jun 2023).
7. Moreau P, Touzeau C. T-cell-redirecting bispecific antibodies in multiple myeloma: a revolution? Blood 2022; 139: 3681-3687.
8. Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ 2020; 370: m3176.
9. Teh BW, Reynolds G, Slavin MA, et al; Medical and Scientific Advisory Group, Myeloma Australia and National Centrefor Infections in Cancer. Executive summary of consensus clinical practice guidelines for the prevention of infection in patients with multiple myeloma. Intern Med J 2023; https://doi.org/10.1111/imj.16100.
10. Dickinson M, Prince HM, Kirsa S, et al. Osteonecrosis of the jaw complicating bisphosphonate treatment for bone disease in multiple myeloma: an overview with recommendations for prevention and treatment. Intern Med J 2009; 39: 304-316.
11. Lee OL, Horvath N, Lee C, et al. Bisphosphonate guidelines for treatment and prevention of myeloma bone disease. Intern Med J 2017; 47: 938-951.
12. Joshy G, Thandrayen J, Koczwara B, et al. Disability, psychological distress and quality of life in relation to cancer diagnosis and cancer type: population-based Australian study of 22,505 cancer survivors and 244,000 people without cancer. BMC Med 2020; 18: 372.
13. Chronic Disease Management Patient Information. Canberra: Department of Health and Aged Care; 2014. Available online at: https://www1.health.gov.au/internet/main/publishing.nsf/Content/mbsprimarycare-chronicdisease-pdf-infosheet.