Head and neck oncology: signs, symptoms and survivorship
Head and neck cancers (HNCs) are a heterogeneous group of malignancies arising in the face, scalp, sinonasal cavity, oral cavity, pharynx and larynx. Their rapid progression makes early diagnosis and referral critical to improving survival rates. Recent therapeutic advances have enabled a greater number of HNC survivors who, with the support of an effective multidisciplinary team, can experience a fulfilling quality of life, even after treatment.
Head and neck cancers (HNCs) are a significant healthcare concern in Australia, accounting for 3.4% of new cancer diagnoses annually, with a total of 4884 cases in 2018.1 They also contribute to about 2.5% of the cancer-related mortality rate every year, and most cases are diagnosed as squamous cell carcinomas (SCCs).
As the nation with the highest rate of cutaneous HNCs worldwide, Australia faces a unique challenge in this realm of oncology that is showing no signs of slowing down. The incidence of melanoma in Australia is already the highest worldwide (36.6 per 100,000 people), reflecting our community’s preponderance for ultraviolet radiation exposure, and the global incidence is expected to increase by a further 50% by 2040.2,3 Furthermore, HNCs grow rapidly by about 70% every four to five weeks, which can correlate to an increase in primary tumour stage roughly every month, with a tumour doubling time of 47 days.4,5 This is much faster than with other solid tumours (e.g. breast and lung tumours and pancreatic adenocarcinoma) in an anatomically and functionally complex and delicate region.
Therefore, it is essential that we diagnose, refer and manage HNCs early to provide patients the best chance at survival. This article presents an overview of head and neck oncology diagnoses, investigations and management approaches, with a focus on important issues to recognise in HNC survivorship.
Risk factors
Traditionally, older men and those who are socioeconomically disadvantaged have been disproportionally affected by HNCs, given the prevalence within these populations of two major risk factors: tobacco smoking and alcohol consumption. Although there has been a decline in smoking in developed countries, 18,414 Australians are predicted to die from smoking-related HNCs (cancers of the larynx, lips, oral cavity and pharynx) between 2020 and 2044.6 However, there has recently been a rapid increase in human papilloma virus (HPV)-associated oropharyngeal cancers, predominately within the younger, unvaccinated population. Other identified risk factors include occupational exposure to materials such as wood dust, betel quid chewing and Epstein–Barr virus exposure. Laryngopharyngeal reflux has been shown to be a risk factor for laryngeal SCC in a large-scale prospective study, with a positive hazard ratio of 1.91 (95% confidence interval [CI], 1.24–2.94);7 however, further studies would be of benefit.
Signs and symptoms
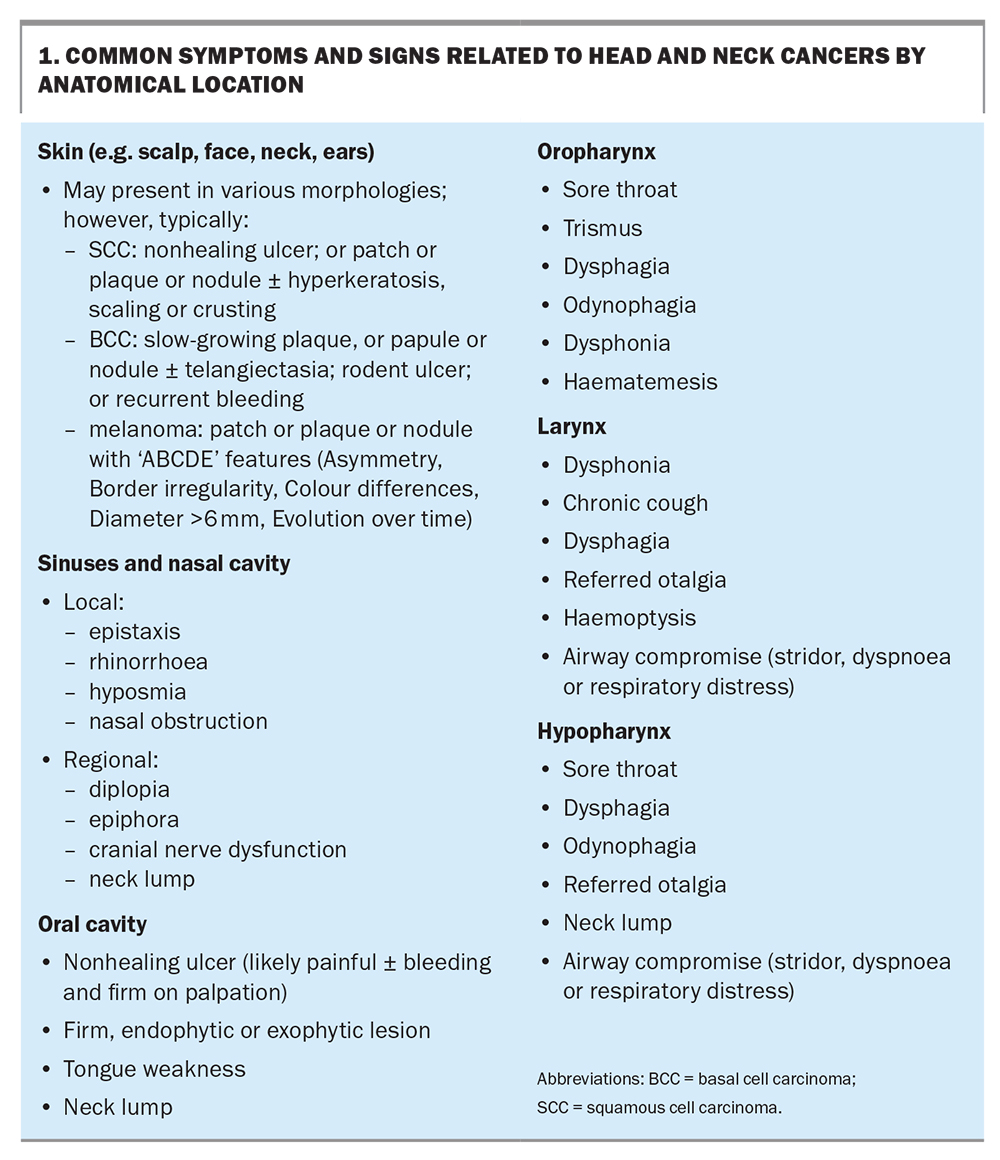
HNCs have different clinical manifestations depending on their anatomical location (Box 1) and may present similarly to various benign head and neck conditions, making early detection a significant challenge. The National Institute for Health and Care Excellence has released guidelines for cases in which urgent referrals should be made, including when any symptom persists for three or more weeks, or on presentation of any symptom in older and high-risk patients.8
Skin malignancies
As the skin on the face, ears, neck and scalp is easily exposed to ultraviolet radiation in daily life, skin carcinogenesis often occurs in these areas. Cutaneous SCCs (cSCCs) and basal cell carcinomas can present in a variety of ways, such as skin lesions with several possible morphologies from nodules and patches to eroding ulcers. GPs may also encounter actinic keratoses, which are precancerous lesions to cSCCs. Melanomas also frequently arise in the head and neck region and are more common in older patients but can present in young adults. Close examination of the skin with dermatoscopy may be indicated to characterise these lesions.9
Australia also has the highest incidence of cutaneous metastasis to the parotid gland. Any new lump in this major salivary gland should be treated with a high degree of suspicion, especially in patients with actinopathies or patients who have had a cSCC or melanoma excised from their face. An associated facial nerve weakness or pain are concerning signs and highly suggestive of malignancy.10
Sinonasal malignancies
An uncommon entity in head and neck oncology, sinonasal cancer is challenging to diagnose given its similarities in presentation to inflammatory sinus pathologies. Local symptoms include epistaxis, nasal obstruction, epiphora and rhinorrhoea. Advanced-stage tumours may involve orbital components and present with visual disturbances, such as diplopia. A high index of clinical suspicion may provide a critical diagnostic tool, especially in older patients presenting with insidious-onset symptoms, no history of sinusitis or rhinitis and evidence of exposure to risk factors such as wood dust or tobacco smoke.11
Oral cavity malignancies
The oral cavity is the most common anatomical location for SCCs to arise in the mucosa of the head and neck region. Patients often present late with nonhealing ulcerations, which may be associated with tenderness, bleeding from friable tissue and palpable submucosal firmness. Trismus or unilateral tongue immobility suggests advanced-stage cancers invading either the muscles of mastication or the hypoglossal nerve, respectively. These lesions may have been preceded by identifiable premalignant changes, including leukoplakia or erythroplakia, which may appear as whitened or erythematous mucosa, respectively. Examination for cervical lymphadenopathy is also crucial to assess for regional spread.12,13
Oropharyngeal malignancies
Oropharyngeal tumours are related to the posterior third of the tongue, soft palate, tonsils and surrounding pharyngeal walls. Not infrequently, HPV-positive oropharyngeal cancer first presents with an incidental neck lump due to nodal metastasis in patients with few risk factors. HPV-negative oropharyngeal cancers are more likely to produce localised symptoms, such as sore throat, dysphagia and odynophagia. It is thus crucial that patients with nonresolving neck lumps are investigated for oropharyngeal cancer, irrespective of the presence of risk factors. Careful history-taking may reveal other risk factors that may distinguish the two, including previous HPV vaccination status, and alcohol consumption and smoking history.14
Laryngeal malignancies
Common symptoms related to laryngeal cancers include dysphonia, hoarseness of the voice, chronic cough, dysphagia, referred otalgia and haemoptysis. Advanced-stage tumours may present with airway compromise, leading to stridor, dyspnoea and respiratory distress.15-17
Hypopharyngeal malignancies
The hypopharynx is bounded superiorly by the oropharynx and inferiorly by the oesophageal inlet. Cancers arising in this region may go unnoticed at early stages because of the large spatial capacity of this region, but large tumours will often cause throat discomfort, dysphagia, odynophagia or referred otalgia. As lymphatic involvement occurs early, patients may also first report a new neck lump. Rarely, advanced lesions may invade anteriorly into the larynx and cause laryngeal symptoms similar to those associated with laryngeal malignancies, notably airway compromise and aspiration.
Investigations
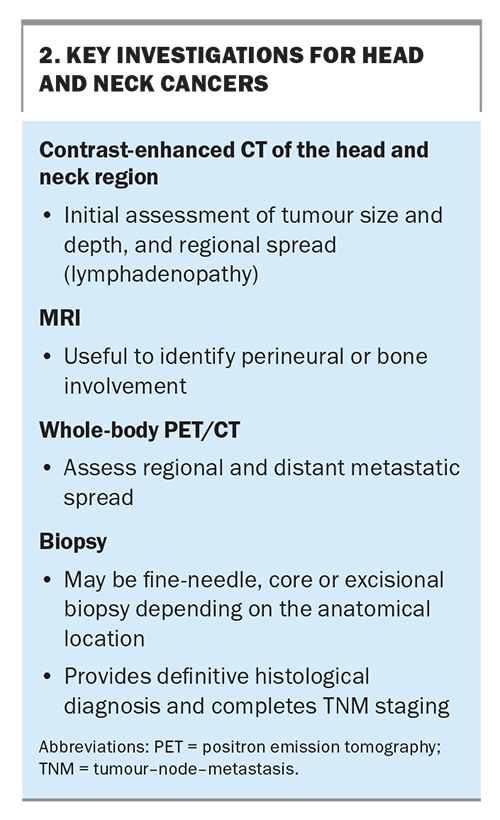
The main investigations for HNCs involve imaging and a biopsy for histological diagnosis (Box 2). Contrast-enhanced CT of the head and neck region is often the initial imaging modality of choice to assess a tumour’s size, depth and regional lymph node or cortical bone erosion. MRI may also assist with identifying perineural or medullary space invasion and is particularly helpful for soft tissue definition, especially delineating tumours within the tongue and oropharynx. Positron emission tomography is used to identify the extent of regional and distant metastases. Following this, a biopsy of the lesion provides a definitive histological diagnosis. With this information, the cancer can be staged according to the appropriate tumour-node-metastasis classification to guide management. Patients presenting with CT or biopsy results are helpful in expediting specialist assessment and management. GPs are encouraged to contact a specialist directly upon identifying an HNC to help guide additional investigations and expedite patient care.
Management
Although the mainstay of managing HNCs primarily involves a combination of surgery, radiotherapy and chemotherapy, multidisciplinary team care involving other allied health members (e.g. dentists, dietitians, speech pathologists, occupational therapists and physiotherapists) provides optimal outcomes for patients while preserving maximal function and quality of life.
Treatment regimens can vary widely depending on patient factors (e.g. age, comorbidities, functional status) and disease factors (e.g. anatomical location, stage). The presence of two or more comorbidities is associated with poor survival and functional outcomes; however, the impact of performance status on prognosis is unclear.18-21 Regardless, HNC treatment inevitably causes functional decline, and it is important that a patient’s pretreatment functional status is carefully considered when deciding on treatment options.
Stage I or II cancers may be curable with surgical excision or radiotherapy alone. Cancers of the sinonasal vault and oral and oropharyngeal cavities can be successfully treated with primary surgery, as there have been developments in minimally invasive surgical techniques, including robotic transoral surgery, which improves surgical access. Cancers of the oropharynx or larynx, on the other hand, may be better targeted in the first instance with radiotherapy for structural and functional preservation, although surgery may be appropriate for some presentations. Surgical or radiotherapy treatment of the cervical lymph nodes may be considered depending on the location of the primary cancer.
More advanced-stage cancers typically have a poorer prognosis and require multimodal therapy. Surgery is again first-line for the oral cavity, followed by adjuvant radiotherapy with or without chemotherapy. At other sites, such as the larynx or hypopharynx, surgery may be considered for smaller tumours and in the setting of more advanced-stage disease, where organ preservation is not viable or when there is disease recurrence.
The Australian Institute of Health and Welfare reported an improvement in the five-year overall survival of patients with HNCs from 61.8% in 1982 to 1987 to 68.2% in 2006 to 2010. However, relative subsite-associated survival can differ:
- oral cavity: 75%
- larynx: 64.8%
- salivary glands: 70.4%
- nasal cavity and paranasal sinuses: 60.3%.22
The three-year overall survival for HPV-associated oropharyngeal SCCs is significantly higher (93.6%; 95% CI, 87.9–96.6%) than that for non-HPV-associated oropharyngeal SCCs (68.9%; 95% CI, 50.5–81.6%).23
In the past, treatment aims for recurrent or metastatic tumours were often palliative, given the poor prognosis in many patients. However, revolutionary advancements have revealed that immune checkpoint inhibitors (ICIs) can be used to induce tumour regression and improve survival rates in a subset of patients with HNCs. Since 2006, the US Food and Drug Administration (FDA) approved several ICIs for the treatment of HNCs, including nivolumab and pembrolizumab, which continue to be used despite the availability of platinum-based chemotherapy.24,25 Nivolumab is TGA approved and PBS listed for recurrent or metastatic SCC of the oral cavity, pharynx or larynx. In 2019, the FDA indication for pembrolizumab was expanded to be the first-line monotherapy for metastatic or recurrent head and neck SCCs with programmed death-ligand 1 (PD-L1) expression or in combination with chemotherapy.26 Pembrolizumub is also TGA approved for this indication and is PBS listed.27 This indication was established based on research showing improved overall survival with the use of pembrolizumab alone for programmed death-1 (PD-1)-positive SCCs and pembrolizumab plus chemotherapy for recurrent or metastatic head and neck SCCs.28 Subsequent trials have further shown that overall response rates to ICIs are higher in patients with advanced-stage head and neck cSCCs (42.3%) compared with mucosal SCCs (30%), with durable disease control and improved patient-reported quality of life.29-32 It is proposed that the improved response in patients with advanced cutaneous SCCs is because of a commonly high tumour mutational burden secondary to sun-related ultraviolet mutagenesis.33
Although trials are ongoing, there are promising data demonstrating that pre-operative (neoadjuvant) cemiplimab (an anti-PD-1 ICI) can ‘shrink’ tumours and, in some cases, demonstrate a complete response in patients with advanced- stage cutaneous SCCs before surgical intervention.34 In HNCs, this may reduce the necessity to remove critically important structures like the eyes, facial nerves or hearing apparatus. In a landmark phase 2 clinical trial, neoadjuvant immunotherapy was shown to significantly improve recurrence-free survival in patients with resectable stage III or IV melanoma when compared with adjuvant immunotherapy (72%; 95% CI, 64–80% vs 49%; 95% CI, 41–59%), raising the possibility for an ongoing or ‘memory-like’ immune response.35 Additionally, it is worth mentioning that HPV- positive oropharyngeal cancers have shown improved response rates to the same treatment regimens compared with HPV-negative cancers, including PD-L1 inhibitors.36 Although no therapies or standardised regimens targeting HPV-positive oropharyngeal cancers specifically have been established, this continues to be an emerging field of interest as the advent of ICI therapy in HNC treatment has galvanised many research efforts into other immunotherapies, including small molecule inhibitors and vaccines, for HPV-positive tumours.3
Supportive care
It is essential that patients are introduced to multidisciplinary care as early as possible after diagnosis, before starting definitive treatment, so that they may be supported holistically throughout their treatment course. Primary care physicians become pivotal points of contact at this stage. For instance, patients should be assisted with ceasing smoking and alcohol consumption.
Additionally, all patients should undergo a pretreatment assessment by a dietitian for malnutrition and the risk of refeeding syndrome to ensure their nutritional status is optimised. Understanding the alcohol dependence risk and early diet optimisation with a dietitian can be beneficial and improve perioperative recovery. A dental evaluation is also important to instate preventative measures against oral complications of HNC treatment, especially radiotherapy. Speech pathologists should also perform a baseline assessment of patients’ speech and swallow function and provide education on the potential effects of HNC treatment. Early referral to and continuous involvement of services, including palliative care, occupational therapy and physiotherapy, can help patients maintain a good quality of life throughout their treatment regimen.
Understandably, this entire work-up and treatment phase can be overwhelming for some patients, and GPs play a key role in co-ordinating care, ensuring ongoing follow up with all parties, and facilitating patients’ access to care, especially within vulnerable or disadvantaged populations. In particular, patients who attend scheduled appointments inconsistently may require further investigation into their barriers to accessing care and a more individualised treatment approach.
Survivorship
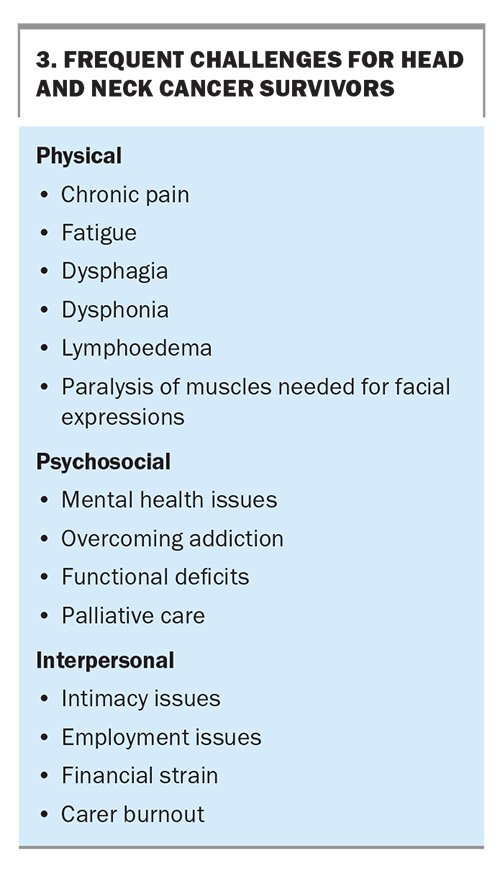
Developments in therapeutic measures for HNCs have significantly improved the prognosis and survival rates for some patients. In line with this trend, there is greater acknowledgement of the unique care needs of cancer survivors and attention towards addressing them (Box 3). The oncology team is closely involved to provide regular cancer surveillance, and a multidisciplinary team is beneficial in the post-treatment phase to help patients regain function and independence.
Physical and psychosocial challenges
Survivors of HNCs are often left with significant physical post-treatment morbidity, which can range from mild functional impairment to debilitating effects, including impairments in speech, swallowing, facial movements, overall appearance and salivary function (causing xerostomia). Xerostomia can often be alleviated with nonpharmacological approaches, including encouraging patients to take regular sips of water, chew sugarfree gum or use artificial saliva. Pilocarpine, a muscarinic receptor agonist, can also be prescribed to increase saliva production.
The role of ongoing speech pathology, physiotherapy and dietetic follow up is important after treatment to ensure patients can adapt and cope with these symptoms to function well in their daily lives. Other common side effects of treatment may include chronic pain and fatigue. Regular contact with a palliative care or chronic pain team for adequate analgesia and symptom relief can help patients regain independence despite these symptoms. Lymphoedema may also present a substantial challenge; patients can be referred to specialised lymphoedema clinics or an appropriate surgeon to determine whether surgical intervention may be beneficial.
From a mental health perspective, many may struggle with elements of acute or chronic depression, especially if they have chronic pain or significant concerns surrounding cancer recurrence. If the patient is unable to access dedicated psycho-oncology services, resources are available to help provide mental health support for patients and their families, such as Head & Neck Cancer Australia (https://www.headandneckcancer.org.au/).
Interpersonal and financial challenges
The interpersonal and financial challenges that patients may encounter during survivorship cannot be understated. Patients’ relationships with loved ones can be greatly affected, for instance, by the development of intimacy issues and carer burnout. Employment issues may also arise because of the after-effects of cancer treatment, placing significant financial strain on patients and their families.
It is important that we do not overlook any collateral damage sustained by a patient’s family and support systems throughout this process, as they are equally important components of the patient’s wellbeing. Engaging a social worker can help patients access local financial support services, such as the New South Wales’ Isolated Patients Travel and Accommodation Assistance Scheme, disability support pensions and carer allowance. Carer support may also be offered in the form of discussions regarding services or respite, and carers should also consult their primary care physicians on a regular basis.
Prevention
From a primary care perspective, there are preventive efforts that can be taken to reduce the risk of HNC development. Encouraging the cessation of alcohol consumption and tobacco smoking can significantly reduce the risk of HNCs. Regarding the use of e-cigarettes and vapes, there is insufficient evidence to demonstrate causation towards the pathogenesis of HNCs, but an association has been identified.38 Cessation may reduce a patient’s exposure to similar carcinogens as those in tobacco, thus reducing the risk of HNCs. Preclinical studies have shown that the use of marijuana can exacerbate tumour cell growth in HPV-positive oropharyngeal cancers; however, pooled clinical observation studies have not confirmed that the use of marijuana is an independent risk factor for HNCs in general.39,40
Vaccination
Given the rising prevalence of HPV-positive oropharyngeal cancers in the past few decades, researchers have questioned whether HPV vaccination might confer the same protective effects against oropharyngeal cancer as those against other HPV-related malignancies.41 Thus far, the evidence suggests that vaccination can effectively prevent oral HPV infections and their associated cellular changes.42
However, their direct impact on HNC incidence has yet to be proven, likely because of the recency of national pangender HPV vaccine implementation and latency of HNC development at an older age. In Australia, the national HPV vaccination program launched in 2007 for girls was only broadened to include boys in 2013. Given the median age of HPV-associated HNC diagnosis is about 53 years, the impact of HPV vaccinations may only be definitively recorded in 40 to 50 years.43 However, the importance of vaccines in preventing HPV-related oropharyngeal cancer is being increasingly recognised, with the FDA updating the indications of their nonvalent HPV vaccine to include this in 2020.44 This may reflect the beginning of a shift towards a global pangender HPV immunisation strategy against HPV-positive oropharyngeal cancer as further research emerges.
Conclusion
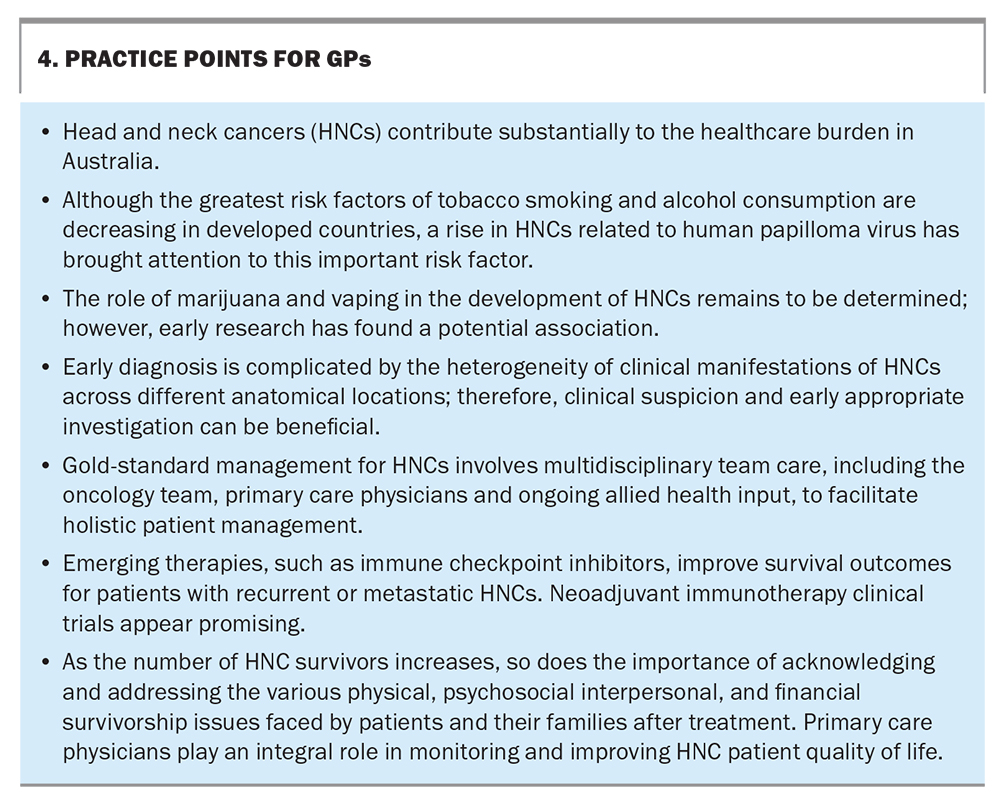
HNCs present a complex and diverse set of challenges, both in terms of their clinical manifestations and management, from diagnosis to survivorship. It is important for the medical community to recognise and address these challenges to provide comprehensive and holistic care for these vulnerable patients. Some practice points for GPs are provided in Box 4. MT
COMPETING INTERESTS: None
References
1. Cancer Australia. Head and neck cancer. Sydney: Cancer Australia; 2023. Available online at: https://www.canceraustralia.gov.au/cancer-types/head-and-neck-cancer/overview (accessed November 2023).
2. Cancer Today. Global cancer observatory. Lyon: International Agency for Research on Cancer; 2020. Available online at: https://gco.iarc.fr/today (accessed November 2023).
3. Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol 2022; 158: 495-503.
4. Terhaard C, Bockel L, Verduyn G, et al. Determination of tumor growth rate in head and neck cancer, correlation with possible prognostic parameters in the diagnostic biopsy. Int J Radiat Oncol Biol Phys 2008; 72: S392.
5. Dejaco D, Steinbichler T, Schartinger VH, et al. Specific growth rates calculated from CTs in patients with head and neck squamous cell carcinoma: a retrospective study performed in Austria. BMJ Open 2019; 9: e025359.
6. Luo Q, Steinberg J, Yu XQ, et al. Projections of smoking-related cancer mortality in Australia to 2044. J Epidemiol Community Health 2022; 76: 792.
7. Wang SM, Freedman ND, Katki HA, et al. Gastroesophageal reflux disease: a risk factor for laryngeal squamous cell carcinoma and esophageal squamous cell carcinoma in the NIH-AARP diet and health study cohort. Cancer 2021; 127: 1871-1879.
8. National Institute for Health and Care Excellence (NICE). Suspected cancer: recognition and referral. Manchester: NICE; 2015. Available online at: https://www.nice.org.uk/guidance/ng12 (accessed November 2023).
9. Gruber P, Zito PM. Skin cancer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
10. Goh RYH, Bova R, Fogarty GB. Cutaneous squamous cell carcinoma metastatic to patois-analysis of prognostic factors and treatment outcomes. World J Surg Onc 2012; 10: 117.
11. Harvey RJ, Dalgorf DM. Chapter 10: Sinonasal malignancies. Am J Rhinol Allergy 2013; 27: S35-S38.
12. Howard A, Agrawal N, Gooi Z. Lip and oral cavity squamous cell carcinoma. Hematol Oncol Clin North Am 2021; 35: 895-911.
13. Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am 2015; 24: 491-508.
14. Chow LQM. Head and neck cancer. N Engl J Med 2020; 382: 60-72.
15. Obid R, Redlich M, Tomeh C. The treatment of laryngeal cancer. Oral Maxillofac Surg Clin North Am 2019; 31: 1-11.
16. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin 2017; 67: 31-50.
17. Ringash J. Survivorship and quality of life in head and neck cancer. J Clin Oncol 2015; 33: 3322-3327.
18. Paleri V, Wight RG, Silver CE, et al. Comorbidity in head and neck cancer: a critical appraisal and recommendations for practice. Oral Oncol 2010; 46: 712-719.
19. Wang JR, Habbous S, Espin–Garcia O, et al. Comorbidity and performance status as independent prognostic factors in patients with head and neck squamous cell carcinoma. Head & Neck 2016; 38: 736-742.
20. Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006; 368: 843-854.
21. Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92: 4-14.
22. Australian Institute of Health and Welfare (AIHW). Head and neck cancers in Australia. Canberra: AIHW; 2014. Available online at: https://www.aihw.gov.au/reports/cancer/head-neck-cancers-australia/summary (accessed November 2023).
23. Taylor A, Eade T, Veivers D, et al. Human papillomavirus and oropharyngeal squamous cell carcinoma: a 12-year retrospective review in a New South Wales tertiary referral centre. Aust J Otolaryngol 2019; 2.
24. US Food & Drug Administration (FDA). Nivolumab for SCCHN. Silver Spring (MD): US FDA; 2016. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-scchn#:~:text=On%20November%2010%2C%202016%2C%20the,after%20a%20platinum%2Dbased%20therapy (accessed November 2023).
25. US Food & Drug Administration (FDA). Pembrolizumab (KEYTRUDA). Silver Spring (MD): US FDA; 2016. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda (accessed November 2023).
26. US Food & Drug Administration (FDA). FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma. Silver Spring (MD): US FDA; 2019. Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-first-line-treatment-head-and-neck-squamous-cell-carcinoma (accessed November 2023).
27. Therapeutic Goods Administration (TGA). KEYTRUDA pembrolizumab (rch) 100mg/4 mL concentrated injection vial (263932) Canberra: TGA; 2016. Available online at: https://www.tga.gov.au/resources/artg/263932 (accessed November 2023).
28. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019; 394: 1915-1928.
29. Mehta NK, Li AR, Nguyen SA, et al. Immune checkpoint inhibitors for advanced cutaneous squamous cell carcinoma: a systematic review with meta-analysis. Target Oncol 2021; 16: 743-752.
30. Hobday SB, Brody RM, Kriegsman B, et al. Outcomes among patients with mucosal head and neck squamous cell carcinoma treated with checkpoint inhibitors. JAMA Otolaryngol Head Neck Surg 2022; 148: 918-926.
31. Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018; 379: 341-351.
32. Migden MR, Khushalani NI, Chang ALS, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol 2020; 21: 294-305.
33. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9: 34.
34. Gross ND, Miller DM, Khushalani NI, et al. Neoadjuvant cemiplimab for stage ii to iv cutaneous squamous-cell carcinoma. N Engl J Med 2022; 387: 1557-1568.
35. Patel SP, Othus M, Chen Y, et al. Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med 2023; 388: 813-823.
36. Xu Y, Zhu G, Maroun CA, et al. Programmed death-1/programmed death-ligand 1-axis blockade in recurrent or metastatic head and neck squamous cell carcinoma stratified by human papillomavirus status: a systematic review and meta-analysis. Front Immunol 2021; 12: 645170.
37. Julian R, Savani M, Bauman JE. Immunotherapy approaches in HPV-associated head and neck cancer. Cancers (Basel) 2021; 13: 5889.
38. Szukalska M, Szyfter K, Florek E, et al. Electronic cigarettes and head and neck cancer risk- current state of art. Cancers (Basel) 2020; 12: 3274.
39. Liu C, Sadat SH, Ebisumoto K, et al. Cannabinoids promote progression of HPV-positive head and neck squamous cell carcinoma via p38 MAPK activation. Clin Cancer Res 2020; 26: 2693-2703.
40 Berthiller J, Lee YA, Boffetta P, et al. Marijuana smoking and the risk of head and neck cancer: pooled analysis in the INHANCE Consortium. Cancer Epidemiol Biomarkers Prev 2009; 18: 1544-1551.
41. Hong A, Lee CS, Jones D, et al. Rising prevalence of human papillomavirus-related oropharyngeal cancer in Australia over the last 2 decades. Head Neck 2016; 38: 743-750.
42. Nielsen KJ, Jakobsen KK, Jensen JS, et al. The effect of prophylactic HPV vaccines on oral and oropharyngeal HPV infection-a systematic review. Viruses 2021; 13: 1339.
43. Windon MJ, D’Souza G, Rettig EM, et al. Increasing prevalence of human papillomavirus–positive oropharyngeal cancers among older adults. Cancer 2018; 124: 2993-2999.
44. Zhou JZ, Jou J, Cohen E. Vaccine strategies for human papillomavirus- associated head and neck cancers. Cancers (Basel) 2022; 14: 33.