Chronic kidney disease: doing simple things well for those most at risk
The incidence of chronic kidney disease (CKD) is increasing worldwide. A holistic approach to management that includes nonpharmacological and pharmacological strategies to manage risk factors, as well as advocating for patients, particularly those most at risk, can significantly slow the progression of CKD. Input from multidisciplinary team members, including a nephrologist, CKD nurse and allied health professionals, can further reduce the progression of CKD, delay kidney failure and avoid CKD-related mortality.
Important update: changes to PBS listing
On 1 April 2024, a new PBS listing for empagliflozin was announced. The article has been updated to include this change and is available here.
- The incidence of chronic kidney disease (CKD) is increasing in Australia and worldwide, placing a significant burden on the healthcare system and economy.
- A thorough weight history, assessment of volume status and use of three essential tests (blood pressure, estimated glomerular filtration rate and spot urine albumin to creatinine ratio) are imperative to the diagnosis of CKD.
- Aboriginal and Torres Straits Islander people are twice as likely to develop CKD and five times more likely to develop kidney failure requiring dialysis compared with non-Indigenous Australians.
- Nonpharmacological measures, including optimising diet and nutrition, regular exercise and managing mental health, and a culturally responsible approach to care contribute to a patient’s overall wellbeing and improved health.
- Risk factors for developing CKD, including diabetes, hypertension, obesity and cardiovascular disease, should be managed aggressively with pharmacotherapy.
- The four pillars of medical management for patients with CKD are renin-angiotensin system inhibitors, sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists and nonsteroidal mineralocorticoid receptor antagonists.
Chronic kidney disease (CKD) is a global public health emergency, with its incidence increasing in parallel with growing rates of obesity, diabetes, hypertension and cardiovascular disease (CVD).1 Over 2 million adults in Australia, representing 11% of the adult population, are estimated to have biomedical signs of CKD, and the number of people progressing to kidney failure requiring dialysis (kidney replacement therapy) more than doubled between 2000 and 2021.2,3 This also comes at a burgeoning cost to public health systems. In 2021, CKD and kidney failure cost the Australian healthcare system $2.38 billion and, internationally, care of people with CKD has threatened to overwhelm the UK’s NHS.2,4,5 Diabetic kidney disease consistently contributes to about 40% of cases of kidney failure, followed by glomerulonephritis and hypertensive kidney disease.6 Aboriginal and Torres Strait Islander people are five times more likely to develop kidney failure requiring dialysis (per population size) than non-Indigenous Australians.2
CKD screening programs in Australia have been simplified to include three essential tests: blood pressure (BP), estimated glomerular filtration rate (eGFR) and spot urine albumin to creatinine ratio (uACR). Screening is widely promoted through Kidney Health Australia via many medical avenues, but is implemented with only partial success.7 Home-based screening methods are being developed to improve population screening for CKD;8 however, translating screening data and a CKD diagnosis into effective CKD therapy poses a further challenge. A recent study showed that pharmacotherapy for CKD management was underprescribed in primary care, with only 4.1% of eligible patients with CKD in Australia prescribed a sodium-glucose cotransporter-2 (SGLT-2) inhibitor.9 Achieving implementation closer to 75% has been estimated to prevent over 3600 cardiorenal events and 1300 kidney failure episodes a year.10
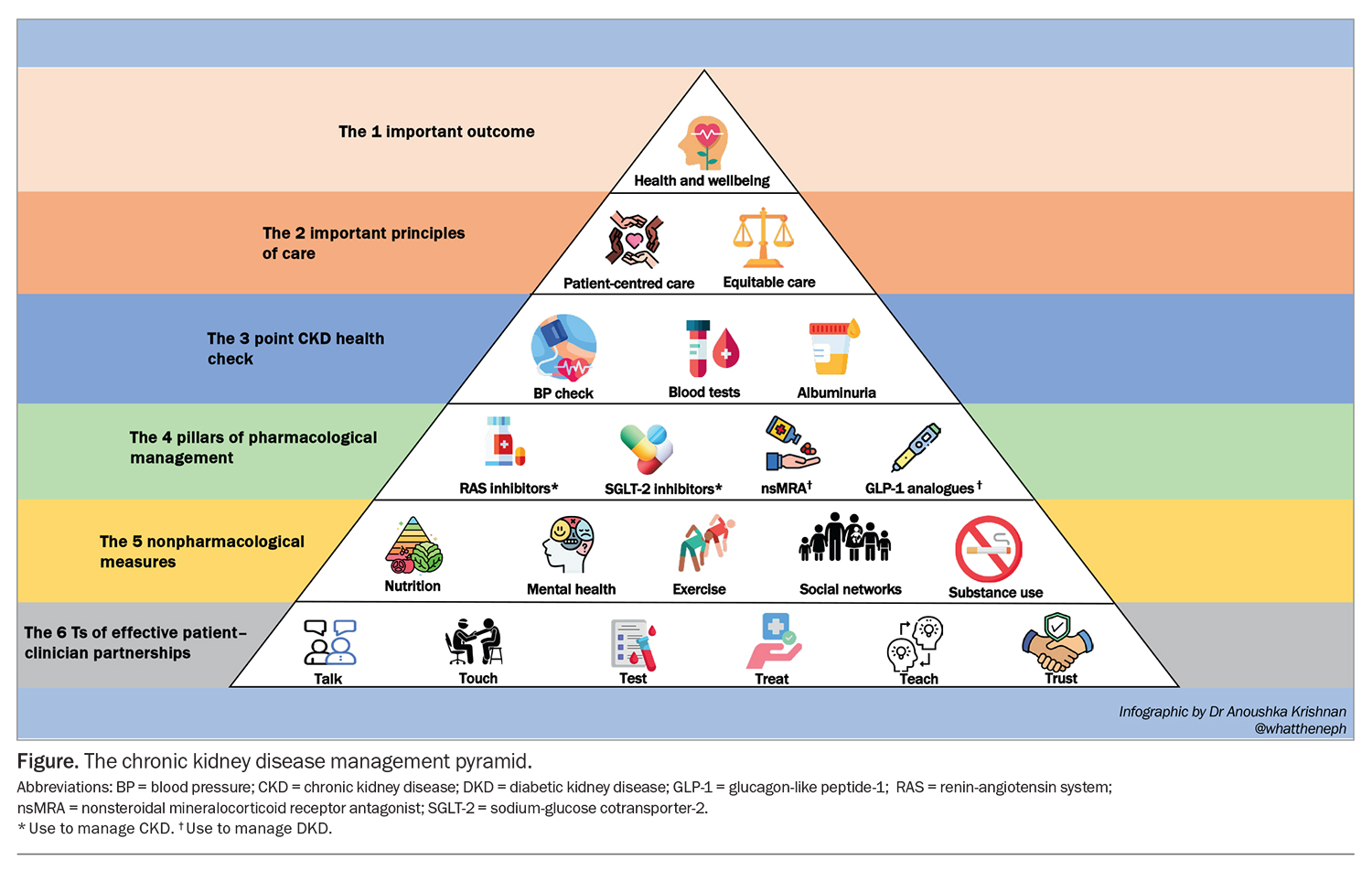
At a population level, effective public health policy and resourced strategies can promote kidney health among communities with high CKD risk.11 The core of public health strategy includes facilitating access to quality nutrition, adequate fitness and physical activity, and providing support to people seeking smoking and alcohol cessation. Effective public health strategies also facilitate access to affordable pharmacological approaches. Recently, several drugs have been developed that show a significant impact on reducing the progression of CKD, both in people with and without diabetes. This article discusses how clinicians can promote conceptually simple actions into highly effective health benefits for patients who are most at risk of kidney disease. The cornerstones of CKD management are summarised in the Figure.
First principles of best care
First principles of best care aim to improve the overall health of people with CKD, particularly at-risk populations. Key aspects of management include providing culturally responsible delivery of kidney care, promoting effective patient–clinician partnerships and delivering targeted care to optimise outcomes. These principles are outlined in Box 1.12
Optimise holistic care
Mindset (mental wellness)
Patients’ regular practices of healthful living are fundamental to kidney health and preventing related conditions associated with CKD. Over-riding social, financial, pain or mental health issues can be temporary or permanent barriers to healthful practices. These factors may cause elevated cortisol levels (hypercortisolism), resulting in insulin resistance, insomnia, hunger, hypertension, central adiposity and proximal muscle weakness, which contributes to accelerating diabetes, CVD and CKD progression.13 Online cognitive behavioural therapy modules, such as those provided by This Way Up (https://thiswayup.org.au), may help patients manage poor sleep and anxiety associated with hypercortisolism, and are best prescribed for couples or households rather than isolated individuals, provided they have the technological access and determination to complete the programs.14
In the authors’ experience, mnemonics can help patient’s focus attention systematically on their chosen problem area, starting with ‘looking after your-SELFF’, an acronym for Sleep, Exercise, Love, Foods and Fluids. Improving the quality of caloric intake takes priority over quantity, and includes prioritising a plant-based diet, avoiding ultraprocessed foods and optimising water intake.15 Referral for formal dietetic assessment and individualised advice is a standard of care but, in the authors’ experience, broad direction can be quickly given as positive suggestions, for example, ‘just eat real food’, ‘keep foods crunchy and colourful’, ‘swap salt flavouring to pepper, garlic, lemon and ginger’ and ‘make your drink a fashion statement traffic light: add mint, strawberry, lemon and orange to plain water for DIY potassium citrate’. Patients keen to avoid similar metabolic issues in their children or grandchildren should be encouraged to avoid smoking and make sure that special treats do not become daily habits.
Metabolic and psychological challenges
Supportive weight management
Avoid introducing assumptions or shame in collaborative health planning around weight management. Appreciating the metabolic and psychological challenges faced by many patients with CKD can sometimes be quickly gleaned by taking a five-point weight history of birth, teen post-puberty, maximum adult, minimum adult and current weight – then asking when and why the extreme values occurred, and with what functional impact. Episodes of extreme weight loss and volume depletion (e.g. teenage anorexia nervosa) can leave lasting kidney damage with osteoporosis. Conversely, for patients who are overweight, the dates of onset of painful arthritis, sleep apnoea, high BP, diabetes and stress incontinence are often precipitated by preceding weight gains.
Routine patient assessment
Routine examination of patients with CKD should include a five-point volume assessment, based on height, weight, postural change in BP and pulse rates, determining the extent of oedema and peripheral perfusion by capillary refill (and where possible, visualisation of jugular venous pressure). Total body weight measurement can be an understandably emotive issue and should be explained to patients as comprising the sum total of fluid, fat, muscle and bone weights, with each component having an individualised optimum range that is mostly genetically determined. Most people understand their weight in kg rather than body mass index (BMI). Therefore, using the rule of thumb that an individual’s normal body weight (in kg) should roughly corresponds to their height (in cm) minus 100 (i.e. a BMI of 25 kg/m2) allows patients to better estimate their target body weight. Investigations for target organ damage from high BP and diabetes is routine and reimbursed by Medicare.
Glycaemic control
Food accessibility, security, affordability and preparation, nutrition quality, eating frequency and culturally-based eating rituals are important considerations in understanding and supporting a patient’s glycaemic control, and a referral to an accredited practicing dietitian may be considered where indicated.16 Along with self-monitoring of blood glucose levels, made easier with the recent availability of continuous glucose monitoring devices (PBS subsidised for people with type 1 diabetes), the Kidney Disease Improving Global Outcomes (KDIGO) guidelines endorse measuring glycated haemoglobin (HbA1c) to monitor glycaemic control, even in individuals with CKD despite limitations associated with concurrent anaemia.17 HbA1c targets are now individualised, and range from below 6.5% to below 8% in patients with diabetes and CKD.17 Higher targets are usually reserved for patients with several comorbidities or at increased risk of hypoglycaemia.
Metformin has been a long-standing high-value, low-cost agent for metabolic improvement without weight gain but needs progressive dose reduction as GFR falls to avoid nausea or diarrhoea, and should be ceased immediately if a patient is unwell. Dipeptidyl peptidase-4 inhibitors are similarly weight-neutral and have few side effects as eGFR falls. Conversely, sulfonylureas and insulin may cause weight gain and increase the risk of hypoglycaemia, especially in patients with CKD stages 4 and 5. A detailed approach to glycaemic management in the context of CKD has been recently published.18 Use of SGLT-2 inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists is discussed below.
Management of hypertension
Optimising BP control is crucial to reducing CKD risk. Nonpharmacological measures include dietary salt reduction (<2 g sodium intake or <5 g sodium chloride per day) and regular exercise (at least 150 minutes per week of moderately intense physical activity as tolerated).19 Weight loss, smoking cessation and reducing alcohol consumption are useful adjunct measures.8 Current target BP is individualised, with a recommended target of at least 130/80 mmHg or lower if tolerated, with the safety caveat to avoid symptomatic hypotension.7 Target BP can be safely maintained by home BP monitoring and taking measurements on sitting and standing, taking medications at night rather than morning, ensuring good hydration during hot weather or physical exertion and providing patients the authority to reduce the dose of medications if low BP is consistently symptomatic.
Cardioprotection
Low kidney function and albuminuria are independently additive major cardiac risk factors, with cardiovascular (CV) events the cause of 95% of deaths in people with CKD before reaching kidney failure. Therefore, aggressive cardioprotection is crucial.20 CKD causes dyslipidaemia (i.e. low HDL-cholesterol and high triglyceride levels) and nephrotic syndrome causes hyperlipidaemia (i.e. raised LDL-cholesterol); therefore, optimising kidney function (by monitoring eGFR) and pursuing antiproteinuric therapies are crucial. Statins do not retard CKD progression or reduce cardiac events in patients who have developed kidney failure; however, they are recommended to reduce CV events in patients with CKD aged under 50 years with just one additional CV risk factor, regardless of lipid level.21,22 Aspirin is no longer recommended for the primary prevention of CVD in people with CKD because of the increased bleeding risk associated with its use and a lack of proven benefit.23,24
Screening
Individuals at risk of developing CKD should undergo a kidney health check every one to two years, incorporating the three essential BP, eGFR and uACR tests promoted by Kidney Health Australia.7 Aboriginal and Torres Strait Islander peoples are twice as likely to develop CKD than non-Indigenous Australians. Therefore an annual health check from 18 years of age that includes measuring BP, uACR and eGFR is recommended to encourage regular surveillance and promote early detection and management, and is reimbursed under MBS item 715.25 Recommendations for culturally safe kidney care in First Nations Australians is a nationally recognised and inaugural guideline carefully informed and endorsed by First Nation people, clinicians and peak health organisations, and is useful resource for clinicians and consumers.26
Targeted therapeutics
RAS inhibitors
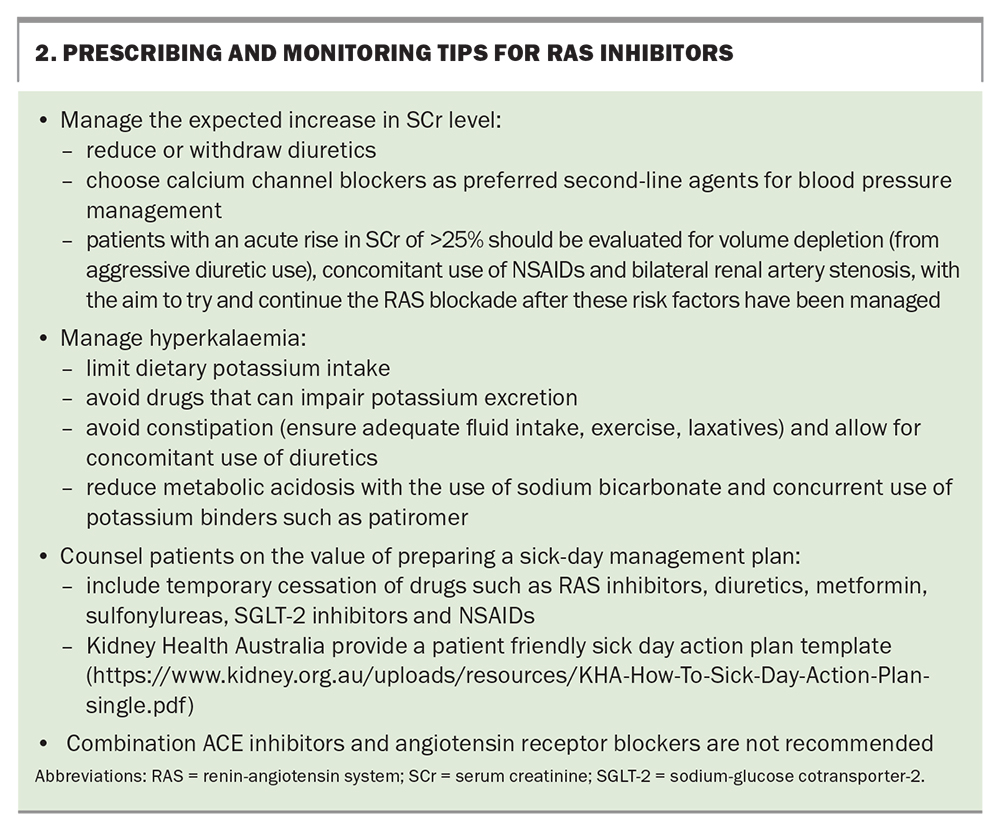
Renin-angiotensin system (RAS) blockade has stood the test of time in CKD management. Several studies over the past three decades have demonstrated the kidney and cardioprotective benefits and albuminurialowering effects of RAS inhibitors.27-31 They are used as first-line agents in the management of proteinuria and hypertension (with or without diabetes). Typically, changes in BP and serum creatinine (SCr) and serum potassium levels are monitored two weeks after initiation of these agents.7 A temporary increase in SCr level less than 25% due to the vasodilatory effects on the glomerular outflow is a predictable effect in the first few days, and the agent should be continued. A decline in kidney function of more than 25% is an indication to cease the drug and consider other factors and referral of the patient to a nephrologist. Additionally, RAS blockade inhibits the action of aldosterone, predisposing patients to hyperkalaemia, particularly in patients with advanced CKD. Tips for prescribing and monitoring RAS inhibitors are highlighted in Box 2.
SGLT-2 inhibitors
SGLT-2 inhibitors were initially used to treat malaria, and later developed as hypoglycaemic agents. Although their impact on glycaemic control is modest, their efficacy in reducing the progression of CKD and heart failure, irrespective of diabetes status, has been a game changer for patients. SGLT-2 inhibitors are now the second-line agent of choice for managing diabetes (after metformin).17 More recently, dapagliflozin and empagliflozin showed a 39% and 28% risk reduction in kidney-related outcomes, respectively.32,33
Dapagliflozin is PBS listed for patients with CKD independent of diabetes status (eGFR 25 to 75 mL/min/1.73 m2 and uACR 22.6 to 565 mg/mmol); empagliflozin is TGA indicated for adults with CKD stages 2 and 3a and a uACR 30 mg/g (3 mg/mmol) or higher, or CKD stages 3b to 5 regardless of uACR. SGLT-2 inhibitors are not approved for use in patients on kidney replacement therapy (dialysis or transplant); however, the RENAL LIFECYCLE trial is currently underway to address the efficacy of dapagliflozin in patients with severe CKD (Clinical Trials Identifier: NCT05374291). SGLT-2 inhibitors are also not indicated for those with polycystic kidney disease or acute glomerulonephritis requiring immunosuppression.
SGLT-2 inhibitors are simple to use and have a low side-effect profile. However, as with RAS inhibitors, SGLT-2 inhibitor use can lead to an acute increase in SCr level in the first four weeks of initiation due to glomerular haemodynamic changes. This dip in kidney function is not associated with a greater long-term decline and is, in fact, associated with a rebound and, ultimately, slower progression of CKD.7 The risk of genital mycotic infections is increased and should be managed with patient education on maintaining meticulous groin hygiene and close monitoring for signs of infection. Euglycaemic diabetic ketoacidosis is also a concerning adverse effect of SGLT-2 inhibitor use, particularly in people with diabetes, and withdrawal of insulin should be considered in those experiencing starvation and acute sickness. Tips for prescribing and monitoring SGLT-2 inhibitors are highlighted in Box 3.
Nonsteroidal MRAs
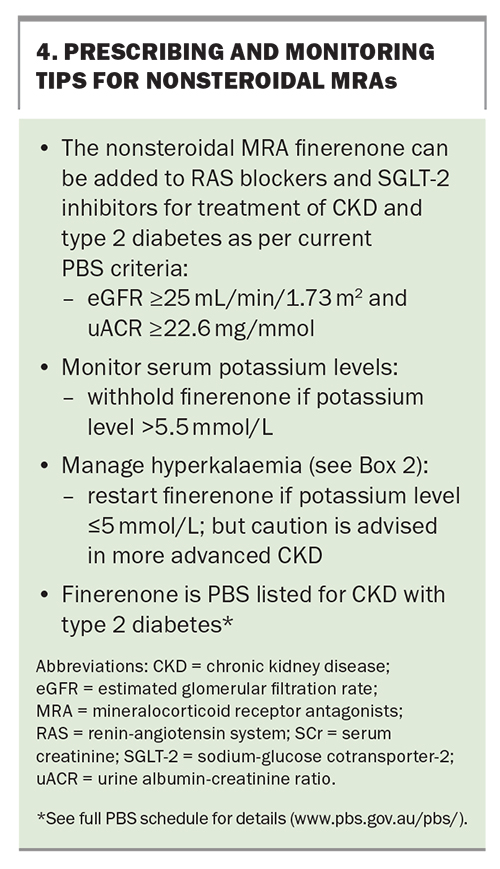
Mineralocorticoid receptor antagonists (MRAs) such as spironolactone have an established role in the management of heart failure and refractory hypertension (especially in patients with low to normal potassium levels), and also reduce albuminuria.34-36 However, the side-effect profile, which includes increased risk of hyperkalaemia, acute kidney injury and gynecomastia, can often limit their use. Finerenone is a nonsteroidal MRA that is more selective for mineralocorticoid receptors. It has a lower risk of hyperkalaemia (similar to that of lower-dose spironolactone). Recent placebo-controlled trials have shown a synergistic effect of finerenone with RAS blockade in reducing the risk of kidney function decline and CV events in patients with type 2 diabetes and albuminuria, but there is a need for electrolyte monitoring as CKD advances.37,38 Finerenone is listed on the PBS for patients with CKD (eGFR of 25 mL/min/1.73 m2 or higher and uACR of 22.6 mg/mmol or higher) with type 2 diabetes in combination with RAS blockers and SGLT-2 inhibitors, unless medically contraindicated or intolerant. Tips for prescribing and monitoring nonsteroidal MRAs are highlighted in Box 4.
GLP-1 receptor agonists
GLP-1 receptor agonists (such as dulaglutide and semaglutide) stimulate glucose-dependent insulin release from pancreatic beta cells and suppress glucagon release from alpha cells. They are now a well-established third-line treatment for type 2 diabetes (after metformin and SGLT-2 inhibitors).16 GLP-1 receptor agonists slow gastric emptying, suppress appetite and inhibit unnecessary hepatic gluconeogenesis, thus aiding weight loss, which further improves insulin sensitivity. They have gastrointestinal side effects and increase the risk of hypoglycaemia, although this risk is low.
Even more effective analogues, such as tirzepatide, the first glucose-dependent insulinotropic polypeptide/GLP-1 receptor agonist (or twincretin), and combination semaglutide-cagrilintide, show promise in achieving improved glycaemic control and weight loss.39,40 Tirzepatide is currently only TGA approved for type 2 diabetes, and semaglutide-cagrilintide is undergoing clinical trials.
Several studies have demonstrated the efficacy of GLP-1 receptor agonists in reducing major cardiovascular events in people with type 2 diabetes and HbA1c below 7%, and in reducing proteinuria, which suggests potential kidney protective benefits.41-43 The recently completed FLOW trial aims to assess the impact of semaglutide on kidney function decline in people with type 2 diabetes, with key finding expected later in 2024.44 GLP-1 receptor agonists are currently only TGA approved for type 2 diabetes. Tips for prescribing and monitoring GLP-1 receptor agonists are highlighted in Box 5.
Multidisciplinary team input
This article aims to support clinicians to care for people with CKD or those who may have risk factors for CKD and does not provide exhaustive advice for managing people with kidney failure and advanced comorbid conditions that occur with CKD. Working within a multidisciplinary team that includes (but is not limited to) a nephrologist, a CKD nurse, a psychologist, endocrinologists, cardiologists and allied health professionals, can assist GPs in developing individualised care plans and in managing people with complex conditions.
Conclusion
The rising incidence of CKD is a significant public health concern. Addressing this growing problem involves a holistic approach to management that includes implementing nonpharmacological and pharmacological therapies, and input from a multidisciplinary team. Regular screening can help with early diagnosis and management. Optimising holistic care through nonpharmacological measures, including nutritional management and supporting lifestyle changes, is a mainstay for improving patient outcomes. Providing culturally responsible care to vulnerable at-risk populations, specifically Aboriginal and Torres Strait Islander people, is important for ensuring that care is effective, accessible, continuous and sustainable. A growing suite of pharmacological therapies are available for CKD and common comorbidities, and should be used to aggressively manage this disease and its associated risk factors. MT
COMPETING INTERESTS: Dr Krishnan and Professor Hughes: None. Professor Dwyer has received lecture fees and advisory board payments from Astra-Zeneca, Bayer, Boehringer-Ingelheim and Eli-Lilly; and seed grant funding from Servier. Dr Thomas has received lecture fees and advisory board payments from Astra-Zeneca, Bayer, Boehringer-Ingelheim and Eli-Lilly.
Acknowledgements: Professor Hughes is supported by a National Health and Medical Research Council Emerging Leadership Fellowhip (GNT 1174758- ‘Yes We Will’ – Implementing Indigenous-led Aboriginal and Torres Strait Islander kidney health in Northern and Central Australia). Professor Hughes and Dr Thomas are clinician-researchers of the NHMRC funded eGFR3 Study (GNT 1184083).
References
1. Australian Institute of Health and Welfare (AIHW). Chronic kidney disease prevalence among Australian adults over time. Updated August 2023. AIHW: Canberra; 2023. Available online at: https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease-prevalence-adults/summary (accessed March 2024).
2. Australian Institute of Health and Welfare (AIHW). Chronic kidney disease: Australian facts. Updated December 2023. AIHW: Canberra; 2023. Available online at: https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease/contents/how-many-people-are-living-with-ckd (accessed March 2024).
3. Australian Institute of Health and Welfare (AIHW). Dialysis. AIHW: Canberra; 2023. Available online at: https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease/contents/treatment-and-management-of-chronic-kidney-disease/dialysis (accessed March 2024).
4. Deloitte. Changing the chronic kidney disease landscape. March 2023. Deloitte, 2024. Available online at: https://www.deloitte.com/au/en/services/economics/analysis/changing-chronic-kidney-disease-landscape.html (accessed March 2024).
5. Kidney Research UK. Kidney disease is a public health emergency that threatens to overwhelm the NHS, major new report reveals. 05 June 2023. Available online at: https://www.kidneyresearchuk.org/2023/06/05/kidney-disease-is-a-public-health-emergency-that-threatens-to-overwhelm-the-nhs-major-new-report-reveals/ (accessed March 2024).
6. Australia and New Zealand dialysis and transplant registry. Annual Report 2022 Chapter 1: Summarising the number of incident patients with kidney failure with replacement therapy in Australia and New Zealand, the rate per million population and the demographic and clinical characteristics of incident patients ANZDATA; Adelaide, 2022. Available online at: https://www.anzdata.org.au/wp-content/uploads/2023/02/c01_incidence_2021_ar_2022_v1.0_FINAL.pdf (accessed February 2024).
7. Kidney Health Australia. Chronic Kidney Disease (CKD) Management in Primary Care (4th edition). Kidney Health Australia, Melbourne, 2020. Available online at: https://kidney.org.au/uploads/resources/CKD-Management-in-Primary-Care_handbook_2020.1.pdf (accessed February 2024).
8. van Mil D, Kieneker LM, Evers-Roeten B, et al. Participation rate and yield of two home-based screening methods to detect increased albuminuria in the general population in the Netherlands (THOMAS): a prospective, randomised, open-label implementation study. Lancet 2023; 402: 1052-1064.
9. Neuen BL, Jun M, Wick J, et al. Estimating the population-level kidney benefits of improved uptake of SGLT-2 inhibitors in patients with chronic kidney disease in Australian primary care. medRxiv 2023; 06.26.23291881
10. George Institute. The wider benefits of SGLT2 inhibitors. George Institute: NSW, 2024. Available online at: https://www.georgeinstitute.org/our-impact/policy-and-recommendations/the-wider-benefits-of-sglt2-inhibitors (accessed March 2024).
11. Chaturvedi S, Bianchi MEV, Bello A, Crowshoe H, Hughes JT. Barriers to optimal kidney health among indigenous peoples. Kidney International Reports 2024; 9: 508-511.
12. Stewart M, Brown JB, Freeman TR. The fourth component: enhancing the patient–clinician relationship. In: Patient-Centered Medicine. Fourth edition. Taylor and Francis: London, 2024.
13. Sagmeister MS, Harper L, Hardy RS. Cortisol excess in chronic kidney disease - a review of changes and impact on mortality. Front Endocrinol (Lausanne) 2023; 13: 1075809.
14. Mason EC, Grierson AB, Sie A, et al. Co-occurring insomnia and anxiety: a randomized controlled trial of internet cognitive behavioral therapy for insomnia versus internet cognitive behavioral therapy for anxiety. Sleep 2023; 46: zsac205.
15. Avesani CM, Cardozo LFMF, Yee-Moon Wang A, et al. Planetary health, nutrition, and chronic kidney disease: connecting the dots for a sustainable future. J Ren Nutr 2023; 33(6S): S40-S48.
16. Hewat C. Can a dietician help? Aust Fam Physician 2009 Jun; 38: 405-407.
17. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney International 2022; 102. Available online at: https://kdigo. org/wp-content/uploads/2022/10/KDIGO-2022-Clinical-Practice-Guideline-for-Diabetes-Management-in-CKD.pdf (accessed March 2024).
18. Dwyer K, Robson B, Figtree P. A GP guide to chronic kidney disease in diabetes. AusDoc 2023. Available online at: https://www.ausdoc.com.au/therapy-update/ a-gp-guide-to-chronic-kidney-disease-in-diabetes/ (accessed March 2024).
19. Dietitian/Nutritionists from the Nutrition Education Materials Online (NEMO). Low salt diet. Queensland Government: Qld, 2015. Available online at: https://www.health.qld.gov.au/__data/assets/pdf_file/0025/150577/renal_lowsalt.pdf (accessed March2024).
20. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021; 143: 1157-1172.
21. Kidney disease improving global outcomes (KDIGO). KDIGO Clinical practice guideline for lipid management in chronic kidney disease. Kidney International Supplements. 2013; 3: 262.
22. Tonelli M, Wanner C; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. Lipid management in chronic kidney disease: synopsis of the Kidney disease: Improving Global Outcomes 2013 clinical practice guideline. Ann Intern Med 2014; 160: 1 82.
23. Taliercio JJ, Nakhoul G, Mehdi A, et al; CRIC study Investigators. Aspirin for primary and secondary prevention of mortality, cardiovascular disease, and kidney failure in the Chronic Renal Insufficiency Cohort (CRIC) Study. Kidney Med 2022; 4: 100547.
24. Major RW, Oozeerally I, Dawson S, Riddleston H, Gray LJ, Brunskill NJ. Aspirin and cardiovascular primary prevention in non-endstage chronic kidney disease: a meta-analysis. Atherosclerosis 2016; 251: 177-182.
25. Kidney Health Australia (KHA). Factsheet. Who needs a health check? KHA; Vic, 2022. https://kidney.org.au/uploads/resources/KHA-First-Nations-Factsheet-Who-needs-a-kidney-health-check.pdf (accessed March 2024).
26. Caring for Australian and New Zealanders with Kidney Impairment (CARI). Recommendations for culturally safe kidney care in First Nations Australians. In: First Nations Australian Guidelines. CARI Guidelines: NSW, 2004. Available online at: https://www.cariguidelines.org/guidelines/management-of-chronic-kidney-disease-among-first-nations/ (accessed March 2024).
27. Giatras I, Lau J, Levey AS. Effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: a meta-analysis of randomized trials. Angiotensin-Converting-Enzyme Inhibition and Progressive Renal Disease Study Group. Ann Intern Med 1997; 127: 337-345.
28. Lewis EJ, Hunsicker LG, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851-860.
29. Kidney disease improving global outcomes (KDIGO). KDIGO 2021 Clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney International Supplements. 2021; 99(3S): S1-S87.
30. Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26: 2827-2847.
31. Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342: 145-153. Erratum in: 2000 May 4;342(18):1376. Erratum in: N Engl J Med 2000; 342: 748.
32. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436-1446.
33. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023; 388: 117-127.
34. Pitt B, Zannad F, Remme WJ, et al; Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 34: 709-717.
35. Acelajado MC, Hughes ZH, Oparil S, Calhoun DA. Treatment of resistant and refractory hypertension. Circ Res 2019; 124: 1061-1070.
36. Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383-1392.
37. Bakris GL, Agarwal R, Anker SD, et al; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219-2229.
38. Pitt B, Filippatos G, Agarwal R, et al; FIGARO-DKD Investigators. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385: 2252-2263.
39. Jastreboff AM, Aronne LJ, Ahmad NN, et al; SURMOUNT-1 Investigators. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022 ;387: 205-216.
40. Becerril S, Frühbeck G. Cagrilintide plus semaglutide for obesity management. Lancet 2021; 397: 1687-1689.
41. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021; 9: 653-662.
42. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834-1844.
43. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019; 394: 131-138.
44. Rossing P, Baeres FMM, Bakris G, et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant 2023; 38: 2041-2051.