Immunoglobulin A nephropathy: a collaborative opportunity for GPs and nephrologists

Immunoglobulin A nephropathy is the leading cause of kidney failure requiring dialysis or transplantation in young adults and is associated with significant morbidity and socioeconomic burden. Available therapies are mainly supportive, but recent years have seen notable developments in risk prediction tools, standardisation of guidelines and an explosion of clinical trials investigating treatments for this common form of glomerulonephritis.
- Immunoglobulin A (IgA) nephropathy is the most common cause of glomerulonephritis worldwide, and up to 40% of patients with this disease will progress to kidney failure and require kidney replacement therapy.
- IgA nephropathy should be considered in patients with microscopic haematuria, with or without proteinuria, renal decline and hypertension.
- Key investigations in primary care include checking for hypertension, urinary studies (microscopy, culture and susceptibility, and proteinuria quantification), a kidney function assessment using the estimated glomerular filtration rate, and renal imaging.
- Early nephrologist input is recommended if IgA nephropathy is suspected, with a renal biopsy required to confirm the diagnosis.
- Management includes dietary salt restriction, lifestyle modification, blood pressure optimisation, cardiovascular risk management and proteinuria suppression therapies; corticosteroid therapy may, with nephrologist input, be considered for selected patients.
- There have been rapid developments in new therapeutic targets to halt the progression of kidney disease in patients with IgA nephropathy.
Immunoglobulin A nephropathy (IgAN) is the most common glomerulonephritis worldwide and has a prevalence in Australia of 10.5 per 100,000 people.1 The hallmark characteristic of IgAN is mesangial deposition of glycosylated IgA1, which can be seen in kidney biopsy specimens. It has a wide clinical presentation, ranging from asymptomatic microscopic haematuria, with or without proteinuria, to macroscopic haematuria, nephrotic syndrome and even rapidly progressive glomerulonephritis. IgAN is often associated with antecedent or concurrent upper respiratory tract or gastrointestinal infections. It most often occurs in young adults in the second and third decades of life. A higher prevalence and more aggressive disease progression are seen in people from East Asian countries.2 This geographical variation may be related to genetic and environmental factors, as well as greater recognition of the disease through active kidney screening programs and a lower threshold for performing kidney biopsies in this region.
For many years, IgAN has been managed with supportive therapies, which involve optimising blood pressure control, restriction of salt intake and lifestyle modifications. However, this approach does not treat the underlying pathogenesis of the disease, and up to 40% of patients progress to kidney failure and require dialysis or transplantation two decades after diagnosis.3 Given that IgAN has an immunological basis, corticosteroid therapy has recently been proven to have a role in managing patients with IgAN who are at high risk of kidney disease progression. However, use of corticosteroids is associated with substantial short-term and long-term adverse effects, resulting in variable uptake by clinicians. Thus, the emergence of several promising therapeutic agents that specifically target the underlying pathogenesis of IgAN represents an exciting potential improvement in IgAN management.
This review summarises recent developments, focusing on our current understanding of IgAN’s pathogenesis, its clinical presentation and diagnosis, existing and emerging therapies, and how GPs and nephrologists can collaborate in its management.
Pathogenesis of IgAN
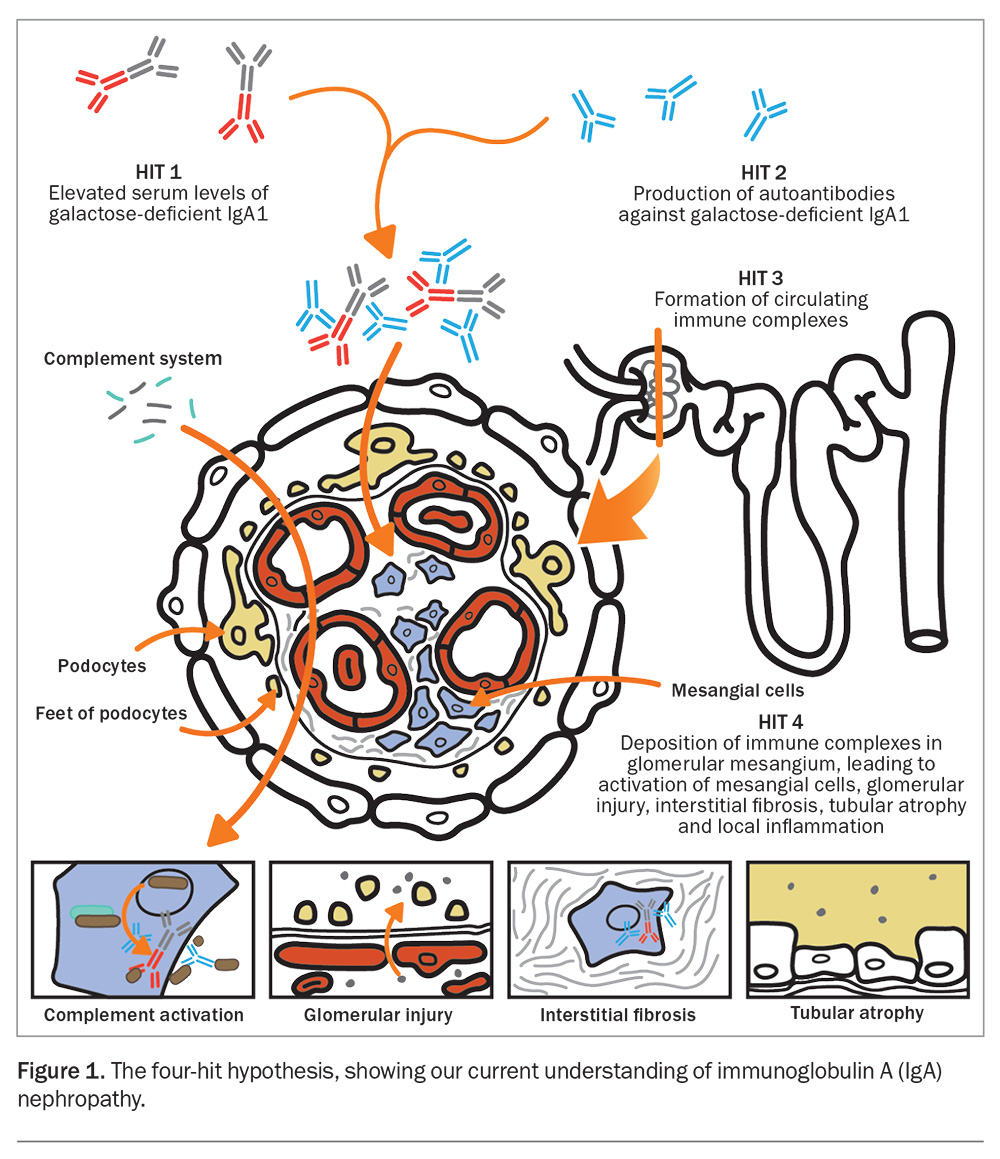
IgAN is an immune-mediated disease, which is influenced by environmental and genetic factors that lead to an abnormal innate and adaptive immune response targeting the glomeruli and mesangium. About 90% of cases are sporadic, whereas 10% of cases are familial, with a strong association with chromosome 6q22-23.4 An aberrant immune response, usually triggered by an infective process, leads to the upregulation of toll-like receptors, complement activation and abnormal B-cell and T-cell activity. This is best described by the ‘four-hit hypothesis’, which ultimately leads to a local inflammatory response in the glomerular mesangium (Figure 1).5,6
The first hit in the four-hit hypothesis involves the generation of circulating galactose-deficient IgA (Gd-IgA1), which is thought to be inherently pathogenic.6,7 This may result from antigen presentation and priming of mucosal B-cells located in lymphoid aggregates within the gut or respiratory mucosal system.8 B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) are key cytokines involved in T-cell-independent activation of B-cells; they have been linked to the perpetual production of circulating Gd-IgA1 by promoting the maturation of B-cells and plasma cell survival.9,10 The alternative and lectin complement pathways have also been implicated, with more than 90% of kidney biopsy specimens from patients with confirmed IgAN showing C3 deposition, and about a third having C4d deposition.11,12
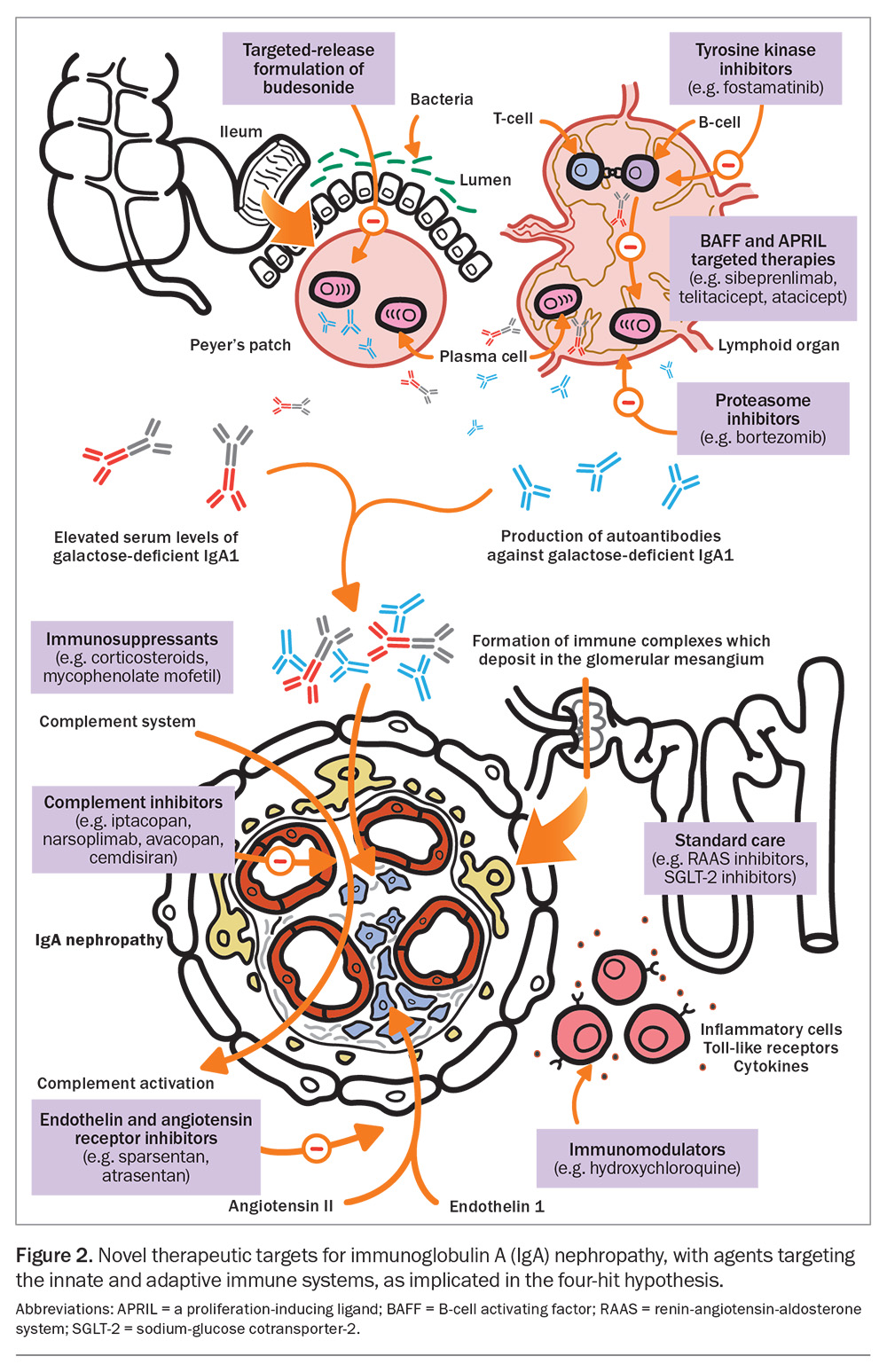
Recent advances in our knowledge of the pathogenesis of IgAN have enabled a wide scope for therapeutic discoveries targeting its pathogenic pathway (Figure 2).
Shared care
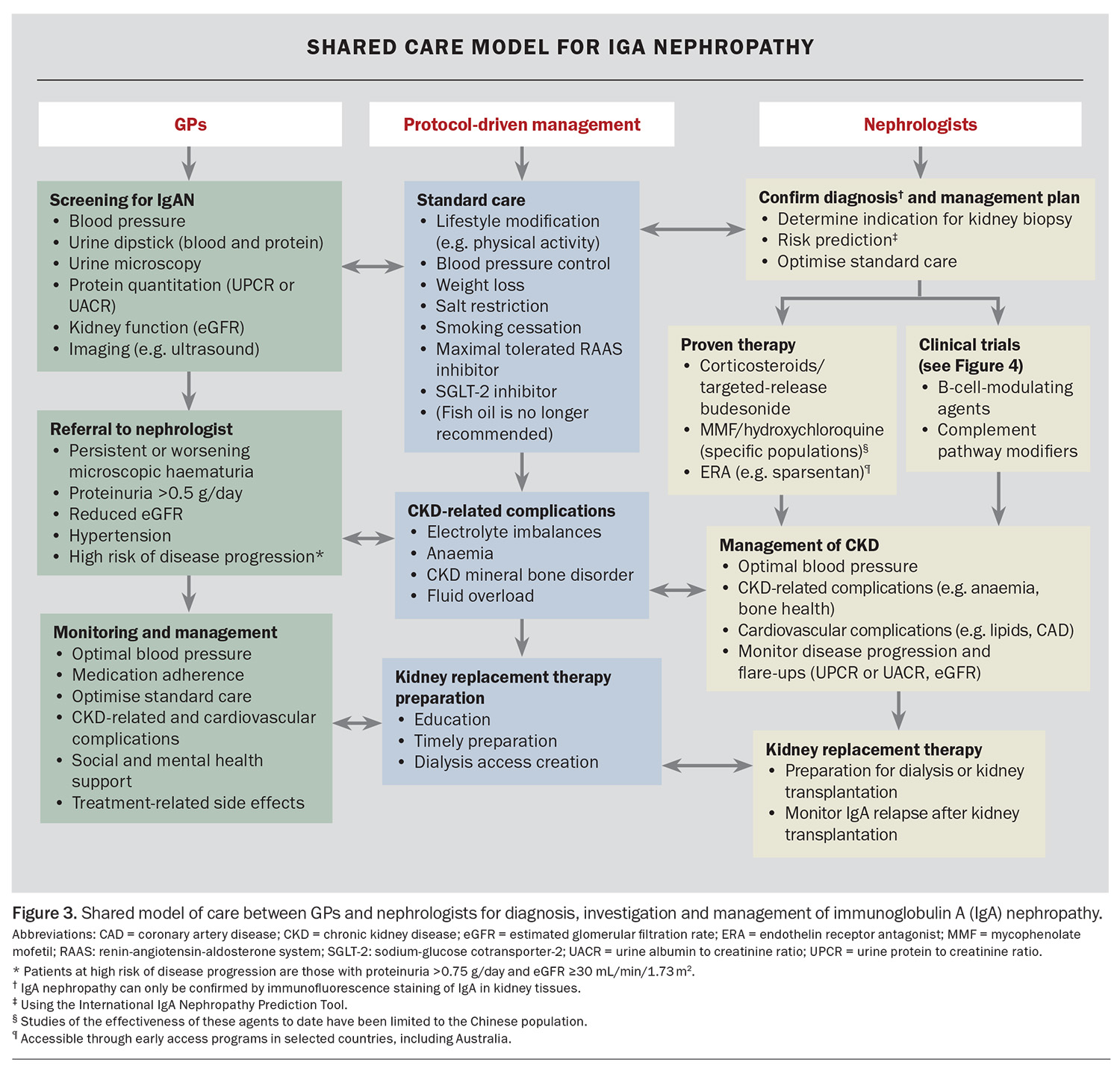
A recommended approach to the investigation, diagnosis, risk assessment and management of IgAN that involves shared care between GPs and nephrologists is outlined in Figure 3. GPs play a crucial role in screening and identifying people at risk of IgAN and can refer patients with suspected IgAN to a nephrologist for confirmation of the diagnosis, risk stratification and initiation of therapy. For patients with confirmed IgAN, GPs have an important ongoing role in optimising standard care, ensuring medication adherence and monitoring for treatment-related side effects.
Clinical presentation, referral and diagnosis
IgAN should be suspected in any patient who presents with persistent microscopic haematuria with or without proteinuria, hypertension and renal impairment. Patients may not present with all features of IgAN, but a combination of these is most suggestive of the disease.
Investigations include:
- blood pressure check
- urine dipstick and urine microscopy, culture and susceptibility
- protein quantitation using a urine protein to creatinine ratio or a urine albumin to creatinine ratio
- kidney function assessment using the estimated glomerular filtration rate (eGFR)
- renal imaging (e.g. ultrasound or noncontrast CT of the renal tract) to check for nonglomerular haematuria causes, size of kidneys (which indicates chronicity of disease) and obstructive causes of renal decline.
If IgAN is suspected, referral to a nephrologist is recommended, especially for patients with persistent or worsening microscopic haematuria, proteinuria of greater than 0.5 g/day, hypertension or a reduced eGFR (Figure 3).
Ultimately, kidney biopsy is needed for a histopathological diagnosis of IgAN. A biopsy provides valuable information about the activity and chronicity of disease, which helps the nephrologist to make therapeutic decisions and determine prognosis.
Disease risk stratification
Although many people with IgAN have an indolent course, up to 40% of patients progress to kidney failure and require dialysis or transplantation about 20 years after diagnosis.3 Risk stratification is therefore vital in determining which patients are at high risk of kidney failure and would benefit most from treatment.
The only validated risk prediction tool for IgAN is the International IgAN Prediction Tool, which can be used up to two years after a kidney biopsy to predict the five-year risk of a 50% decline in eGFR or kidney failure that will require dialysis or transplantation. It incorporates 12 clinical and histological variables (including race and Oxford MEST-C scores).13,14 This prediction tool is freely available to all clinicians online (https://qxmd.com/calculate) or using an app called Calculate by QxMD (available for iOS and Android). There is a similar but separate version of the tool for paediatric patients. This prediction tool is recommended by the international Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.15 However, it does have limitations, including its reliance on a recent kidney biopsy, lack of validity outside the time frame of prediction and an inability to predict treatment response to a specific therapy. Nevertheless, the tool allows clinicians to engage with patients and communicate their likelihood of disease progression, to allow informed decision making about treatment options.
The degree of proteinuria and eGFR decline have been proven in epidemiological and patient-level data analyses to be strong and modifiable risk factors for disease progression.16,17 Given the prolonged time course to kidney failure in IgAN, the use of proteinuria and eGFR slope as surrogate markers for disease progression has had a significant impact on trial designs and has renewed commercial interest in therapeutic developments for the disease.17,18
Treatment of IgAN
Standard care for IgAN includes proteinuria suppression, optimal blood pressure control, cardiovascular risk management and lifestyle modifications, such as salt restriction, weight loss, smoking cessation and physical activity (Figure 3).15 The goal of treatment is to delay progression of kidney disease and, ultimately, prevent kidney failure that would require dialysis or kidney transplantation.
Proteinuria suppression therapies
Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers are the preferred agents for proteinuria suppression, as there is strong evidence that they prolong kidney survival regardless of their blood pressure-lowering effects.19-21 The use of fish oil and other supplemental medicines, such as vitamins, is no longer recommended, as there is only weak evidence that they improve kidney outcomes.15
Sodium-glucose cotransporter-2 inhibitors are also now accepted as an effective proteinuria suppression treatment for patients with IgAN. There is good evidence of their benefit in this population, with trials consistently showing reduced proteinuria and improved kidney survival.22
More recently, the PROTECT trial showed that sparsentan, a dual inhibitor of the renin-angiotensin and endothelin-1 system, is effective in reducing proteinuria and delaying progression of chronic kidney disease.23 Sparsentan has been approved by European regulatory authorities for treatment of adults with IgAN.24 It can be accessed through early access programs in several countries, including Australia.
Corticosteroid therapy
Corticosteroids can be considered for patients at high risk of progression to kidney failure, despite maximal supportive care, after a thorough discussion of individualised risks and benefits.
The Therapeutic Evaluation of Steroids in IgA Nephropathy Global (TESTING) study showed that a six- to nine-month course of oral methylprednisolone reduced the risk of a composite outcome of decline in kidney function, kidney failure and death due to kidney disease by 47% after a median follow up of 4.2 years.25 However, the use of corticosteroids was associated with a substantial increased risk of severe adverse events, particularly infection, among those who received a full dose of methylprednisolone (0.6 to 0.8 mg/kg/day).26 On the other hand, the Supportive versus Immunosuppressive Therapy for the Treatment Of Progressive IgA Nephropathy (STOP-IgAN) study, which used a combination of intravenous methylprednisolone and oral prednisolone, did not show any beneficial short-term or long-term kidney outcomes, despite a reduction in proteinuria in the first 12 months.27,28
Common to both these trials is the finding that considerable caution should be exercised with use of corticosteroids, as they consistently increased adverse effects, including fatal infection-related complications. The KDIGO 2021 guidelines recommend that corticosteroids should ideally be avoided in patients with an eGFR less than 30 mL/min/1.73 m2, diabetes, obesity, latent infections, secondary diseases such as cirrhosis, active peptic ulceration, uncontrolled psychiatric illness or severe osteoporosis.15
Targeted-release budesonide is considered a disease-modifying agent and is taken orally, with limited systemic absorption. Budesonide is delivered to the distal ileum, where it targets mucosal B-cells within the Peyer’s patches. The Efficacy and Safety of Nefecon in Patients With Primary IgA Nephropathy (NefIgArd) trial showed that a nine-month course of 16 mg of budesonide daily resulted in a 48% reduction in proteinuria at 12 months and a slower decline in eGFR, which extended to at least two years.29,30 Compared with placebo, budesonide had an acceptable side effect profile, with common side effects including hypertension, peripheral oedema, muscle spasms and acne. The number of serious adverse events was low, with similar rates of infection as placebo, an absence of fractures and osteonecrosis and generally unchanged glycated haemoglobin levels. However, budesonide remains unavailable under the PBS for kidney indications in Australia, and its off-label use is costly.
Other immunosuppressant therapies
Mycophenolate mofetil (1.5 g twice daily for 12 months), in addition to optimised standard care, has been found to significantly reduce the risk of doubling of serum creatinine level, kidney failure or death from kidney or cardiovascular causes in a Chinese population.31 There was no significant difference in adverse events compared with standard care, although infections, such as pneumonia, were more common in the mycophenolate mofetil group.
An early-phase study of oral hydroxychloroquine (200 to 400 mg daily according to eGFR) has shown a significant reduction in proteinuria and stabilisation of eGFR.32 Several randomised controlled trials evaluating hydroxychloroquine’s use for treating IgAN have shown it has an acceptable safety profile; however, mucocutaneous, gastrointestinal and anaphylactic reactions have been noted in a small number of patients.33
To date, studies of the effectiveness of these agents have been limited to the Chinese population. Guidelines discourage the use of other immunosuppressants for IgAN, including calcineurin inhibitors, azathioprine, rituximab and cyclophosphamide, as they lack demonstrated efficacy.15
Emerging therapies
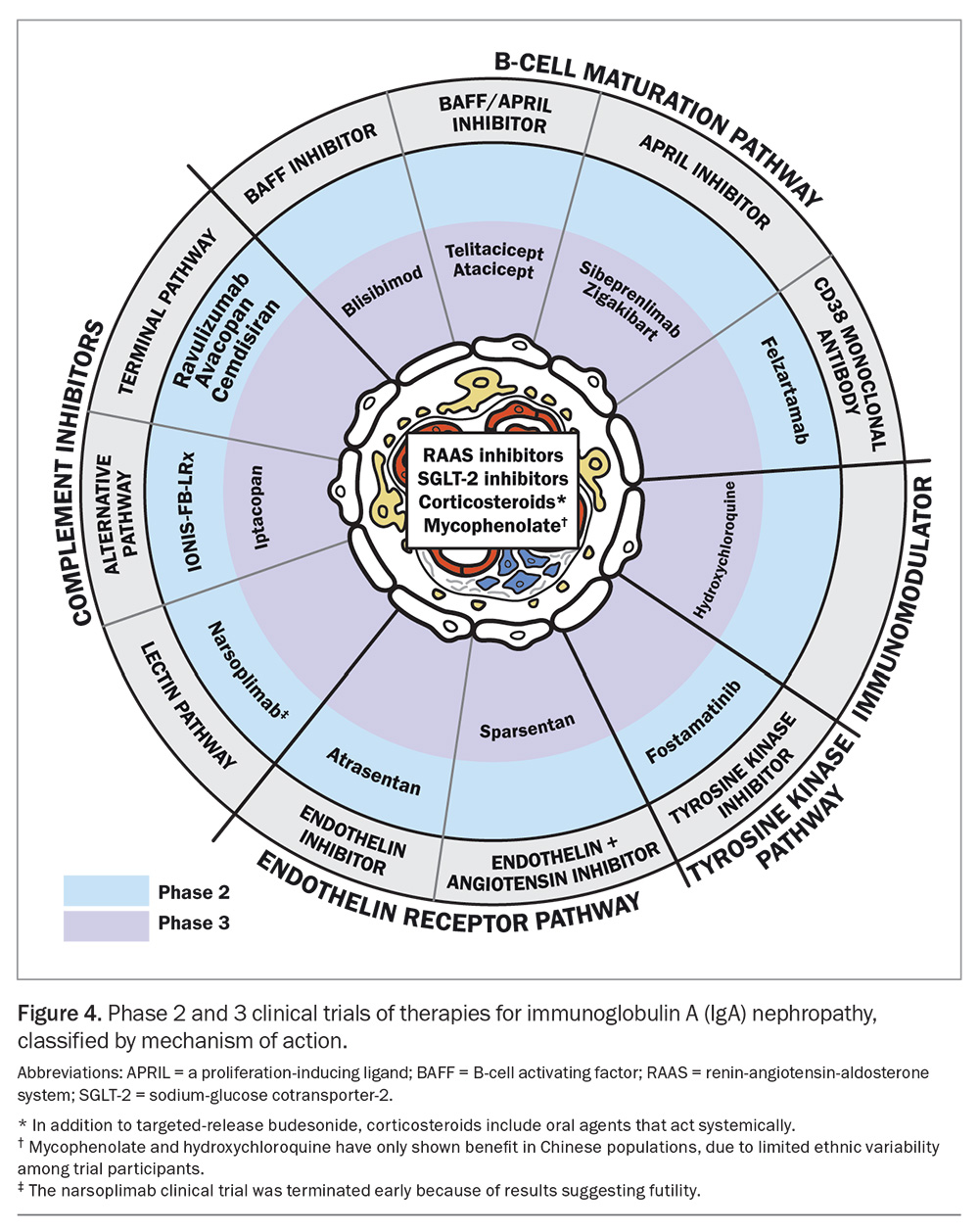
In the past few years, there has been an explosion of clinical trials of novel therapies for IgAN (Figure 4). Emerging therapies are usually trialled in patients at high risk of disease progression, with proteinuria more than 0.75 g/day and an eGFR of 30 mL/min/1.73 m2 or more. Among the phase 2 and higher clinical trials discussed here, agents targeting B-cells show promising results.
Sibeprenlimab is a monoclonal antibody that binds to and neutralises APRIL, which is responsible for B-cell proliferation. A phase 2 trial (ENVISION) showed that a 12-monthly dose of intravenous sibeprenlimab (2 mg/kg, 4 mg/kg or 8 mg/kg) reduced proteinuria compared with placebo, particularly for the higher doses. The placebo group showed progressive decline in eGFR at 12 months, whereas the sibeprenlimab groups showed preserved eGFR.34 The placebo and sibeprenlimab-treated groups had comparable safety profiles. More than 5% of patients receiving sibeprenlimab developed febrile illnesses (including COVID-19), nasopharyngitis, headaches, hypertension, diarrhoea or muscle spasms.34 Notably, the COVID-19 vaccine response was not attenuated by sibeprenlimab.35
Other B-cell therapies, such as zigakibart, which is an anti-APRIL agent, and atacicept, povetacicept and telitacicept, which are anti-APRIL and anti-BAFF agents, have also shown encouraging early-phase trial results.36,37 A phase 3 clinical trial of atacicept, povetacicept and telitacicept is underway.
Complement pathway modifiers are another novel therapeutic target that have generated much interest. Iptacopan is a potent selective complement factor B inhibitor that is involved in the alternative complement pathway. In a phase 2 study, patients were randomly assigned to receive 10 mg, 50 mg, 100 mg or 200 mg of iptacopan twice daily for three or six months. There was a significant dose-response effect, with a 23% reduction in urine protein to creatinine ratio seen in those taking the highest dose at three months, and continued reduction at six months.38 There was no significant difference in side effects between the iptacopan and placebo groups, and most adverse effects were mild or moderate (most often headache and back pain). Iptacopan is now in phase 3 trials and has recently been granted accelerated approval by the European Medicines Agency and US Food and Drug Administration because of its promising results.39
Finerenone is a nonsteroidal mineralo-corticoid receptor antagonist that has established protection against progressive chronic kidney disease in patients with diabetes. A phase 3 clinical trial (NCT05047263) is underway in patients with nondiabetic proteinuric kidney disease, and its findings may be applicable to the population with IgAN.
Conclusion
After decades of limited effective therapy for people with IgAN, a plethora of promising therapeutic options has emerged in the past few years. The new treatments are aimed at halting disease progression and reducing the morbidity and socioeconomic burden associated with IgAN. These developments have occurred in parallel with a growing understanding of the pathogenesis of the disease. Many of these emerging agents have shown an acceptable safety profile, although long-term efficacy and safety data are needed.
GPs can play a crucial role in identifying people at risk of IgAN, using simple screening tools such as blood pressure monitoring, urine dipsticks and kidney function tests. There should be a high level of suspicion for IgAN in patients with persistent microscopic haematuria, hypertension or reduced eGFR. Identifying these features will enable timely referral to a nephrologist and performance of a kidney biopsy to confirm the diagnosis. After a diagnosis of IgAN, nephrologists can facilitate early intervention using evidence-based therapy or novel therapeutic agents through clinical trials. For ongoing management, GPs have a vital role in optimising standard care, medication adherence and monitoring for important treatment-related side effects. A shared care model between nephrologists and GPs should span the course of disease from initial presentation to kidney replacement therapy (if ultimately required). It also involves monitoring disease activity, where proteinuria and reduced eGFR are the most useful indicators of disease severity, and managing chronic kidney disease-related complications. Given that IgAN does not always follow a benign disease course and typically affects younger people, the ultimate aim is to delay progression of kidney disease.
It is likely that, in future, a combined therapeutic approach to target the various aspects of IgAN’s pathogenic pathways will be necessary. Clinicians also need better validated tools to identify and stratify those at higher risk of disease progression, to inform treatment choices. As new therapies emerge, the collaboration between GPs and nephrologists will no doubt strengthen. MT
COMPETING INTERESTS: Associate Professor Wong has received fees for serving on advisory boards or steering committees or for scientific presentations from Travere Therapeutics, Baxter, Amgen, AbbVie, Chinook Therapeutics, Dimerix, Otsuka, GlaxoSmithKline, CSL Behring, Eledon Pharmaceuticals, Alpine, Kira Pharmaceuticals, George Clinical, Vera Therapeutics, AstraZeneca, Boehringer Ingelheim, Bridge Healthcare and Eli Lilly. Dr Qian and Dr Faigl: None.
References
1. Briganti EM, Dowling J, Finlay M, et al. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant 2001; 16: 1364-1367.
2. Petrou D, Kalogeropoulos P, Liapis G, Lionaki S. IgA nephropathy: current treatment and new insights. Antibodies 2023; 12(2): 40.
3. Wong K, Pitcher D, Braddon F, et al; RaDaR consortium. Effects of rare kidney diseases on kidney failure: a longitudinal analysis of the UK National Registry of Rare Kidney Diseases (RaDaR) cohort. Lancet 2024; 403: 1279-1289.
4. Woo KT, Lau YK, Choong HL, et al. Genomics and disease progression in IgA nephritis. Ann Acad Med Singap 2013; 42: 674-680.
5. Lai KN, Tang SC, Schena FP, et al. IgA nephropathy. Nat Rev Dis Primers 2016; 2: 16001.
6. Gentile M, Sanchez-Russo L, Riella LV, et al. Immune abnormalities in IgA nephropathy. Clin Kidney J 2023; 16: 1059-1070.
7. Moldoveanu Z, Wyatt RJ, Lee JY, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 2007; 71: 1148-1154.
8. Huang X, Xu G. An update on targeted treatment of IgA nephropathy: an autoimmune perspective. Front Pharmacol 2021; 12: 715253.
9. Sallustio F, Curci C, Chaoul N, et al. High levels of gut-homing immunoglobulin A+ B lymphocytes support the pathogenic role of intestinal mucosal hyperresponsiveness in immunoglobulin A nephropathy patients. Nephrol Dial Transplant 2021; 36: 1765. Erratum to: Nephrol Dial Transplant 2021; 36: 452-464.
10. Xin G, Shi W, Xu LX, Su Y, Yan LJ, Li KS. Serum BAFF is elevated in patients with IgA nephropathy and associated with clinical and histopathological features. J Nephrol 2013; 26: 683-690.
11. Maillard N, Wyatt RJ, Julian BA, et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol 2015; 26: 1503-1512.
12. Jiang Y, Zan J, Shi S, et al. Glomerular C4d deposition and kidney disease progression in IgA nephropathy: a systematic review and meta-analysis. Kidney Med 2021; 3: 1014-1021.
13. Barbour SJ, Coppo R, Zhang H, et al; International IgA Nephropathy Network. Application of the International IgA Nephropathy Prediction Tool one or two years post-biopsy. Kidney Int 2022; 102: 160-172.
14. Trimarchi H, Barratt J, Cattran DC, et al; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017; 91: 1014-1021.
15. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Int 2021; 100(4S): S1-S276.
16. Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 2007; 18: 3177-3183.
17. Thompson A, Carroll K, Inker LA, et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol 2019; 14: 469-481.
18. Inker LA, Heerspink HJL, Tighiouart H, et al. Association of treatment effects on early change in urine protein and treatment effects on GFR slope in IgA nephropathy: an individual participant meta-analysis. Am J Kidney Dis 2021; 78: 340-349.e1.
19. Konishi Y, Okada N, Okamura M, et al. Sodium sensitivity of blood pressure appearing before hypertension and related to histological damage in immunoglobulin a nephropathy. Hypertension 2001; 38: 81-85.
20. Praga M, Gutiérrez E, González E, Morales E, Hernandez E. Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol 2003; 14: 1578-1583.
21. Lv J, Zhang H, Chen Y, et al. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomised controlled trial. Am J Kidney Dis 2009; 53: 26-32.
22. Wheeler DC, Toto RD, Stefansson BV, et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int 2021; 100: 215-224.
23. Heerspink HJL, Radhakrishnan J, Alpers CE, et al; PROTECT Investigators. Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet 2023; 401: 1584-1594.
24. Travere Therapeutics. Travere Therapeutics and CSL Vifor announce European Commission approves FILSPARI (sparsentan) for the treatment of IgA nephropathy. 24 April 2024. Available online at: https://ir.travere.com/news-releases/news-release-details/travere-therapeutics-and-csl-vifor-announce-european-commission (accessed August 2024).
25. Lv J, Wong MG, Hladunewich MA, et al. Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA 2022; 327: 1888-1898.
26. Wong MG, Lv J, Hladunewich MA, et al; TESTING Study Group. The Therapeutic Evaluation of Steroids in IgA Nephropathy Global (TESTING) Study: trial design and baseline characteristics. Am J Nephrol 2021; 52: 827-836.
27. Rauen T, Eitner F, Fitzner C, et al; STOP-IgAN Investigators. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015; 373: 2225-2236.
28. Rauen T, Wied S, Fitzner C, et al; STOP-IgAN Investigators. After ten years of follow-up, no difference between supportive care plus immunosuppression and supportive care alone in IgA nephropathy. Kidney Int 2020; 98: 1044-1052.
29. Barratt J, Lafayette R, Kristensen J, et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int 2023; 103: 391-402.
30. Lafayette R, Kristensen J, Stone A, et al; NefIgArd trial investigators. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. Lancet 2023; 402: 859-870. Erratum in: Lancet 2023; 402: 850.
31. Hou FF, Xie D, Wang J, et al; MAIN Trial Investigators. Effectiveness of mycophenolate mofetil among patients with progressive IgA nephropathy: a randomized clinical trial. JAMA Netw Open 2023; 6: e2254054.
32. Liu LJ, Yang YZ, Shi SF, et al. Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am J Kidney Dis 2019; 74: 15-22.
33. Stefan G, Mircescu G. Hydroxychloroquine in IgA nephropathy: a systematic review. Ren Fail 2021; 43: 1520-1527.
34. Mathur M, Barratt J, Chacko B, et al; ENVISION Trial Investigators Group. A Phase 2 trial of sibeprenlimab in patients with IgA nephropathy. N Engl J Med 2024; 390: 20-31. doi: 10.1056/NEJMoa2305635.
35. McCafferty K, Follman K, Pasetti M, et al. Covid vaccine responses during sibeprenlimab treatment of IgA nephropathy (IgAN): an interim analysis. Nephrol Dial Transplant 2023; 38 (Suppl 1): gfad063a_3347.
36. Barratt J, Kooienga L, Hour B, et al. MO212: Updated interim results of a Phase 1/2 study to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and clinical activity of BION-1301 in patients with IGA nephropathy. Nephrol Dial Transplant 2022; 37 (Suppl 3): gfac067.011.
37. Barratt J, Tumlin J, Suzuki Y, et al; JANUS study investigators. Randomized Phase II JANUS study of atacicept in patients with IgA nephropathy and persistent proteinuria. Kidney Int Rep 2022; 7: 1831-1841.
38. Zhang H, Rizk DV, Perkovic V, et al. Results of a randomized double-blind placebo-controlled Phase 2 study propose iptacopan as an alternative complement pathway inhibitor for IgA nephropathy. Kidney Int 2024; 105: 189-199.
39. McGovern G. FDA grants accelerated approval to iptacopan for the reduction of proteinuria in adults with IgAN. Pharmacy Times 2024; Aug 8. Available online at: https://www.pharmacytimes.com/view/fda-grants-accelerated-approval-to-iptacopan-for-the-reduction-of-proteinuria-in-adults-with-igan (accessed August 2024).