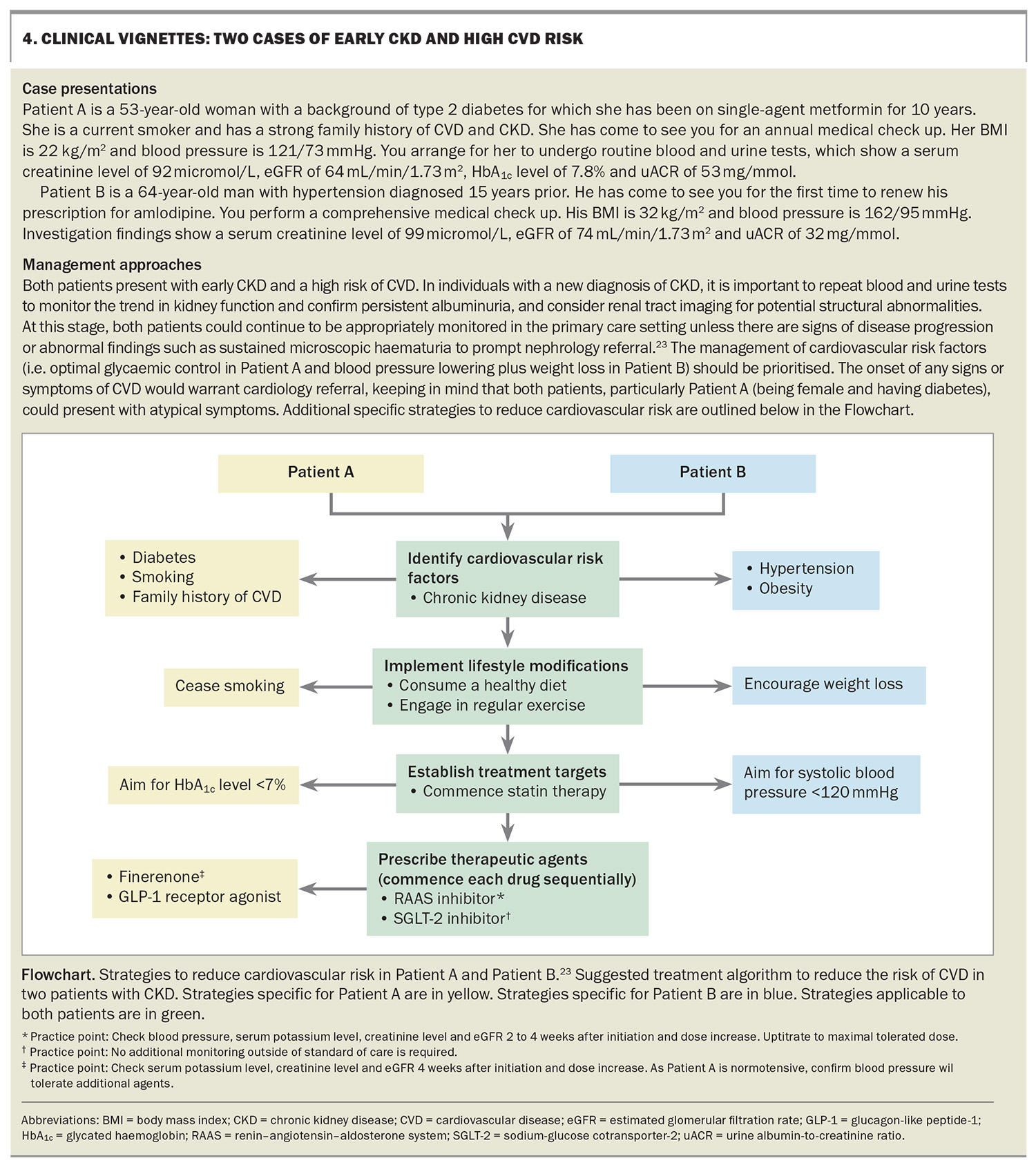
Reducing cardiovascular risk in people with chronic kidney disease
People with chronic kidney disease are at an increased risk of cardiovascular disease. The early recognition of this risk and implementation of effective risk-lowering strategies can be initiated in primary care using a multidisciplinary approach.
Correction
A correction for this article appears in the August 2024 issue of Medicine Today. The online version and the full text PDF of this article (see link above) are correct.
- Chronic kidney disease (CKD) is associated with an increased risk of cardiovascular disease (CVD) and CVD-related death independent of other traditional risk factors.
- Individuals with moderate-to-severe CKD have an estimated five-year CVD risk of at least 10%, placing them in the highest risk category.
- GPs play a key role in the multidisciplinary care required to reduce cardiovascular risk in individuals with CKD, which includes lifestyle modifications, the management of traditional risk factors (e.g. hypertension, diabetes mellitus, hyperlipidaemia) and preventing progressive CKD.
- First-line pharmacotherapy includes renin–angiotensin–aldosterone system (RAAS) inhibitors and sodium-glucose cotransporter-2 (SGLT-2) inhibitors.
- Adjunct therapy for diabetic kidney disease includes nonsteroidal mineralocorticoid receptor antagonists (MRAs) and glucagon-like peptide-1 receptor agonists.
- If medications such as RAAS inhibitors, SGLT-2 inhibitors and MRAs have been ceased in patients with CKD consider recommencing as soon as appropriate to maximise their long-term cardioprotective effects.
Chronic kidney disease (CKD) affects over 10% of the global population and is unequivocally associated with increased risks of cardiovascular disease (CVD) and cardiovascular mortality.1-4 Specifically, a reduced estimated glomerular filtration rate (eGFR) and the presence of albuminuria or proteinuria portend greater cardiovascular events independent of one another and in addition to other potential confounding factors, such as the presence of diabetes and hypertension.1,5 Compared with individuals with normal kidney function, the risk of cardiovascular death is twice and three times as high in those with eGFR 30 to 59 mL/min/1.73 m2 and 15 to 29 mL/min/1.73 m2, respectively. CVD is the main cause of death in people with kidney failure requiring kidney replacement therapy (maintenance dialysis or kidney transplantation).6,7
The strong association between CKD and CVD, alongside prevalent shared comorbidities highlight the importance of adopting a multidisciplinary approach to cardiovascular risk reduction. GPs play a pivotal role in fostering patient engagement and facilitating effective care co-ordination. Individuals with early stages of CKD do not require specialist involvement and usually present in primary care settings; thus, understanding the risk of CVD and implementing long-term risk-reducing treatments remain crucial. As more efficacious therapeutic options become available to curb cardiovascular events and delay CKD progression, it becomes increasingly important to ensure they are used appropriately. This article summarises the underlying mechanisms linking CVD and CKD, discusses screening for CVD in people with CKD and outlines lifestyle modifications and pharmacological interventions aimed at mitigating CVD risk in those with CKD. Additionally, two clinical vignettes illustrate the practical application of these strategies in real-world scenarios.
Risk factors for cardiovascular disease in chronic kidney disease
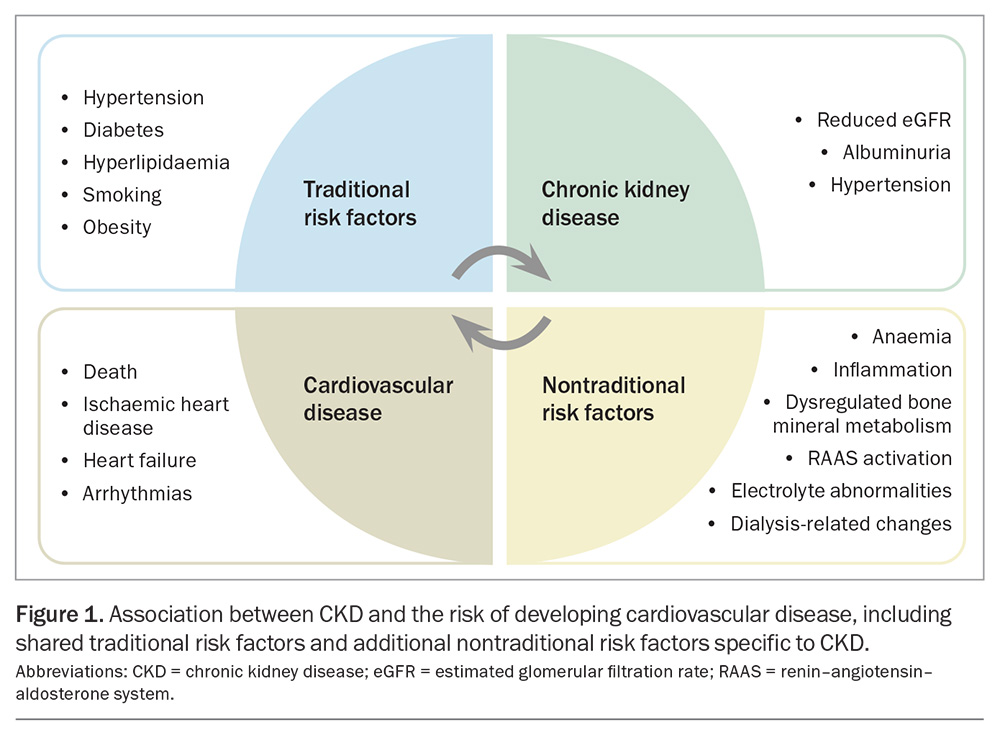
CKD is associated with a range of CVD subtypes resulting in outcomes such as ischaemic heart disease, heart failure, arrhythmias, sudden cardiac death, peripheral vascular disease, cerebrovascular disease and venous thrombosis.8,9 This spectrum reflects the number of underlying mechanisms contributing to CVD in people with CKD, which include shared traditional risk factors such as hypertension and diabetes (the two leading causes of CKD), as well as nontraditional factors unique to patients with CKD, such as those listed below and illustrated in Figure 1.
- Anaemia: this is a significant complication of CKD that can lead to adverse cardiovascular outcomes (e.g. heart failure)
- Inflammation: CKD can induce inflammation, reflected by elevated levels of inflammatory markers (e.g. ferritin, C-reactive protein, interleukin-6, tumour necrosis factor), which is associated with cardiac remodelling and fibrosis, cardiomyopathy, left ventricular hypertrophy and diastolic dysfunction
- Dysregulation in bone mineral metabolism: elevated levels of fibroblast growth factor 23, seen even in early stages of CKD, and elevated parathyroid hormone and phosphate levels; in addition, 1,25-dihydroxyvitamin D deficiency drives vascular calcification and increases arterial stiffness and potentially the risk of cardiac fibrosis and heart failure
- Overactivation of the renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system: this contributes to hypertension, vasoconstriction and an increased risk of CVD
- Electrolyte abnormalities: hyperkalaemia is particularly prevalent in advanced CKD and can lead to an increased risk of cardiac arrhythmias
- Dyslipidaemia: patients with CKD typically exhibit hypertriglyceridaemia and low levels of HDL cholesterol, with increased atherogenic qualities of these lipids
- Dialysis-related shifts: patients with kidney failure receiving chronic haemodialysis are at particular risk of sudden cardiac death, precipitated by intradialytic hypotension, hypoxaemia and rapid electrolyte and volume shifts.8,10,11
Assessment of cardiovascular risk in chronic kidney disease
Screening
There are insufficient data to support routine screening for coronary artery disease in asymptomatic patients with CKD, as there is no evidence it improves cardiovascular outcomes or alters management.9,12 This is further emphasised by the results of the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches-Chronic Kidney Disease (ISCHEMIA-CKD), which found no difference in a composite outcome of death or nonfatal myocardial infarction in patients with advanced CKD (eGFR <30 mL/min/1.73 m2) and moderate- to-severe ischaemia on stress testing randomised to medical therapy compared with invasive coronary revascularisation.13 Therefore, CVD risk assessment and appropriate risk factor modification in all patients with CKD are recommended in the first instance.
Risk assessment of cardiovascular disease
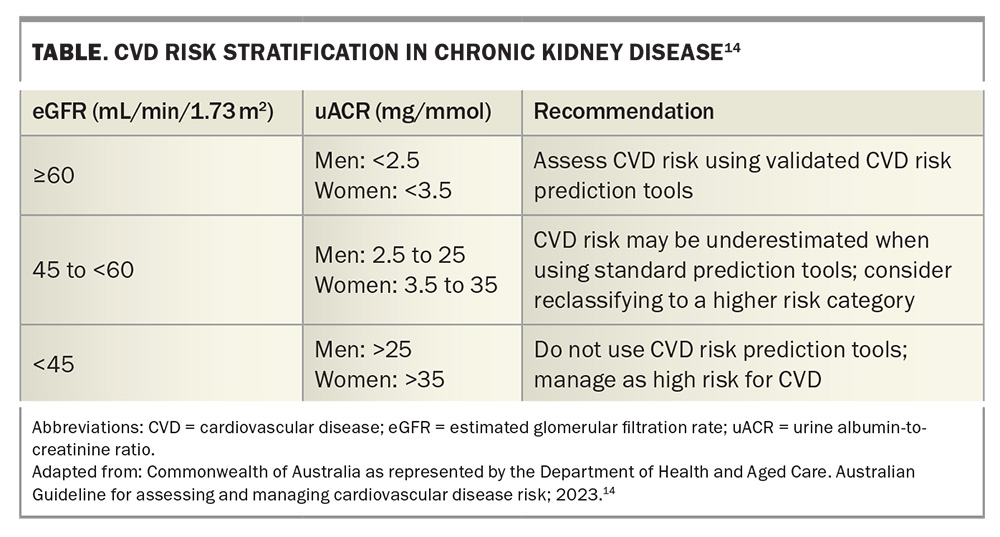
Most cardiovascular risk prediction calculators do not consider the eGFR or presence of albuminuria and, thus, largely underestimate CVD risk in patients with CKD. These calculators are not validated for use in people with advanced CKD or kidney failure requiring dialysis or transplantation.5,9 As such, current Australian guidelines recommend individuals with an eGFR less than 45 mL/min/1.73 m2 or macroalbuminuria (urine albumin-to-creatinine ratio [uACR] >25 mg/mmol in men and >35 mg/mmol in women) be regarded at high CVD risk with a five-year risk of 10% or greater (Table).14
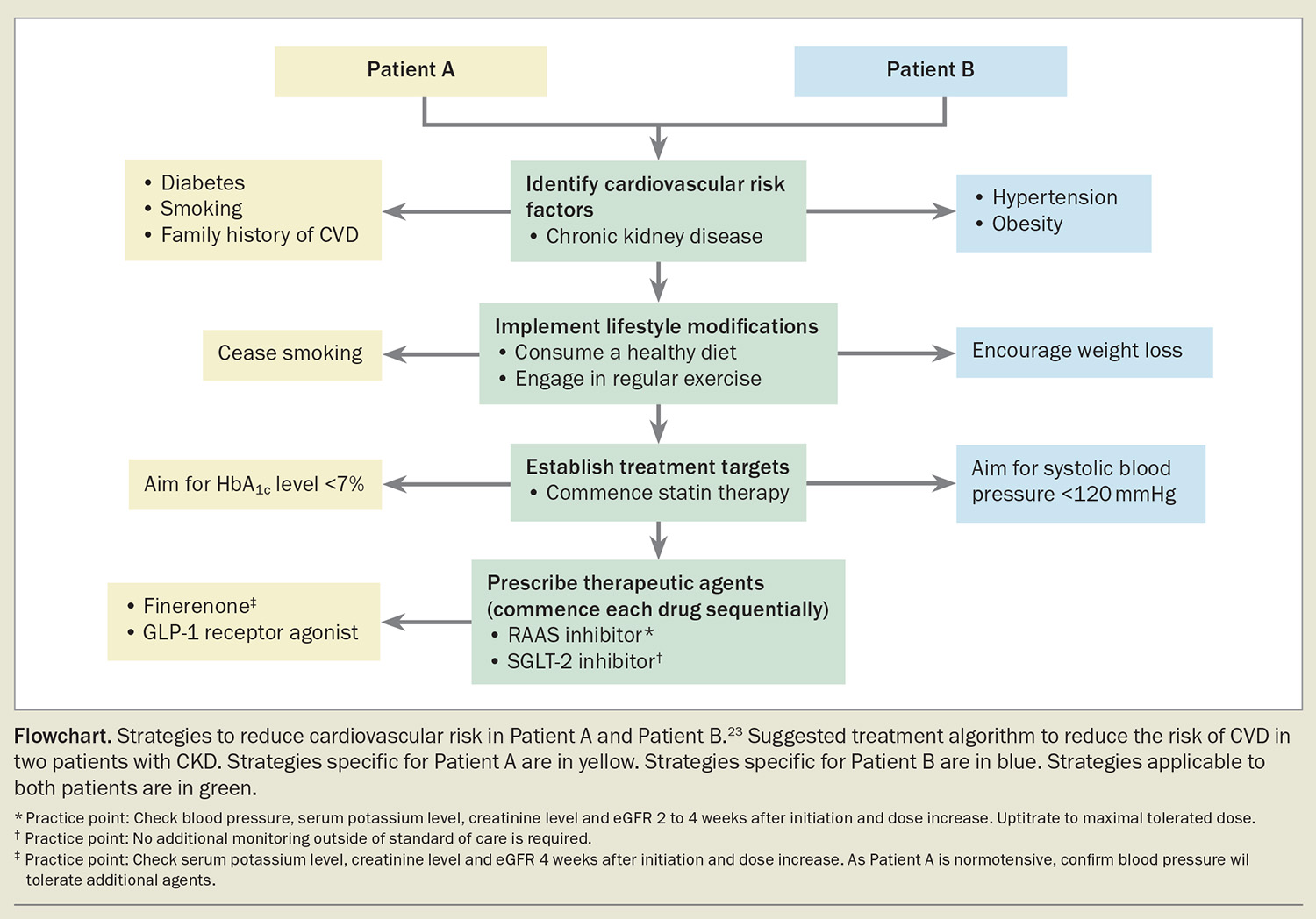
Approach to reducing cardiovascular risk in chronic kidney disease
The management approach to reducing cardiovascular risk in patients with CKD should include a multidisciplinary team to address lifestyle modifications and common traditional risk factors, such as hypertension, hyperlipidaemia and diabetes, and consider additional pharmacological therapies where appropriate. As CKD itself is a significant cardiovascular risk factor, efforts to prevent kidney disease progression by reducing albuminuria and slowing the rate of eGFR decline should also be prioritised in mitigating CVD risk. It must be recognised that in an increasingly comorbid and elderly population, the treatment targets and recommendations are general in nature. A holistic, patient-centred approach is advised, taking into consideration factors such as tolerability, safety and polypharmacy.
Lifestyle factors
Clear and effective communication of an individual’s cardiovascular risk, along with behavioural strategies and continual patient engagement, are essential for the successful implementation of lifestyle modifications. There are limited data regarding lifestyle modifications aimed at reducing cardiovascular risk specifically in CKD populations, so these guidelines are largely extrapolated from studies conducted in general populations and expert opinions. Some recommended lifestyle modifications are listed in Box 1.
Diet
A well-balanced diet with increased fruit and vegetable intake and limited intake of processed meats, refined carbohydrates and sweetened beverages is recommended.15-17 Dietary interventions, such as consuming a Mediterranean diet, increasing plant-based intake and restricting carbohydrate intake, appear to lower blood pressure and are associated with improved kidney function.18 Salt restriction in patients with CKD has been shown to significantly lower blood pressure and albuminuria, as well as increase the efficacy of RAAS inhibitors.19 Therefore, a sodium intake of less than 2 g/day is recommended.
Exercise
Regular exercise in adults with CKD reduces albuminuria, increases aerobic capacity, lowers blood pressure and improves quality of life.20,21 Moderate-intensity physical activity is recommended for 30 minutes, five times per week, or 150 minutes per week; however, this should be tailored to the individual patient’s tolerance and cardiovascular fitness levels.22,23
Smoking cessation
Chronic smoking is associated with an increased risk of irreversible proteinuria independent of the daily or cumulative number of cigarettes smoked, and an increased risk of kidney failure in CKD.24,25 On this basis, along with robust evidence indicating that smoking cessation is associated with a reduction in cardiovascular risk in the general population, the cessation of smoking and tobacco products is recommended in patients with CKD.22
Obesity
Obesity is associated with an increased risk of CKD and CVD. People with a body mass index greater than 25 kg/m2 should be encouraged to lose weight, taking into consideration their other medical comorbidities, overall health and physical activity levels.23
Management of traditional risk factors
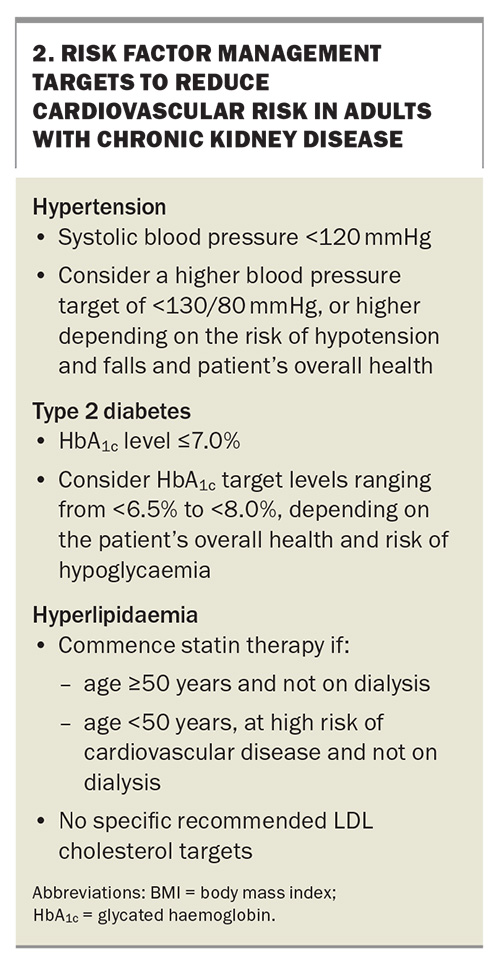
The treatment targets of traditional risk factor management are outlined in Box 2.
Blood pressure control
The lowering of blood pressure in patients with hypertension significantly reduces the risk of CVD and mortality in patients with CKD; however, optimal treatment targets remain contentious.26-28 Current international CKD guidelines suggest aiming for a systolic blood pressure less than 120 mmHg, but to consider a more liberal approach depending on the patient’s overall health, frailty and risk of postural hypotension and falls.29 Other guidelines, including the Kidney Health Australia CKD Management in Primary Care handbook, recommend higher targets of blood pressure (<130/80 mmHg).17 These guidelines do not apply to patients on dialysis, in whom ideal blood pressure targets are even more unclear.
Glycaemic control
Type 2 diabetes is a significant shared risk factor of CKD and CVD. Australian guidelines recommend a glycated haemoglobin (HbA1c) target of 7.0% or less in patients with CKD.9 Tight glycaemic control has not been shown to reduce the risk of kidney failure or cardiovascular death in CKD, although it may help reduce microalbuminuria and nonfatal myocardial infarction.30 The safety of tight glycaemic control has also not been confirmed in CKD, particularly in frail patients with comorbidities. International guidelines have more recently recommended individualised treatment parameters, with HbA1c targets ranging from less than 6.5% to less than 8.0% to balance the risk of hypoglycaemia and long-term benefits of glycaemic control depending on the patient.15 Of note, HbA1c measurements can be unreliable in people with advanced CKD or end-stage kidney disease because of anaemia and anaemia treatments including erythropoietin-stimulating agents, iron replacement and blood transfusions.15 Specific medications, such as sodium-glucose cotransporter-2 (SGLT-2) inhibitors, nonsteroidal mineralocorticoid receptor antagonists (MRAs) and glucagon-like peptide 1 (GLP-1) receptor agonists, may reduce the risk of cardiovascular events and slow CKD progression in type 2 diabetes.
Lipid management
The Study of Heart and Renal Protection (SHARP) trial clearly demonstrated that primary prevention with simvastatin plus ezetimibe reduced the risk of major atherosclerotic events in patients with CKD compared with placebo.31 The cardiovascular effects of statin therapy do not appear to extend to patients on dialysis, despite significantly lowering LDL cholesterol levels, and the benefits in terms of major vascular events and mortality diminish as the eGFR declines.32,33 Specific LDL treatment targets for these populations have not been defined.
Statin-based therapy is currently recommended in adults with CKD:
- aged 50 years and older not treated with chronic dialysis or kidney transplantation
– in those with an eGFR less than 60 mL/min/1.73 m2, a statin or statin plus ezetimibe combination should be considered - younger than 50 years of age at high risk of CVD, defined as having one or more of the following:
– known coronary disease
– diabetes mellitus
– prior ischaemic stroke
– estimated 10-year risk of coronary death or myocardial infarction greater than 10%.23,34
Pharmacotherapy
The landscape of available therapies to reduce cardiovascular risk and slow the rate of eGFR decline in CKD is evolving, with the relatively recent development of drugs including SGLT-2 inhibitors, MRAs and GLP-1 receptor agonists.
Aspirin
Overall, aspirin has been shown to reduce the risk of myocardial infarction in people with CKD when used for both primary and secondary prevention, while also increasing the risk of bleeding.35 When studied for primary prevention alone, the risk of bleeding from aspirin appeared to outweigh the potential cardiovascular benefits of treatment.36 Therefore, aspirin is only recommended for the secondary prevention of recurrent ischaemic cardiovascular events in people with CKD and established coronary artery disease.
Renin–angiotensin–aldosterone system inhibitors
RAAS blockade slows the rate of eGFR decline, lowers proteinuria and reduces the risk of kidney failure and cardiovascular events independent of its blood pressure-lowering effects.37-40 RAAS inhibition with ACE inhibitors and angiotensin receptor blockers has been the cornerstone of CKD treatment for many years. Discontinuation of RAAS inhibitors in patients with CKD is associated with elevated risks of all-cause mortality and cardiovascular events; thus, these agents should be continued if safe and feasible, or promptly recommenced after any period of discontinuation, such as during acute illness or episodes of acute kidney injury.41
RAAS inhibitors are recommended for the following patients with CKD:
- patients requiring first-line treatment for hypertension
- patients with type 2 diabetes and uACR greater than 3 mg/mmol
- patients without type 2 diabetes and uACR greater than 30 mg/mmol
– consider in patients with uACR between 3 and 30 mg/mmol.15,29,42
Sodium-glucose cotransporter-2 inhibitors
The cardio- and renoprotective class effects of SGLT-2 inhibitors demonstrated in studies (e.g. the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation [CREDENCE], Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease [DAPA-CKD] and Study of Heart and Kidney Protection with Empagliflozin [EMPA-KIDNEY] trials) conducted in patients with CKD with and without diabetes are overwhelmingly positive.43-46 SGLT-2 inhibitors have been shown to lower albuminuria and reduce the risk of kidney failure, acute kidney disease and cardiovascular events, including cardiovascular death and hospitalisation for heart failure.
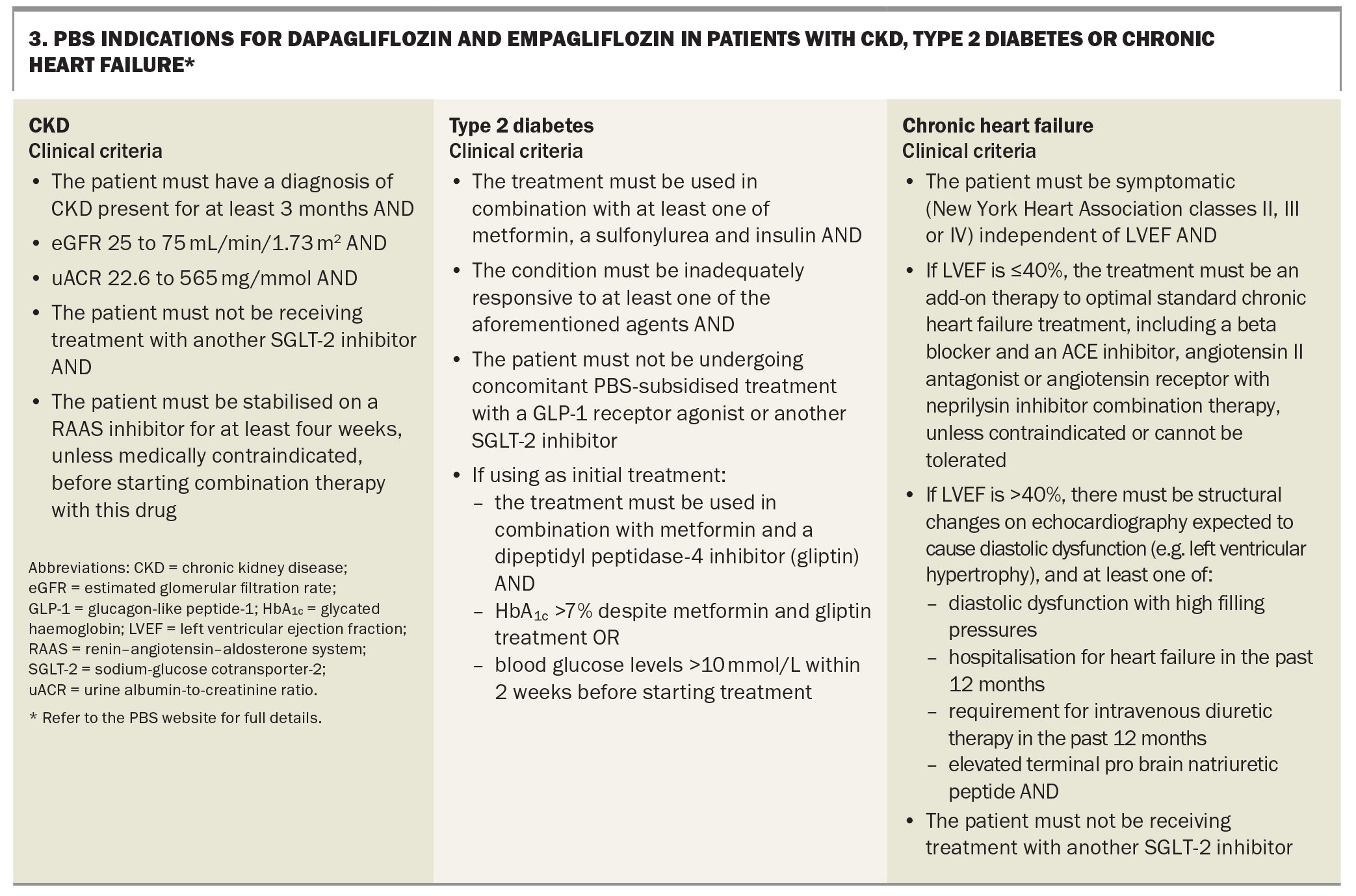
Although the benefits of SGLT-2 inhibitors may extend beyond these groups and international guidelines recommend their use across broader indications,23 dapagliflozin and empagliflozin are listed on the PBS for the indications listed in Box 3.
Finerenone
Finerenone is a selective, nonsteroidal MRA that has been shown to reduce the risk of kidney failure and cardiovascular events in patients with type 2 diabetes and CKD in the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trials.47-49 A class effect has not been confirmed with other selective and nonselective MRAs.
Finerenone is PBS listed for patients who have CKD with type 2 diabetes, plus all the following:
- absence of known significant nondiabetic renal disease
- eGFR 25 mL/min/1.73 m2 or greater
- uACR of 22.6 mg/mmol or greater
- stabilised on a RAAS inhibitor for at least four weeks unless medically contraindicated, before starting combination therapy with this drug
- treatment must be in combination with an SGLT-2 inhibitor unless medically contraindicated or intolerant
- must not be receiving treatment with another selective nonsteroidal MRA, renin inhibitor or potassium-sparing diuretic
- must not have established heart failure with reduced ejection fraction with an indication for MRA treatment.
Refer to the PBS website for full details.
GLP-1 receptor agonists
GLP-1 receptor agonists reduce the risk of major cardiovascular events in patients with type 2 diabetes, including in those with an eGFR less than 60 mL/min/1.73 m2.50 More recently, the findings from the Evaluate Renal Function with Semaglutide Once Weekly (FLOW) trial, specifically studying the effects of semaglutide on renal and cardiovascular outcomes in patients with type 2 diabetes and CKD, have been published.51 Adults with type 2 diabetes, an HbA1c level of 10% or less and CKD with albuminuria and who were established on maximal tolerated RAAS blockade were included in the study, regardless of treatment with other concomitant glucose-lowering agents. The study found that semaglutide lowered the risk of the primary outcome (a composite of kidney failure, 50% reduction in the eGFR or death from kidney-related or cardiovascular causes) by 24% and major cardiovascular events by 16% compared with placebo over a median follow-up period of 3.4 years.
Long-acting GLP-1 receptor agonists are currently recommended as an additional glucose-lowering agent in patients with CKD and type 2 diabetes who are yet to achieve their individualised glycaemic targets despite use of metformin and SGLT-2 inhibitor therapy, or in those who are unable to use these drugs.15 However, based on the results of the FLOW trial, the indications for use in CKD may broaden in the future.
Practical prescribing considerations
Considering the acute effects of treatment, it is generally recommended to commence each drug sequentially after establishing a period of stability for at least four weeks. RAAS inhibitors, SGLT-2 inhibitors and finerenone can induce haemodynamic changes resulting in a reversible decline in eGFR, referred to as the ‘eGFR dip’, which typically occurs within the first four weeks of treatment.52 If the reduction in the eGFR exceeds 30% or continues to decline beyond the initial dip following drug commencement, alternative causes should be considered. RAAS inhibitors and finerenone may cause hyperkalaemia, particularly in advanced kidney disease (eGFR <30 mL/min/1.73 m2); therefore, serum potassium levels should be monitored following drug initiation and dose escalation in line with the Australian Medicines Handbook guidelines. Notably, concurrent therapy with SGLT-2 inhibitors may lower this risk of hyperkalaemia, and finerenone is not recommended in individuals with an eGFR less than 25 mL/min/1.73 m2 or serum potassium level greater than 5.0 mmol/L.53 All these agents also exhibit blood pressure-lowering effects, which should be monitored. These potential adverse effects are likely to be heightened in the setting of acute illness and hypovolaemic states, and therefore may require additional monitoring or drug suspension during these periods. Two clinical vignettes are presented in Box 4, with management approaches outlined in the Flowchart.23
Conclusion
Patients with CKD face a heightened risk of CVD, significantly worsening their health outcomes. Combatting this risk requires a multidisciplinary approach, leveraging various existing and emerging therapies. However, the long-term benefits of some strategies may be limited in advanced CKD. Therefore, early CKD diagnosis, risk assessment and prompt management initiated in primary care are crucial. MT
COMPETING INTERESTS: Dr Kim has received an RACP Jacquot Research Entry Scholarship. Associate Professor Kotwal has received payments from Chinook Therapeutics and Novartis for SC membership, payments from Dimerix for National Leader duties and speaker fees from Amgen.
References
1. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073-2081.
2. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLOS One 2016; 11: e0158765.
3. Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15: 1307-1315.
4. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med 2004; 351: 1296-1305.
5. Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015; 3: 514-525.
6. van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79: 1341-1352.
7. Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008; 371: 2173-2182.
8. Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol 2022; 18: 696-707.
9. Sarnak MJ, Amann K, Bangalore S, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol 2019; 74: 1823-1838.
10. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021; 143: 1157-1172.
11. Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet 2016; 388: 276-284.
12. McCallum W, Sarnak MJ. Screening for cardiovascular disease in CKD: COMMENTARY. Kidney360 2022; 3: 1839-1841.
13. Bangalore S, Maron DJ, O’Brien SM, et al. Management of coronary disease in patients with advanced kidney disease. N Engl J Med 2020; 382: 1608-1618.
14. Australian Guideline for assessing and managing cardiovascular disease risk. Canberra: Commonwealth of Australia, as represented by the Department of Health and Aged Care; 2023. Available online at: https://www.cvdcheck.org.au/ (accessed May 2024).
15. Kidney Disease Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98: S1-S115.
16. Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis 2020; 76: S1-S107.
17. Kidney Health Australia. Chronic Kidney Disease (CKD) Management in Primary Care. 5th ed. Melbourne: Kidney Health Australia; 2024. Available online at: https://kidney.org.au/health-professionals/ckd-management-handbook (accessed May 2024).
18. Palmer SC, Maggo JK, Campbell KL, et al. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst Rev 2017; 4: Cd011998.
19. McMahon EJ, Campbell KL, Bauer JD, Mudge DW, Kelly JT. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev 2021; 6: Cd010070.
20. Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 2011; 2011: Cd003236.
21. Robinson ES, Fisher ND, Forman JP, Curhan GC. Physical activity and albuminuria. Am J Epidemiol 2010; 171: 515-521.
22. Gansevoort RTD, Correa-Rotter RP, Hemmelgarn BRP, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. The Lancet (British edition) 2013; 382: 339-352.
23. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2024; 105: S117-S314.
24. Halimi JM, Giraudeau B, Vol S, et al. Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int 2000; 58: 1285-1292.
25. Hallan SI, Orth SR. Smoking is a risk factor in the progression to kidney failure. Kidney Int 2011; 80: 516-523.
26. Malhotra R, Nguyen HA, Benavente O, et al. Association between more intensive vs less intensive blood pressure lowering and risk of mortality in chronic kidney disease stages 3 to 5: a systematic review and meta-analysis. JAMA Intern Med 2017; 177: 1498-1505.
27. Aggarwal R, Petrie B, Bala W, Chiu N. Mortality outcomes with intensive blood pressure targets in chronic kidney disease patients. Hypertension 2019; 73: 1275-1282.
28. Ninomiya T, Perkovic V, Turnbull F, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. BMJ 2013; 347: f5680.
29. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int 2021; 99: S1-S87.
30. Ruospo M, Saglimbene VM, Palmer SC, et al. Glucose targets for preventing diabetic kidney disease and its progression. Cochrane Database Syst Rev 2017; 6: Cd010137.
31. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377: 2181-2192.
32. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360: 1395-1407.
33. Herrington WG, Emberson J, Mihaylova B, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol 2016; 4: 829-839.
34. Wanner C, Tonelli M. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 2014; 85: 1303-1309.
35. Natale P, Palmer SC, Saglimbene VM, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev 2022; 2: Cd008834.
36. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373: 1849-1860.
37. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851-860.
38. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861-869.
39. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355: 253-259.
40. Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: A Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis 2016; 67: 728-741.
41. Tang C, Wen XY, Lv JC, et al. Discontinuation of renin-angiotensin system inhibitors and clinical outcomes in chronic kidney disease: a systemic review and meta-analysis. Am J Nephrol 2023; 54: 234-244.
42. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3: 1-150.
43. Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022; 400: 1788-1801.
44. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295-2306.
45. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436-1446.
46. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023; 388: 117-127.
47. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219-2229.
48. Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385: 2252-2263.
49. [no authors]. Finerenone for chronic kidney disease associated with type 2 diabetes with albuminuria. Aust Prescr 2023; 46: 68-69.
50. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021; 9: 653-662.
51. Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, Baeres FMM, Idorn T, Bosch-Traberg H, Lausvig NL, Pratley R; FLOW Trial Committees and Investigators. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med 2024 May 24; e-pub (https://doi.org/10.1056/NEJMoa2403347).
52. Heerspink HJL, Cherney DZI. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol 2021; 16: 1278-1280.
53. Neuen BL, Oshima M, Agarwal R, et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation 2022; 145: 1460-1470.