Mild cognitive impairment and dementia: detection and diagnosis

Dementia affects one in 12 people aged over 65 years. GPs and practice nurses have an important role in detection and assessment, and GPs in the diagnosis of dementia.
- Over 400,000 people in Australia had dementia in 2023, and an estimated 50% of these do not have a formal diagnosis.
- GPs and practice nurses have a key role in detection and assessment, and GPs in the diagnosis of dementia.
- The presentation might include functional difficulties or behavioural changes, in addition to cognitive complaints. These might be noticed by the GP, practice nurse or receptionist, or reported by the person themselves or by a family or friend.
- Any member of the primary care team may identify issues and raise concerns that prompt the need to investigate further whether a person has mild cognitive impairment or dementia. An annual health assessment for people aged older than 75 years is a good opportunity to identify issues and concerns that could be related to mild cognitive impairment or dementia.
- A comprehensive assessment can take place over several visits and include both a personal and collaborative history, physical examination and investigations, including blood tests and CT scans.
- Any reversible and treatable causes, such as delirium, depression, drugs or other organic causes of symptoms, should be excluded.
- If uncertain, mild cognitive impairment may be considered as a diagnosis and the patient monitored over time.
Dementia affects one in 12 people aged over 65 years. Population ageing means that more Australians will live with dementia. An estimated 411,100 people in Australia were living with dementia in 2023, and this number is projected to more than double by 2058.1 In 2022, dementia was the second leading cause of burden and injury, and the second leading cause of death in Australia. In 2021, dementia caused 10% of all deaths in Australia.1
Only half of all people living with dementia have received a formal diagnosis. We estimate that about 50% of people in Australia with dementia have not received a formal diagnosis (i.e. between 231,720 and 283,200 people). An Australian primary care study found 55% of people with dementia were undiagnosed and international meta-analysis pooling data from 23 studies reported that 62% of people with dementia were not diagnosed.2,3
This article provides an overview of the detection and diagnosis of mild cognitive impairment (MCI) and dementia.
Dementia: types and associated neuropathology
Dementia is an umbrella term for neurodegenerative diseases resulting in cognitive decline and functional difficulties. Alzheimer’s disease is the most common form of dementia (affecting 50 to 70% of cases). Vascular dementia (affecting up to 20% of cases), Lewy body dementia (affecting 10 to 15% of cases) and frontotemporal dementia (affecting 2% of cases) are also common forms. Each of these types of dementia is characterised by a different type of neuropathology. Amyloid plaques and neurofibrillary tangles are hallmarks of Alzheimer’s disease; white matter ischemia and infarctions reflect vascular dementia; focal frontal lobe degeneration occurs in frontotemporal dementia; and Lewy bodies (as seen in Parkinson’s disease), amyloid plaques and neurofibrillary tangles are seen in Lewy body dementia. Notably, neuropathological studies show that most community-dwelling older people with dementia have a mixture of different types of neuropathology.4
18F-fluorodeoxyglucose positron emission tomography (PET) brain scans (which are Medicare Benefits Schedule [MBS] rebatable) are sometimes used to determine the aetiology of a dementia. Amyloid PET brain scans (which are not MBS rebatable) are used in specialist clinics to determine or confirm whether a dementia is caused by Alzheimer’s disease. This will likely be a prerequisite once monoclonal antibodies become available in Australia. Amyloid PET is also starting to be used to help diagnose preclinical Alzheimer’s disease, although the benefits and harms of patients being given a preclinical diagnosis are not clear.5
Mild cognitive impairment
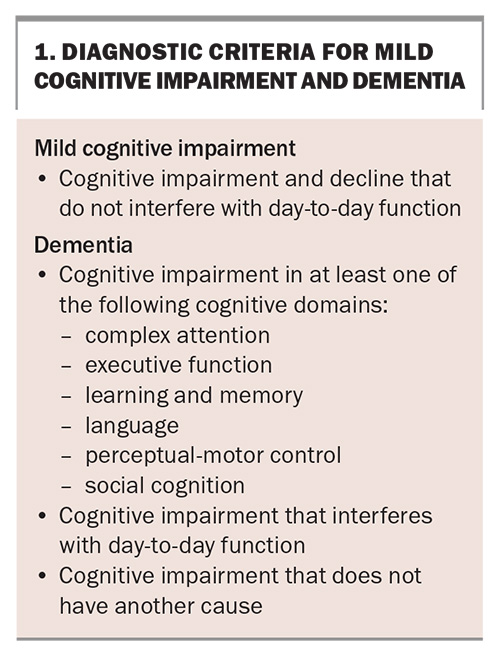
MCI is a syndrome in which an individual has subjective cognitive complaints and objective impairment, but the impairment is not sufficient to cause significant difficulty in day-to-day function.6,7
A meta-analysis showed that people with MCI are 3.3 times (95% CI, 2.5-4.5) more likely to progress to dementia compared with others their age with normal cognition.6 Furthermore, of those diagnosed with MCI in population samples, 14.9% (95% CI, 11.6%-19.1%) developed dementia within two years of diagnosis.6 However, 14 to 38% returned to normal cognition and the remainder were stable.6 MCI represents an opportunity to intervene earlier to address treatable causes or slow underlying disease processes before the onset of functional impairment.
Only about 8% of patients in primary care older than 65 years of age with MCI have a formal diagnosis of MCI.8 Confirming the diagnosis of MCI allows the primary care team, together with the patient and their family, to implement brain health strategies and to monitor cognition and function regularly. Although there may be negative effects when patients are informed about cognitive decline, providing active risk reduction strategies and emphasising the uncertainty of progression can help alleviate negative effects, such as anxiety, and allow patients and their families to take control of their brain health.
Diagnosis of mild cognitive impairment or dementia
The diagnostic criteria for MCI and dementia are presented in Box 1.
Benefits of a timely diagnosis
A timely diagnosis occurs when the cognitive or functional changes begin to affect the individual or their families.9 Diagnosing MCI provides an opportunity to acknowledge the possibility of cognitive changes while using this often negatively perceived diagnosis as an opportunity to improve general health, rationalise medication use, forward plan, normalise discussions about cognitive changes and possibly resolve the presentation.
The benefits of a dementia diagnosis include:
- an explanation for the symptoms (some say it is a relief to know the cause)
- access to available treatments, including rehabilitation to improve quality of life
- lifestyle changes that may slow disease progression
- access to education and support for people living with dementia and their families or carers
- access to services to support independent living at home
- the opportunity for management planning
- the ability to consider cognitive impairment in the management of other medical conditions.10
Possible negative effects of a diagnosis
Dementia is the second most feared health condition because of the anticipated social, practical, emotional and legal impacts.11 Some people with dementia and their families react strongly negatively when given the diagnosis, including grief and fear relating to actual or anticipated losses.12 After the diagnosis, people can reduce their social activities and engagement.13 Self-stigma related to dementia means that people with dementia are at a higher risk of suicide, particularly immediately after diagnosis.14 Diagnosis has particularly strong negative impacts on people with young-onset dementia, as the diagnosis has effects on continued employment and finances.1
Diagnosis should be timely, and cognitive concerns should not be dismissed when they are raised. There may be instances where it is reasonable to not formally diagnose MCI or dementia (i.e. if the patient or their family is not expressing any concerns related to cognition, function or behaviour, or to avoid the negative effects of diagnosis). However, this choice must be weighed against the potential benefits of confirming a diagnosis. There has been a gradual increase in the availability of dementia support services and nonpharmacological interventions in Australia that can help mitigate some of the negative effects. Some examples are the MCI Thinking Ahead program offered by Dementia Australia (https://www.dementia.org.au/get-support/mild-cognitive-impairment-thinking-ahead), and the 12-week, multicomponent, Sustainable Personalised Interventions for Cognition, Care and Engagement (SPICE) rehabilitation program for people with dementia offered by Canberra Health Service (https://www.canberrahospitalfoundation.org.au/blog/spice_program). The arguments for and against using these programs may change over time, particularly with the availability of more effective treatments, such as monoclonal antibodies.1
Clinical presentations that can suggest mild cognitive impairment and dementia
Cognitive difficulties often manifest as functional difficulties, although people with MCI may apply compensatory strategies to overcome such difficulties (e.g. written reminders, routines, etc.).17 The person may complain about having trouble concentrating on tasks, completing tasks or with mood regulation. Dementia may also present as changes in behaviour and personality, such as a loss of interest and initiation in activities they previously enjoyed, loss of insight or blunting of affect. Primary care staff may notice that the person is forgetting appointments, having trouble managing their medications and other healthcare tasks, having trouble understanding instructions and relying on family members increasingly.
It is important that GPs and practice nurses detect signs of possible cognitive impairment and raise concerns when they are noticed, even if the person does not raise the concerns themselves. People with dementia often minimise or deny difficulties. Anosognosia (lack of awareness of deficits) is a symptom of dementia. It may also be because of embarrassment or shame, or fear of having dementia and losing independence.
Carers and family are the most common initiators of medical help-seeking for dementia. Family members are also in the position to notice subtle changes in function, behaviour and personality, which might not be observable during a brief medical appointment. Some carers contact the GP directly and ask that the GP not mention this to the patient to protect the patient–carer relationship. GPs might ask the carer to come in with the patient and raise concerns during an appointment, or seek an opportunity to suggest an assessment.
Understandably, GPs can find it difficult to raise the issue of possible cognitive impairment.18 Raising concerns may require graduated conversations over several visits. Suggesting a general health check, such as an annual health assessment for those aged 75 years and older, may be less alarming to patients than a memory or dementia assessment. The conversation might be framed around issues that the patient is worried about and emphasise that treatments and support are available once a cause is identified. Box 2 lists some examples of wording that might be used by GPs to suggest an assessment.
Assessments for suspected dementia or mild cognitive impairment
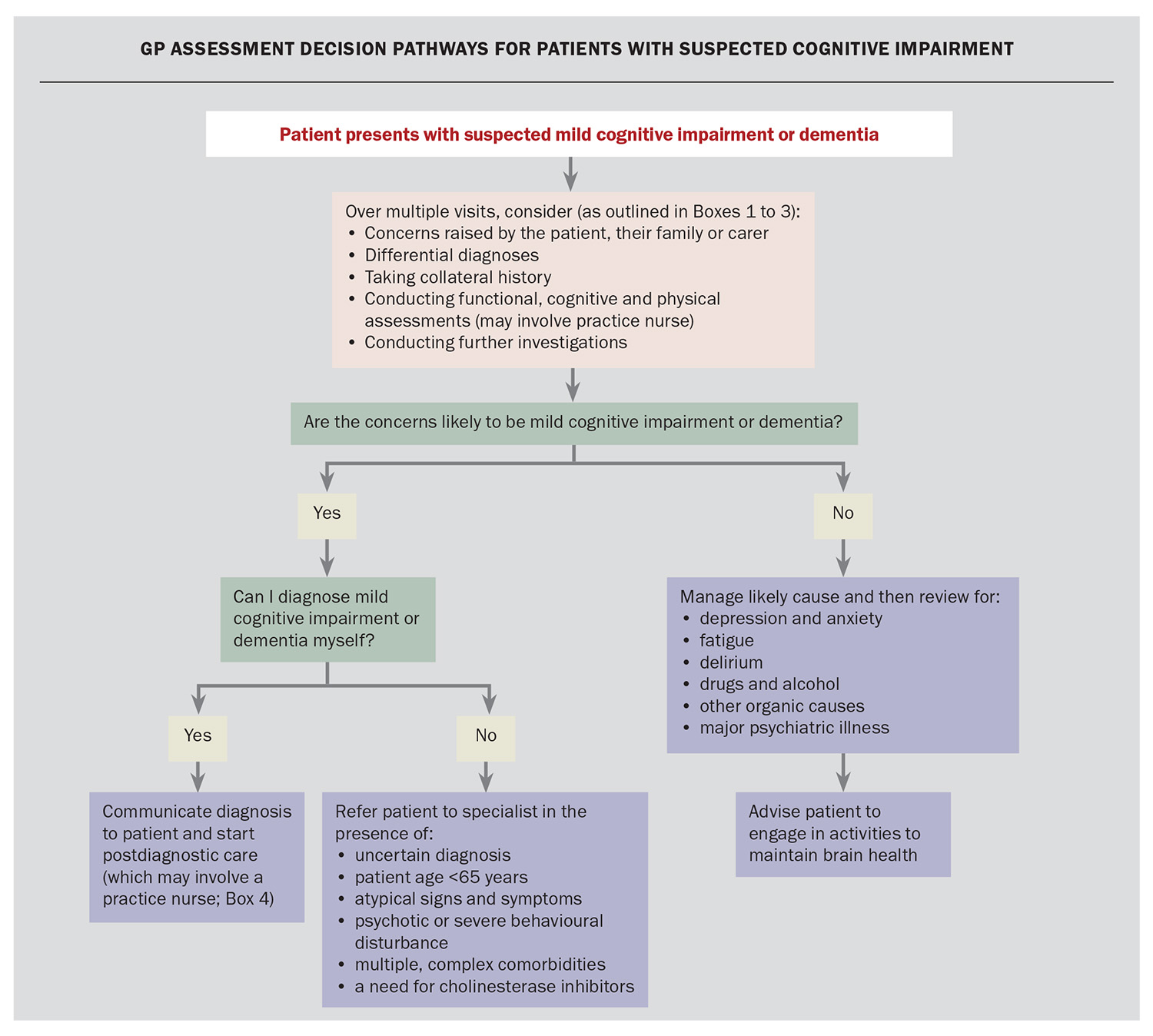
Assessment decision pathways for patients with suspected cognitive impairment are presented in the Flowchart. Assessment usually takes place over several visits and ideally includes history taking with a family member or support person who knows the patient well, preferably together and then separately. Some points to ask about are the following:
- changes in memory or thinking
- time course of changes – gradual onset and persistent over months or years, or sudden onset
- changes in function – including the use of technology (e.g. phones, computers, remote controls), shopping, driving and transport, banking, hobbies, medications, etc.
- mood changes and any changes in behaviours
- impacts of any changes on patient and family quality of life
- history of head injury, alcohol consumption, infections (e.g. COVID-19), etc.
Other assessments include physical examination, cognitive assessments, medications review, blood and urine laboratory tests and brain imaging (Box 3).19-23
Making the diagnosis of dementia or mild cognitive impairment
GPs and practice nurses are critical in recognising possible cognitive and functional changes and initiating assessment. GPs are encouraged to develop the confidence to diagnose dementia, particularly in regions where there is limited access to specialists.
After reviewing the findings of the assessments, consider differential diagnoses, such as delirium and depression, and reversible causes of cognitive impairment. These may include the use of medications that can impair cognition, polypharmacy, thyroid excess or deficiency, vitamin B12 deficiency, infection, toxicity, iron deficiency and electrolyte imbalances.
GPs report that they find delivering a dementia diagnosis difficult and complex.24,25 GPs are encouraged to invite the patient to bring a family supporter to the appointment but talk directly to the patient. Some practice points for communicating the diagnosis of dementia are presented in Box 4.
If a GP feels confident that the patient meets the criteria for dementia, then they can deliver the diagnosis themselves. GPs may consult with specialists to support their diagnosis, such as a memory clinic specialist, geriatrician, old age psychiatrist or neurologist. GPs may decide to refer a patient to a specialist for the following reasons:
- Mini-Mental State Examination (MMSE) score of 25 or higher, with a history of cognitive or functional decline
- age younger than 65 years with concerns of cognitive impairment
- atypical clinical presentation
- psychotic features or severe behavioural disturbance – these patients should be referred to an old age psychiatrist
- multiple complex comorbidities – these patients should be referred to a geriatrician
- need for specialist confirmation for suspected Alzheimer’s dementia or mixed dementia including Alzheimer’s dementia, in order to prescribe antidementia medications.
Confirmation of a diagnosis by a specialist is required for prescribing cholinesterase inhibitors or memantine for patients with a diagnosis of Alzheimer’s dementia or mixed dementia including Alzheimer’s dementia, as listed on the PBS. Prescriptions of donepezil, galantamine and rivastigmine require an MMSE score of 10 or higher, and memantine requires an MMSE score of 10 to 14, inclusive. After six months, a report of improvement is required for treatment continuation.
Conclusion
Timely diagnosis of MCI and dementia accompanied by ongoing management and support by the primary care team is beneficial for the patient and their family while mitigating some of the negative consequences of being given the diagnosis. The primary care team must be alert for possible signs of cognitive difficulties in order to detect dementia when the issue is not raised by patients themselves. GPs can diagnose MCI and dementia and refer more complex cases to specialists. MT
COMPETING INTERESTS: Associate Professor Yates is a Member of the Australian Ministers Dementia Advisory Group and the Nutricia Advisory Board. Dr Daly has received fees from Dementia Training Australia (DTA) for educational materials; has received a grant/contract on Biomarker with the Australian Dementia Network (ADNeT); checks modules by the Royal Australian College of General Practitioners (RACGP); is an Associate Investigator under a Face Dementia grant for BrainTrack (Dementia Australia); has received consulting fees from ADNeT for the Biomarker project, from DTA for education and from Medicines Australia for the Brain Health Collective; is an Advisory Board Member with Eisai; is Chair of the Dementia Special Interest Group, RACGP; is a member of the ADNeT Registry Steering Committee; is Chair of the Adelaide Clinical Council; and is Member of the Women in General Practice Committee, RACGP. Dr Long has received fees from DTA for this article; has received payment from the Australasian Menopause Society, Dementia Australia and HealthED; has received support for attending meetings by DTA and Dementia Australia; and is a Board Member of the Australasian Menopause Society. Professor Pond has received funding to the University of Newcastle from the Valley to Coast Charitable Trust; has received consulting fees from Sydney North Primary Health Network, HNECC Primary Health Network, SW Sydney Primary Health Network, Australian Department of Health and Aged Care, NSW Health, RACGP, DTA, Palliative Care Australia, the University of Sydney, Monash University, Biogen, Roche and Medicines Australia; has received payment from DTA, the Sydney North Health Network and In Vivo Academy; has received payment from Legal Aid NSW; has received support for attending meetings by RACGP and Palliative Care Australia; is Provost in the NSW Faculty of the RACGP, Vice President of the Doctors Reform Society, Chair of the WONCA Special Interest Group in Ageing and Health, Board Member of the Hunter Postgraduate Medical Institute and Chair of the REC-R Research Committee. Professor Brodaty has received support from the Brain Health Collective expert group; and has received consulting fees from Biogen, Eisai, Eli Lilly, Roche, Skin2Neuron, Cranbrook Care and Montefiore Homes. Ms Gibson received fees from DTA for the development of educational material and is a member of the Federal Government Dementia Expert Reference Group. Her PhD study is supported by a University of Newcastle Postgraduate Research Scholarship from the Australian Community of Practice in Research in Dementia (AcCORD), which is funded by a Dementia Research Team Grant from the National Health and Medical Research Council. Professor Low has received support from MRFF for this article; has received grants to institution from NHMRC and Dementia Australia; has received royalties or licenses from Elsevier and Exise Publishing; has received payment from Roche; and is a Member of the Federal Government Dementia Expert Reference Group, Chair of the Sydney Dementia Network and a Member of the Aged Care Diversity Consultative Diverse Committee.
References
1. Australian Institute of Health and Welfare (AIHW). Dementia in Australia. Canberra: AIHW; 2024. Available online at: https://www.aihw.gov.au/reports/dementia/dementia-in-aus/contents/population-health-impacts-of-dementia/prevalence-of-dementia (accessed May 2024).
2. Pond D, Mate K, Stocks N, et al. Effectiveness of a peer-mediated educational intervention in improving general practitioner diagnostic assessment and management of dementia: a cluster randomised controlled trial. BMJ Open 2018; 8: e021125.
3. Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open 2017; 7: e011146.
4. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69: 2197-2204.
5. Sabbagh MN, DeCourt B. Biomarker-based diagnosis of preclinical Alzheimer disease: time for the clinic? Nat Rev Neurol 2023; 19: 71-72.
6. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment. Neurology 2018; 90: 126-135.
7. Tangalos EG, Petersen RC. Mild cognitive impairment in geriatrics. Clinics Geriatr Med 2018; 34: 563-589.
8. Liu Y, Jun H, Becker A, Wallick C, Mattke S. Detection rates of mild cognitive impairment in primary care for the United States Medicare population. J Prev Alzheimers Dis 2024; 11: 7-12.
9. Hughes J. Ethical issues and decision-making in dementia care. In: Scullin ACT. Alzheimer's Australia. 2010.
10. Rasmussen J, Langerman H. Alzheimer’s disease – why we need early diagnosis. Degener Neurol Neuromusc Dis 2019; 9: 123-130.
11. Watson R, Sanson-Fisher R, Bryant J, Mansfield E. Dementia is the second most feared condition among Australian health service consumers: results of a cross-sectional survey. BMC Public Health 2023; 23: 876.
12. Aminzadeh F, Byszewski A, Molnar FJ, Eisner M. Emotional impact of dementia diagnosis: exploring persons with dementia and caregivers’ perspectives. Aging Ment Health 2007; 11: 281-290.
13. Amano T, Reynolds A, Scher C, Jia Y. The effect of receiving a diagnosis of dementia on social engagement among older adults. Dement Geriatr Cogn Disord 2021; 50: 401-406.
14. Alothman D, Card T, Lewis S, Tyrrell E, Fogarty AW, Marshall CR. Risk of suicide after dementia diagnosis. JAMA Neurol 2022; 79: 1148-1154.
15. Kilty C, Cahill S, Foley T, Fox S. Young onset dementia: implications for employment and finances. Dementia 2023; 22: 68-84.
16. Ebell MH, Barry HC, Baduni K, Grasso G. Clinically important benefits and harms of monoclonal antibodies targeting amyloid for the treatment of Alzheimer disease: a systematic review and meta-analysis. Ann Family Med 2024; 22: 50-62.
17. Lin P, LaMonica HM, Naismith SL, Mowszowski L. Memory compensation strategies in older people with mild cognitive impairment. J Int Neuropsychol Soc 2020; 26: 86-96.
18. Borson S, Small GW, O’Brien Q, Morrello A, Boustani M. Understanding barriers to and facilitators of clinician-patient conversations about brain health and cognitive concerns in primary care: a systematic review and practical considerations for the clinician. BMC Primary Care 2023; 24: 233.
19. Cacho J, Benito-León J, García-García R, Fernández-Calvo B, Vicente-Villardón JL, Mitchell AJ. Does the combination of the MMSE and clock drawing test (mini-clock) improve the detection of mild Alzheimer's disease and mild cognitive impairment? J Alzheimers Dis 2010; 22: 889-896.
20. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695-699.
21. Brodaty H, Pond D, Kemp NM, et al. The GPCOG: A new screening test for dementia designed for general practice. J Am Geriatr Soc 2002; 50: 530-534.
22. Rowland JT, Basic D, Storey JE, Conforti DA. The Rowland Universal Dementia Assessment Scale (RUDAS) and the Folstein MMSE in a multicultural cohort of elderly persons. Int Psychogeriatr 2006; 18: 111-120.
23. LoGiudice D, Smith K, Thomas J, et al. Kimberley Indigenous Cognitive Assessment tool (KICA): development of a cognitive assessment tool for older indigenous Australians. Int Psychogeriatr 2006; 18: 269-280.
24. Phillips J, Pond CD, Paterson NE, et al. Difficulties in disclosing the diagnosis of dementia: A qualitative study in general practice. Br J Gen Pract 2012; 62: e546-e53.
25. Yates J, Stanyon M, Samra R, Clare L. Challenges in disclosing and receiving a diagnosis of dementia: a systematic review of practice from the perspectives of people with dementia, carers, and healthcare professionals. Int Psychogeriatr 2021; 33: 1161-1192.