The stepwise management approach to paediatric asthma

The updates to both national (Australian Asthma Handbook) and international (Global Initiative for Asthma) guidelines over recent years have included major updates to evidence and advice on managing asthma in infants and children. Enhanced adherence to these guidelines is crucial for efforts to address the ongoing concern about preventable asthma deaths in Australia.
- The stepwise approach to the management of childhood asthma differs by age group (0 to 12 months, 1 to 5 years, 6 to 11 years and 12 years and older).
- Before any stepwise increase in asthma preventer treatment, clinicians should reconsider the diagnosis of asthma, check the parents’ and child’s understanding of asthma management and treatment adherence, and minimise exposure to triggers and environmental tobacco smoke exposure.
- Asthma should be considered a chronic remitting and relapsing condition, with any hospital presentation representing evidence of inadequate disease control.
- Asthma exacerbations should be considered a red flag and prompt review of current management.
- Children should be referred for specialist paediatric assessment if they have:
– continued poor symptom control or persistently abnormal spirometry: expiratory airflow limitation or airway hyper-reactivity, despite prescription of an appropriate asthma preventer
– more than two courses of systemic corticosteroids per year or more than one hospital presentation per year
– limitations on school attendance or participation in activities
– any life-threatening episode of asthma.
Around 2.8 million (11%) people in Australia were estimated to be living with asthma in 2022, with asthma being the leading cause of total disease burden in children aged 1 to 9 years. The hospitalisation rate among children aged 0 to 14 years is nearly three times higher than in people aged older than 15 years (225 vs 70 per 100,000 population).1 These data underscore the continued need for the appropriate management of acute episodes of asthma and for longitudinal disease monitoring and management of children with asthma among primary care and specialist healthcare providers.
In 2018, the National Asthma Strategy was released, outlining a co-ordinated health response to asthma and included the development of national asthma indicators (https://www.nationalasthma.org.au/strategy). The 2024 indicator data showed no change in paediatric asthma-related deaths or asthma prevalence since the program’s inception.1 Asthma remains a leading cause of paediatric emergency presentation and outpatient referral to a paediatrician.2,3
The National Asthma Council Australia publishes treatment guidelines in the Australian Asthma Handbook (AAH), based on evidence, where available, and on expert consensus opinion. Versions released over the past five years (versions 2.0 to 2.2, last updated April 2022) included major updates to evidence and advice on managing asthma in infants and children.4 Changes affecting the use of preventers as reliever treatment (i.e. Single Maintenance and Reliever Treatment [SMART]) for adolescents included in these updates are also discussed here. These changes reflect similar changes that have occurred in the major international guidelines such as the Global Initiative for Asthma (GINA) guidelines over the same time period.5
Despite widespread availability of national and international asthma management guidelines, clinician adherence to guidelines remains inadequate and inferior to the reported adherence for many other chronic conditions.6 Most clinicians asked patients and carers about the frequency of rescue inhaler use, but the impact of asthma on normal daily activities and frequency of nocturnal symptoms were less monitored.7 Provision of a written asthma plan, observation of inhaler technique, use of preventer medication and the approach to ‘difficult-to-control’ asthma have been identified as areas for improvement for primary care clinicians. It has been estimated that only 34% of people (adult and children) have a written asthma plan.1,7,8 Families identify GPs among their most trusted resources for asthma management, reinforcing their important role.9
This article summarises the current stepwise approach to asthma management in children recommended in AAH version 2.2 and outlines age-specific aspects that GPs should consider. It also discusses key updates in the latest GINA strategy report published in 2024.5 This article updates a previous review of age-specific management of asthma in children, published in the September 2020 issue of Respiratory Medicine Today.10
There continues to be ongoing concern that asthma deaths, which should be viewed as preventable, occur far too often and that poor asthma control or recent hospitalisation should be recognised as significant ‘red flags’ (i.e. risk factors) for poor outcomes.11-14 Finally, key aspects of the management of difficult-to-treat asthma at tertiary paediatric institutions are described to provide context for the medications and approaches GPs may encounter as they share management in primary care.
Treatment guidelines by age
The stepwise treatment approach by age in the AAH categorises children into those aged 0 to 12 months, 1 to 5 years, 6 to 11 years and adolescents, with the last age category classed together with adults. Several pertinent aspects of the acute management of wheeze and ongoing maintenance asthma preventer treatment have broad relevance across all age groups.
Early childhood wheeze is a highly heterogeneous condition, with several wheeze phenotypes described in important longitudinal cohort studies.15 These wheeze phenotypes are identifiable by the early temporal pattern of wheeze and whether symptoms persist into school age.
In the past, two different approaches to classifying early childhood wheeze phenotypes, occurring in the first six years of life, have been proposed:
- a symptom-based classification – ‘episodic viral wheeze’, in which wheeze occurs in discrete time periods predominantly associated with upper respiratory tract infection with a lack of symptoms between episodes, and ‘multiple-trigger wheeze’, in which wheezing occurs between these episodes and may be caused by a range of triggers (e.g. change in weather, activity, emotion)
- a temporal-based classification – ‘transient wheeze’ (onset and resolution of symptoms within the first three years of life), ‘persistent wheeze’ (onset before 3 years and continuation of symptoms beyond 6 years of age) and ‘late-onset wheeze’ (symptom onset after the age of 3 years).
However, both approaches have limitations, with neither performing well when applied prospectively, and there is ongoing work within large birth cohort studies to identify improved approaches for clinical use.16,17 A more recent example of this work is the CHILDhood Asthma Risk Tool (CHART) which was developed, and subsequently validated, for its ability to identify children with asthma or persistent symptoms at 5 years of age based on identified factors associated with asthma at 3 years of age (timing and number of wheeze or cough episodes, use of asthma medications, and emergency department visits or hospitalisations for asthma or wheeze).18 These tools have yet to be incorporated into clinical care.
Infants aged 0 to 12 months
Infants aged 0 to 12 months are considered separately from older children in the AAH. This change acknowledges the differences in wheeze mechanisms between infants and preschool-aged children and seeks to avoid potential harm due to inappropriate treatment.
Management of acute wheeze in infants
Bronchiolitis is the leading cause of acute wheezing. Repeat wheeze episodes are more likely in children who have been hospitalised with bronchiolitis, although most do not require subsequent hospitalisations. In a longitudinal cohort study of more than 20,000 infants in the UK, 80% of those with relatively severe bronchiolitis (i.e. requiring hospitalisation in the first year of life) did not have further wheezing episodes within the study period, which followed up children to the age of 8 years.19
This year (2024) has seen the introduction and roll out of nirsevimab, an injectable long-acting monoclonal antibody that protects against respiratory syncytial virus (RSV) infection, the most common cause of hospitalisation for bronchiolitis in infants. In clinical trials, nirsevimab reduced the incidence of hospitalisation for RSV-associated lower respiratory tract infection by 76 to 78% among preterm to term infants entering their first RSV season compared with placebo.20 It has been estimated that avoiding RSV infection during infancy may reduce asthma diagnosis rates at 5 years of age by about 15%.21
The recent evidence-based Australasian guidelines on bronchiolitis from the PREDICT research collaborative, recommend against routinely trialling short-acting bronchodilator or systemic corticosteroid therapy in infants aged 12 months and younger with bronchiolitis.22 In this group, the predominant aetiology of wheeze is not airway hyperresponsiveness, but is associated with small airway calibre, further narrowed by oedema and increased mucus secretion, for which beta2 agonists are ineffective.23 Furthermore, there is good evidence, including a Cochrane review, that beta2 agonists are of no benefit (for hospitalisation rate or length of stay) and in fact led to increased adverse events (tachycardia, hypertension, tremor and decreased oxygen saturation) for infants with bronchiolitis compared with placebo.24,25
The negative findings of this Cochrane review were based on large cohort studies; however, there is likely a small subset of infants who respond to beta2 agonists. The current recommendation from the National Asthma Council Australia is to consult a paediatrician before a therapeutic trial of a bronchodilator or corticosteroid (either inhaled or systemic).4 Identifying which infants should trial bronchodilators remains challenging, and for most infants, supportive care (respiratory support and adequate hydration) is all that is required. However, in older infants and those with a history of atopy and a strong family history of asthma, a trial of beta2 agonists may be considered. Important considerations when trialling beta2 agonists are discussed in the older age groups. There is no role for routine corticosteroid therapy in infants with bronchiolitis, nor in the subset of infants with a positive response to beta2 agonists.
Asthma preventer therapy in infants
Transient early wheeze is the most common wheeze phenotype in infants. It is generally not distressing for the infant, who thrives despite these symptoms. Cough and difficulty breathing are uncommon, and wheeze may be more prominent with viral illnesses. It is associated with lower initial lung function (which later improves) and is believed to be associated with the smaller calibre of the airways. The turbulent airflow in the small to medium-sized airways associated with wheeze is therefore not reversible and does not respond to bronchodilator treatment or other asthma preventer medication. This is confirmed by randomised controlled trial data confirming no benefit from intermittent inhaled corticosteroid (ICS) on preventing progression from episodic to persistent wheezing in later life and no short-term benefit during these episodes of wheeze.26
Infants at increased risk of wheeze include:
- those born preterm (especially those with bronchopulmonary dysplasia)
- those with antenatal or environmental exposure to tobacco smoke
- those with previous bronchiolitis caused by RSV or recurrent rhinovirus infections.24
In these infants, viral infections continue to be the most common cause of exacerbations, but other triggers such as environmental tobacco smoke, aeroallergens and air pollution should be considered, and exposure minimised.27
Children aged 1 to 5 years
The AAH has moved away from using the temporal nature of symptoms (i.e. classifying as infrequent intermittent, frequent intermittent or persistent) to guide management, preferring to describe clinical severity as:4
- mild (salbutamol as needed at home)
- moderate–severe (requiring systemic corticosteroids and/or emergency department presentation)
- life-threatening (requiring hospitalisation or intensive care).
The recommended preventer medicines in this age group are ICS and montelukast (in children aged 2 years and older).
Wheeze is common in the preschool age group, with nearly one-third of preschool-aged children having intermittent wheezing.28 Despite the high morbidity of early childhood wheeze, only 30% of toddlers with recurrent wheeze are estimated to progress to asthma at 6 years of age, and fewer still continue to have wheeze as adults.15
Management of acute wheeze in children aged 1 to 5 years
Salbutamol can be used on an ‘as-needed’ basis to relieve symptoms during wheezing episodes if a therapeutic trial shows it is beneficial. A child with wheeze who is eating well and playing, without signs of a prolonged expiratory phase or increased respiratory effort, may not require any treatment. When a trial of a bronchodilator is appropriate, the markers of clinical improvement should be considered a priori.
The most useful markers of a response include improvements in oxygen saturation, heart rate and respiratory rate, combined with subjective examination findings such as the child’s level of interaction, ability to speak or vocalise without limitation, use of accessory muscles, pronounced expiratory phase and changes in air entry or wheeze throughout the lung fields. Repeated clinical assessment is essential, and the response, or lack thereof, should be well documented in the clinical record and may indicate an alternative diagnosis.29,30
In the 1- to 5-year age group, viral-induced wheeze does not reliably respond to systemic corticosteroids in the same way as observed in older age groups. Two randomised placebo-controlled trials of oral corticosteroids (OCS) to treat viral-induced wheeze are worth mentioning. A 2009 UK study found no difference in hospital admission rates between OCS and placebo treated groups.31 Similarly, a 2018 Australian study reported a reduced time to discharge in cases managed with OCS versus placebo, although the difference was less than three hours and of questionable clinical significance.32 These differing results may reflect potential inclusion of infants with bronchiolitis in the first study.33 In the acute inpatient setting, OCS use should be reserved for those with a clinical response to bronchodilators and a high clinical suspicion of allergic asthma.34,35
Asthma preventer therapy in children aged 1 to 5 years
Episodic viral-induced wheeze is the most common wheeze presentation, with children having no symptoms between discrete episodes. Multiple-trigger wheeze describes wheeze with additional triggers, such as exercise, allergens, cold air and smoke.36 Multiple-trigger wheeze is more likely to persist into older age groups and is associated with greater impairment of lung function and atopy (asthma, allergic rhinitis, eczema or food allergies) than viral-induced wheeze.37
As in other age groups, environmental tobacco smoke exposure must be acknowledged to families as an important trigger, and support offered for smoking cessation. The potential for negative effects in those at risk of asthma can occur early: in the offspring of asthmatic mothers, in utero tobacco smoke exposure has been shown to be associated with impaired lung function shortly after birth, with these two factors mediating later risk of wheeze during the first year of life.38 A meta-analysis reported the effects of environmental tobacco smoke exposure on asthma morbidity in children aged 0 to 18 years; environmental tobacco smoke was associated with worsening lung function, increased wheeze symptoms and nearly double the rates of hospitalisation for acute asthma compared with children not exposed.39
The stepwise approach to maintenance asthma preventer treatments for children aged 1 to 5 years is shown in Figure 1. A trial of preventer therapy is indicated in children with:
- recurrent episodic symptoms at least once per week or less often if associated with more severe symptoms (OCS treatment or hospital presentation)
- severe symptoms (OCS treatment or hospital presentation)
- a demonstrated response to bronchodilator therapy, and
- clinical features associated with increased risk of asthma.4
ICS is the preferred first-line preventer treatment for those with frequent symptoms or infrequent but severe exacerbations. A leukotriene receptor antagonist (LTRA) may be considered if the child is unable to use a spacer and MDI or parents decline ICS or are significantly concerned of the side effects (likely to have poor adherence).40 In preschool-aged children with persistent wheeze, both ICS and LTRA have been shown to reduce SABA and OCS use, as well as the frequency of asthma symptoms.41 The adverse effects of ICS and LTRA are described in the next section but are also applicable to this age group. LABA are not licensed for this age group, and the effects of LABA plus ICS have not been studied systematically in children younger than 4 years of age.
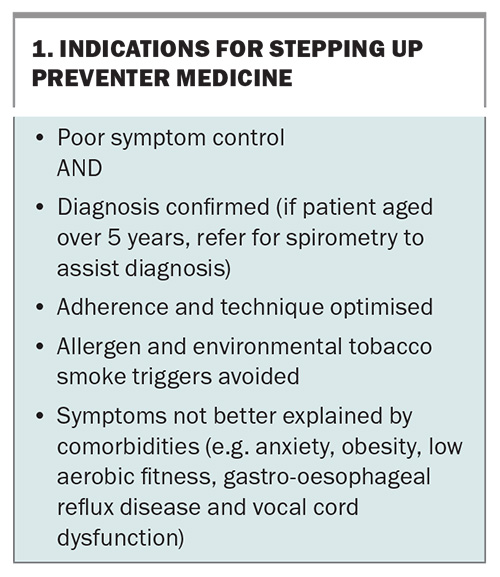
The indications for stepping up preventer therapy are listed in Box 1. Before any increase in preventer therapy, clinicians must:
- confirm symptoms are associated with asthma rather than a concurrent or alternative diagnosis
- assess patient and family understanding of asthma management and the role of reliever and preventer medicines
- assess adherence to current preventer medicine
- assess spacer technique
- minimise exposure to asthma triggers and environmental tobacco smoke.
Children aged 6 to 11 years
In children aged 6 years and older, asthma can be diagnosed with more certainty, as identifying reversible expiratory airflow limitation using spirometry becomes more feasible, and the incidence of other early childhood wheeze phenotypes has reduced by this age. In children aged 5 years, the feasibility of spirometry is generally above 85%.42
The stepwise approach to maintenance treatments for children aged 6 to 11 years is shown in Figure 2. For children of all ages, the aim of maintenance asthma preventer therapy is to achieve good control of asthma symptoms at the lowest step necessary. Good control of asthma symptoms is considered as:
- infrequent daytime symptoms (two days a week or less)
- no nocturnal symptoms
- no limitation on play, physical activity or school attendance.4
Although asthma control is typically assessed by the criteria listed above over the previous four weeks (using tools such as the Asthma Control Test), it is important that any evaluation of asthma control (in this and older age groups) also includes an assessment of future risk factors for adverse outcomes. The full list of factors is listed in the 2024 GINA report (Box 2) but includes risk factors for exacerbation (including history of exacerbations), developing persistent airflow obstruction and medication side effects.5
Before any increase in preventer therapy, clinicians should ensure the diagnosis of asthma is correct. The 2024 GINA report re-emphasises that overdiagnosis and underdiagnosis are common.5 The diagnosis of asthma is based on clinical characteristics consistent with an episodic, reversible, expiratory airflow obstruction. Best practice involves spirometry to confirm airflow obstruction and bronchodilator reversibility. Treatment with ICS is indicated, in the setting of poor asthma control, where spirometry confirms the diagnosis. If spirometry is not available, peak expiratory flow measurement (pre and post bronchodilator) has a role in demonstrating variable expiratory flow, and is an objective measure to monitor, particularly in a poorly resourced setting. Where there is diagnostic uncertainty and no spirometry or peak expiratory flow measurement available, a trial of ICS can be useful if other diagnoses seem unlikely (e.g. chronic suppurative lung disease, inhaled foreign body, allergic rhinitis, congenital heart disease). Failure to respond to ICS or persistent severe symptoms should prompt referral to paediatric services.
For medication delivery, a pressurised metered dose inhaler (pMDI) and spacer is an appropriate first choice for most children.43 pMDI via spacers and nebulisers are equally effective means of delivering beta2 agonists to children with acute asthma. Ongoing parental supervision of medication dosing remains important for school-aged children as they are unlikely to use their devices correctly without careful training and repeated checking of their technique.44 Asking the child to demonstrate how they use their metered dose inhaler and spacer can offer useful insights into the family’s retention of asthma education and home practices. A useful checklist to detect the most common errors in use of metered dose inhalers and spacers is shown in Box 2.9
Leukotriene receptor antagonist vs inhaled corticosteroid
An ICS is recommended as the first-line preventer in Step 2 of the asthma management algorithm for children aged 6 to 11 years. This is based on comparative trials favouring ICS over LTRAs for greater efficacy, symptom reduction, exacerbation prevention and lung function improvement.45,46 However, an LTRA may be more effective for some patients, and some may find adherence to LTRAs easier than to ICS.47,48
Inhaled corticosteroids
At higher doses, ICS have been shown to have a relatively flat dose–response curve, with increasing systemic side effects such as adrenal suppression at doses over 400 mcg/day fluticasone propionate equivalent, without increased efficacy.49 A 2004 Cochrane review suggested that commencing an ICS at a moderate dose is as effective as commencing it at a high dose and then reducing the dose while monitoring symptoms.50
Growth suppression associated with ICS is dose-dependent, and different age groups differ in their susceptibility to growth effects, with children aged 4 to 10 years being more susceptible than pubertal children.51 A randomised placebo-controlled trial of ICS in children aged 5 to 13 years reported a mean decrease of about 1 cm in height, typically with onset within two years, that was sustained at follow up through to adulthood.52 In children with persistent asthma requiring maintenance ICS treatment to achieve good asthma control, we recommend that this small effect on final adult height should be viewed as an acceptable trade-off for better asthma control.
The 2024 GINA report suggests that clinicians have a low threshold for commencing ICS, as a preventer, in children with asthma, even for those with infrequent SABA. The risks of SABA alone are now well established and have been shown to be associated with an increased risk of asthma exacerbations, airway hyper-reactivity and reduced bronchodilator effect in multiple studies.53,54 For infrequent symptoms managed with SABA, it is suggested ICS is used, if not daily then at least regularly through the days the SABA is used. There is a lack of paediatric evidence for intermittent ICS added to SABA during symptomatic periods, with this approach extrapolated from data in benefit in adolescents and adults showing a reduction in severe exacerbations.
Leukotriene receptor antagonists
LTRAs are effective for exercise-induced bronchoconstriction, with studies in both adults and children reporting a superior response when compared to other preventers (ICS and ICS–LABA combination). Patients with exercise-induced bronchoconstriction tend to have a smaller drop in expiratory airflow during exercise and a better response to SABA after exercise when using LTRAs compared with ICS–LABA combined.55-58
When considering LTRA, it is important to discuss with parents the potential for neuropsychiatric adverse drug reactions. These have been of concern and have attracted media attention, including a 2018 TGA safety review. These adverse reactions are more common in children than adults and include aggression and sleep disorders in younger children and headaches and depression or anxiety in adolescents.59,60 A retrospective cohort study in children reported the onset of neuropsychiatric adverse drug reactions usually within the first two weeks of commencing an LTRA; treatment cessation was not typically required but led to resolution in those who chose to cease.61 Clinicians should be aware of these associations, discuss them openly with parents and be vigilant for adverse events, while recognising the benefits of LTRA in asthma control.
Individual responses to Step 3 treatment
Step 2 treatment with an ICS alone is effective in the vast majority of children with mild persistent asthma.62 Step 3 treatment is considered for those with poor symptom control despite a low-dose ICS (250 mcg/day fluticasone proprionate equivalent or less), where adherence is optimised. Step 3 treatment options include: high-dose ICS (500 mcg/day fluticasone proprionate equivalent or more), ICS–LABA combination or an ICS and LTRA.
These three treatment options were compared in a prospective, crossover blinded randomised trial in children aged 6 to 18 years with asthma inadequately controlled by a low-dose ICS (twice daily 100 mcg fluticasone proprionate equivalent).63 Patients were randomly allocated to high-dose ICS (twice daily 250 mcg fluticasone proprionate equivalent), low-dose ICS plus LABA or low-dose ICS plus LTRA in a sequential manner. The study results showed that the optimal treatment differed between individual children. ICS plus LABA therapy was significantly more likely to provide the best response (compared with high-dose ICS or ICS plus LTRA). However, as the composite outcome measure included any improvement in FEV1, and inclusion criteria included a demonstrable FEV1 response to a bronchodilator, the study design may have favoured LABA-containing medications.
These findings highlight the need to consider alternatives in Step 3 of the stepwise management algorithm when asthma control is not achieved despite optimal adherence and education. Other factors such as cost may influence decisions. Currently, LTRA is not PBS-subsidised for patients aged over 15 years, although it may be within financial reach of families as generic montelukast options recently became available.
Adolescents aged 12 to 18 years
The prevalence of asthma declines from about 16% among primary school-aged children to 11% in adolescents.64 The management of asthma during adolescence involves navigating challenges as the young person learns to take responsibility for their own health. It is widely accepted this period is associated with reduced compliance with daily asthma medications.65 Concern of side effects, decreased parental supervision and peer approval are contributors to reduced compliance.66 Consequently the management of asthma becomes more challenging, complicated further by under-recognition of symptoms, erratic self-medication (i.e. decreased adherence), denial of disease severity and higher rates of risk-taking behaviours.67
Preventing asthma deaths
Asthma mortality in Australia remains higher than in other comparable developed countries such as Canada, France, Japan and Italy.12 Adolescents are over-represented among asthma deaths, comprising up to 60% of cases.13,14
Audits of asthma deaths from the UK and Australia, triggered by increasing concern over preventable and escalating mortality, describe children with a pattern of excessive use of reliever medication and underfilled prescriptions for preventer medications, a history of hospital presentations with poor follow-up care, repeated courses of OCS and frequent missed school days.13,14,68,69 Children from vulnerable families are prominent among paediatric asthma deaths, with high rates of family breakdown, parental substance abuse, domestic violence and child protection involvement.13
Damningly, the UK national review of asthma deaths reported that 46% of deaths could have been avoided if patients had been better managed in the year before they died.69 Patients did not receive key areas of routine care, with only 4% managed in line with current national guideline recommendations. Prescribing errors were widespread, acute asthma was poorly managed, and severe cases were not referred to specialist centres.
Leading physicians have advocated for a frameshift in the perception of asthma exacerbations, arguing that each acute exacerbation episode should be viewed as a significant marker of poorly controlled disease and prompt a careful review of management.11 Acute asthma attacks carry a risk of further attacks and death. The AAH currently encourages clinicians to increase disease monitoring around these flare-ups and to refer patients with more than one emergency visit in a year or repeated OCS treatments to a specialist centre.4 Recommendations about the key role of primary care physicians from the UK National Review of Asthma Deaths are shown in Box 3.14
SMART: Single Maintenance and Reliever Treatment
The underuse of preventer medication and overuse or reliance on SABA treatment has emerged as a key issue in asthma management. The shift away from using SABA only for mild asthma in paediatrics stems from two areas of concern. Firstly, 30 to 40% of severe exacerbations arise from patients with ‘mild’ asthma.70 Secondly, adult data indicates that SABA-only therapy is associated with increased risk of asthma exacerbations.71
The 2019 GINA report raised serious concerns about the overuse of SABA reliever treatments paired with the underuse of ICS preventer treatment among adolescents and other age groups. The guidelines within the 2024 GINA report go one step further to recommend ICS–formoterol (SMART) as the first line management approach.5 This treatment simplifies management, avoids SABA overuse without concomitant ICS administration and is also suitable as a step-down approach for patients whose asthma is well controlled on regular ICS or LTRA.
Four studies have evaluated an as-needed combination ICS plus fast onset LABA in mild asthma. Together these studies include almost 10,000 patients (adults and adolescents) and show ICS plus formoterol to be superior to as-needed SABA plus daily ICS treatment for symptom control, and to be more effective in exacerbation prevention.72-75 Adolescent specific insight comes from a post-hoc pooled analysis of the SYGMA 1 and 2 trials performed including only those aged between 12 and 18 years (a total of 889 patients).76 The authors reported no difference in severe exacerbation rate comparing maintenance ICS versus as-needed ICS plus LABA (p=0.66); however, ICS plus LABA as needed was associated with reduced median corticosteroid exposure and improved linear growth. The results of this study are a useful insight to support SMART as a treatment option for adolescents; however, the study does not demonstrate this to be a superior option regarding severe exacerbation rate, FEV1 pre-bronchodilator and symptom control. Caution should be shown in adopting this approach universally and abandoning daily ICS preventer therapy for adolescents.77 Another important consideration is ensuring that adolescents achieve their optimal lung function at entry to adulthood, given its importance in determining later adult health trajectories.78-80
GINA guidelines now recommend this treatment approach also for the 6- to 11-year age group. The latest Australian guidelines have adopted the SMART recommendation as a treatment strategy for adolescents and adults but not as a routine approach for younger children. There is a lack of high-grade evidence in younger patients and little data on the pharmacokinetics and pharmacodynamics of LABAs in younger children. Further research could evaluate the adequacy of extrapolating from older age groups.81
GINA guidelines advocate children and adolescents should receive an ICS whenever a SABA is required, either as a separate ICS inhaler alongside SABA or using combination ICS plus formoterol (a fast-onset LABA). SABA-only treatment is no longer recommended.82 This recommendation is in part derived from large randomised trials mostly in adults, with some adolescent representation, comparing standard treatment with combination ICS plus fast-onset LABA. In these studies, the ‘maintenance and reliever therapy’ (MART) approach was shown to reduce rates of severe exacerbations and overall ICS dosing.72,83,84 As discussed earlier, post-hoc pooled analysis of the SYGMA 1 and 2 trials has provided further adolescent- specific evidence.76
In Australia, the PBS provides access to combination budesonide plus formoterol for children aged 12 years and older for step 2 treatment, or younger if management is directed by a respiratory physician or paediatrician. Also, only an ICS plus fast-onset LABA combination (budesonide and formoterol) is suitable for this approach; other ICS plus LABA combinations (e.g. salmeterol and vilanterol) are not to be used for this indication.
Acute SMART
Implementation of SMART for acute asthma exacerbations presenting to emergency department is now being adopted, as SMART becomes more widely used. For adolescents, guidance on use in the acute setting has been published by the National Asthma Council Australia (Using Symbicort Turbuhaler (budesonide–formoterol) inhaler for an asthma attack [https://www.nationalasthma.org.au/asthma-first-aid/symbicort-turbuhaler-budesonide-formoterol-inhaler]), and recommendations for children aged 6 to 11 years have been produced by the Royal Children’s Hospital in Melbourne (Clinical Practice Guidelines: Acute Asthma [https://www.rch.org.au/clinicalguide/guideline_index/Acute_asthma/]). For moderate or severe exacerbations, management reverts to SABA, ipratropium bromide and systemic corticosteroids, with other agents as required.
Other important management aspects across age groups
Stepping down asthma treatment
Asthma is considered well controlled when symptoms are absent or mild, occur during the daytime only and quickly respond to a SABA (used less often than twice per week). The patient should have no limitations on play, sport or school attendance; no nocturnal symptoms (AAH); and no recent history of exacerbations managed with OCS or hospitalisation. The indications for stepdown of asthma preventer medicines are listed in Box 4.
In patients prescribed ICS plus an additional non-ICS preventer (montelukast, combination ICS with LABA, or a biologic agent), a reduction in ICS dosing while maintaining the other preventer has superior outcomes compared with weaning onto ICS alone. This suggests that tapering ICS dosage by 50% before LABA or LTRA discontinuation may be a preferable approach. Seasonal effects are noted, with greater success in spring and summer than at other times.85 There is a lack of data to guide the rate of reduction, and the lowest dose of ICS needed to control asthma and any reduction needs to consider the individual’s potential for exacerbation, the severity of previous exacerbations and the carer’s ability to manage such an occurrence.
Parent education and asthma plans
The provision of an asthma action plan is not only an important clinical communication between care providers and families but also an important opportunity for focused education. Indeed, research suggests that it is not the asthma plan per se but the education that accompanies it that is associated with better disease control.86 Important aspects for clinicians to address include the pathophysiology of the child’s disease, the use and role of reliever and preventer medicines, expectations of treatment and the threshold for medical review.87 Children provided with education and written action plans have been shown to have significantly fewer asthma exacerbations, OCS prescriptions, loss of school days, nocturnal wakenings and overall symptom scores.86,88
School plans
Schools are importantly placed to support the management of children with asthma. The Asthma Friendly Schools Initiative from North America suggested the development of resources for schools to improve awareness of asthma-related issues and a standardised approach to handling worsening asthma in a child.89 Schools should have an annual process for identifying students with asthma, ensure easy access to inhalers (preferably carry their own), identify and reduce common asthma triggers within the environment and support communication and collaboration between carers, students, educators and healthcare workers.89,90 Asthma education for school staff increases asthma knowledge and preparedness.91 It is important that teachers receive guidance and training in appropriate asthma care. Schools should also encourage a policy of exercise for all students with asthma.91,92
Clinicians should be aware of the phenomenon of back-to-school asthma flare-ups; each year, emergency department presentations and hospitalisations surge during the first months of the school year.93 It is important that clinicians recognise the start of the school term as a time to avoid stepping down treatment.4 It is also helpful to plan reviews towards the end of the school holidays, which ensures the opportunity to review and update the asthma action plan and encourage children to communicate their symptoms to teaching staff with the support of their parents.
Medication adherence
Barriers to medication adherence in school-aged children include a lack of motivation, difficulties remembering and social barriers. Children report the paradox that they find parental reminders annoying but that prompting improves adherence.94
It is important to provide practical support to families by openly and compassionately exploring the understanding of and obstacles to treatment, and where possible offering strategies to overcome these challenges. Evidence-based strategies to promote adherence include:
- the use of rewards to reinforce adherence
- including treatment within other routines (e.g. mealtime, brushing teeth)
- determining reasonable, specific goals defined by the patient
- using visual or auditory reminders
- using a measure of adherence to benchmark achievement at home
- simplifying treatment regimens (e.g. prescribing once-daily ICS for children over 6 years of age).94-96
Monitoring devices
Electronic monitoring devices can be fitted to a range of inhalers, and the data downloaded remotely to provide a clear picture of adherence and how it impacts on asthma control and exacerbations. This technology may be useful in children with persistent symptoms and difficulties understanding treatment adherence, to avoid unnecessary investigations or overtreatment.97 Currently, these devices are typically used only by tertiary centres for assessment of patients with more severe asthma.
The development of smartphone apps to support adherence is an emerging area. The opportunity exists for digital media to detail symptom scores, medication adherence information and even basic measures of lung function, and to communicate these directly to healthcare providers.98 Studies in adults with asthma found that the use of mobile apps improved medication adherence and asthma control.99 The issues with app development include the profit motives of developers that are placed ahead of the health needs of users. In addition, there is an oversupply of low-quality information apps that either are not effective or do not meet patient needs, or that fail to comply with existing evidence-based clinical practice.100
Complex asthma and the role of biologic agents
An estimated 5 to 10% of children with asthma remain symptomatic despite receiving large amounts of asthma preventer treatment.101,102 Problematic severe asthma (PSA) is the umbrella term used to describe all children who present with ongoing persistent symptoms or frequent attacks despite high-intensity treatment.103 It is thought to comprise the following three subgroups of children.
- Severe therapy-resistant asthma: in which children continue to have poor control, or asthma becomes uncontrolled with a reduction in treatment, once modifiable factors have been identified and addressed (e.g. adherence, reduction in allergen exposure or treatment of comorbidities). It is recommended these children are phenotypically characterised and assessed as to their eligibility for biologic treatment.
- Difficult-to-treat asthma: in which modifiable factors are the cause of poor control, and once corrected, treatment can be weaned to the lowest possible dose to maintain good asthma control. In this cohort, management of treatable traits is a key strategy in symptom control.
- Refractory difficult asthma: despite the identification and correction of modifiable factors, disease remains intractable due to additional factors, including but not limited to psychosocial issues such as safeguarding concerns, an adverse home environment, poor parental supervision or comorbidities such as obesity, which are largely beyond the control of the child. The increased risk of severe, life-threatening asthma attacks in this subgroup means that consideration for biological therapy is also justified.103
Although patients with PSA represent a small proportion of those with asthma overall, they consume a high proportion of healthcare resources.103
Management of problematic severe asthma
Patients with PSA require a systematic approach that:
- confirms the diagnosis and the contribution of asthma to symptoms
- identifies barriers to effective treatments
- manages comorbidities (anxiety, obesity, vocal cord dysfunction, gastro-oesophageal reflux, allergy and rhinosinusitis)
- optimises treatment and monitors for adverse effects.103
Within complex asthma clinics, this care is co-ordinated by a respiratory physician in conjunction with some or all of: an asthma nurse specialist, allergy and immunology physician, respiratory scientist, psychologist, physiotherapist, speech pathologist and ear, nose and throat surgeon.104 This multidisciplinary approach is starting to emerge within tertiary paediatric institutions, with encouraging evidence of benefit for important outcomes such as rates of exacerbation, hospitalisation and oral corticosteroid exposure.105
Severe asthma is considered in patients with a confirmed asthma diagnosis, comorbidities, optimal adherence and a continued need for high-dose ICS plus a second preventer to maintain control or asthma that remains uncontrolled despite this therapy.106 Children with severe asthma have poor quality of life, limitations on activities and frequent asthma attacks and are at high risk of treatment side effects.106,107
Biologic agents: indications and limitations
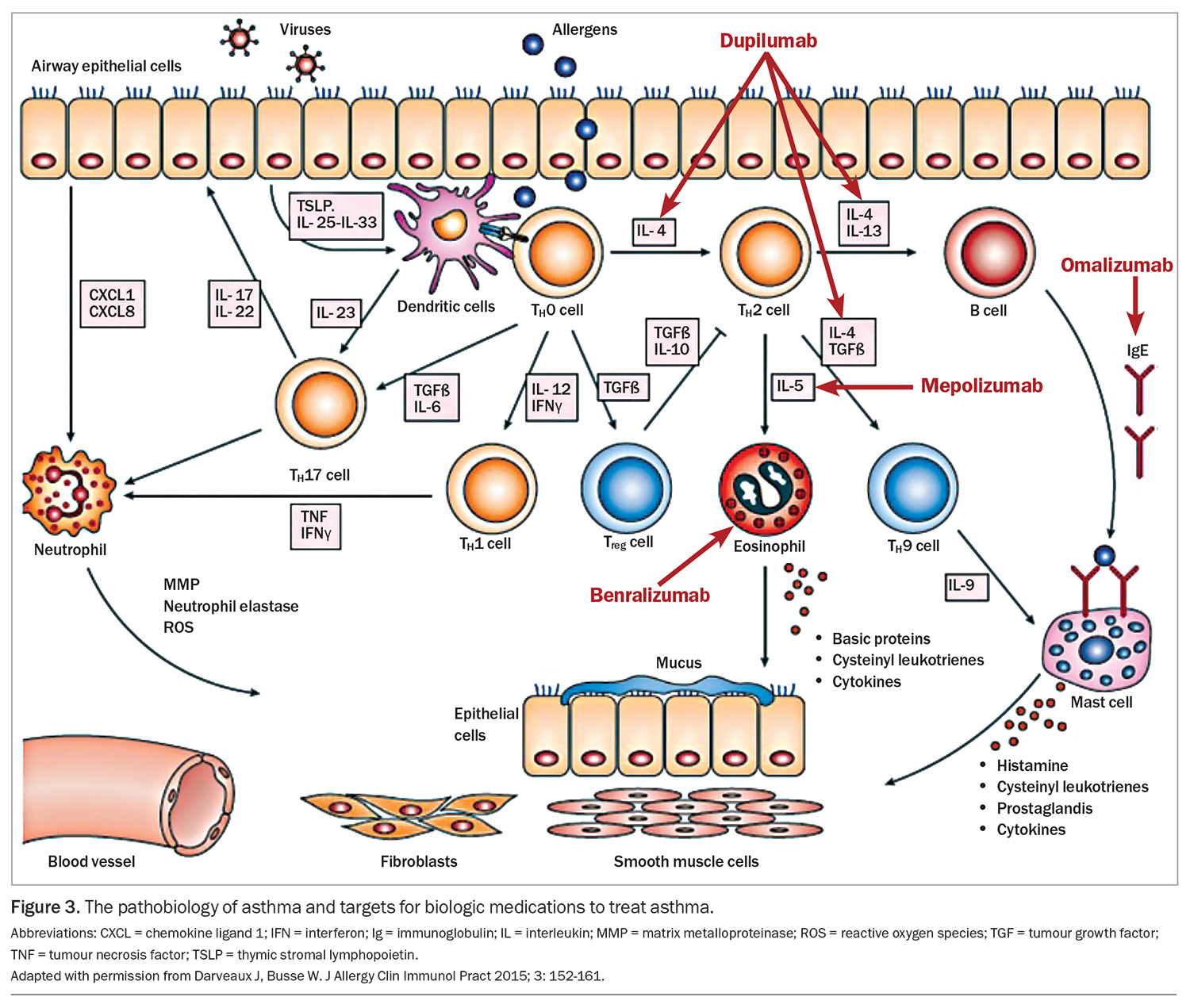
Severe asthma is recognised as a heterogeneous condition, and cohort studies have begun to define phenotypes within this patient group that may allow effective biomarker-driven treatments. The best described phenotypes include the allergic and eosinophilic types which are described according to the pattern of inflammatory response. Although no specific phenotypes are agreed on, there appears to be a clustering of an early-onset allergic phenotype, a later-onset obese (primarily female) phenotype and a later-onset eosinophilic phenotype. Identifying eosinophilic inflammatory biomarkers is helpful in considering targeted therapy such as biological agents.108-113
Traditional asthma medications broadly aimed to reduce airway inflammation and reverse bronchoconstriction. Biologic medications now offer a targeted and personalised treatment approach. Measured against placebo, the biologic agents have demonstrated reductions in severe exacerbation rate (about 50%), corticosteroid use, lung function and health related quality of life.106-111
In Australia, the following monoclonal antibody treatments are approved for the treatment of severe asthma:
- omalizumab: antisoluble IgE, prevents IgE binding to mast cells, has been used since 2002 for the treatment of uncontrolled severe asthma and has been approved for children aged 6 years and older from 2016, administered subcutaneously two- to four-weekly
- benralizumab: antibody directed to interleukin-5 receptor, expressed on eosinophils and basophils reducing the production and survival of eosinophils, available for adolescents aged 12 years and older, administered subcutaneously four-weekly, then progressed to eight-weekly
- mepolizumab: antisoluble interleukin-5, reducing the production and survival of eosinophils, available for adolescents aged 12 years and older, administered subcutaneously four-weekly
- dupilimab: antibody directed to soluble interleukin-4 and -13, approved for the management of severe asthma and atopic dermatitis (separate indications) in children aged 12 years and older and 6 years and older for atopic dermatitis
- tezepelumab: antithymic stromal lymphopoietin, indicated for any asthma subtype for adolescents 12 years and older (not currently TGA approved).
The complex inflammatory cascade of asthma and site of action of these agents is shown in Figure 3.
Treatment algorithms include certain biomarkers to inform the choice of biologic agents. Key markers include blood eosinophil count, serum IgE and measured exhaled nitric oxide. These indices have been extrapolated from the inclusion criteria of the placebo-controlled trials. To be eligible for a PBS subsidy for monoclonal antibody treatment, patients must be known to a respiratory specialist or severe asthma clinic and meet specified biomarker and clinical criteria.4
The short-term safety profiles are generally excellent for the biologics, the most common reaction being local injection site reactions. Specific agents are associated with other mild adverse effects. Serious rare adverse effects, including anaphylaxis and polyangiitis, are reported and should be monitored for depending on the specific agent used. Long-term safety data is lacking, specifically concerning the effect on the developing immune system, vaccine responsiveness and the possible emergence of antidrug antibodies.
Comparative effectiveness and optimal choice is unknown as there have been no RCTs directly comparing asthma biologics for efficacy and safety. The TREAT trial is a UK industry independent RCT comparing mepolizumab and omalizumab for severe paediatric asthma (https://doi.org/10.1186/ISRCTN12109108).
Conclusion
Management recommendations for paediatric asthma vary by age. However, important principles, such as accurate diagnosis and titration of treatment to maintain adequate control at the lowest preventer medication dose, are applicable to all age groups. Patients with well-controlled asthma should have little need of reliever medication, no limitations on activity and no nocturnal symptoms; this should be the goal for the primary physicians of all patients with asthma. Routine review of patients with well-controlled asthma should focus on revising action plans and reinforcing treatment adherence and drug delivery technique. Patients who have poor day-to-day symptom profiles, require more than one course of OCS per year or are not responding to guideline-recommended treatment must be recognised as a concern and referred for specialist assessment and reviewed more regularly.
Recent asthma mortality reviews have identified important risk factors, such as over-reliance on reliever and underuse of preventer medications. Worryingly, UK research suggests that these patients are not being well identified in primary care. Asthma deaths remain highly preventable, and better recognition of at-risk groups and improved adherence to current management guidelines, such as those outlined in the AAH, is crucial to efforts to reduce asthma mortality. Options to improve adherence are increasing, including once-daily administration options for ICS and ICS plus LABA, asthma-focused apps and electronic monitoring devices. SMART is featuring more prominently in international guidelines as the recommended treatment for adolescents, and more recently, children aged 6 to 11 years. AAH recommendations have yet to adopt this approach for the younger age group. For patients with difficult-to-treat asthma and severe asthma, the armamentarium is expanding with novel biologic agents targeted against precise disease pathways. Understanding of how best to identify the phenotypes most likely to respond is improving.
In a Lancet article entitled ‘After asthma: redefining airway disease’, several thought-provoking recommendations were made.114 Asthma should be considered an umbrella term, encompassing a collection of distinct airway disease phenotypes alongside comorbidities that may contribute to symptoms. Better appreciation of these components and the developmental trajectories of airway disease will allow clinicians to tailor an individualised approach to asthma management for each patient, trialling and evaluating therapies that have led to a clinical improvement, or not, and considering the treatment of comorbidities that influence disease or influence the perception of symptoms. Lastly, clinicians must adopt a ‘zero tolerance’ approach toward asthma attacks, recognising them as a ‘red flag’, if future efforts to reduce the unacceptable ongoing asthma mortality are to be successful. MT
COMPETING INTERESTS: None.
References
1. Australian Institute of Health and Welfare (AIHW). Chronic respiratory conditions: Asthma. Canberra: AIHW; 2024. Available online at: https://www.aihw.gov.au/reports/chronic-respiratory-conditions/asthma (accessed November 2024).
2. Acworth J, Babl F, Borland M, et al. Patterns of presentation to the Australian and New Zealand Paediatric Emergency Research Network. Emerg Med Australas 2009; 21: 59-66.
3. Hiscock H, Roberts G, Efron D, et al. Children Attending Paediatricians Study: a national prospective audit of outpatient practice from the Australian Paediatric Research Network. Med J Aust 2011; 194: 392-397.
4. National Asthma Council Australia. Australian Asthma Handbook, version 2.2. Melbourne: National Asthma Council Australia; 2022. Available online at: https://www.asthmahandbook.org.au (accessed November 2024).
5. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, 2024. Fontana, WI: GINA; 2024. Available online at: https://www.ginasthma.org (accessed May 2024).
6. Braithwaite J, Hibbert PD, Jaffe A, et al. Quality of health care for children in Australia, 2012-2013. JAMA 2018; 319: 1113-1124.
7. Cloutier MM, Salo PM, Akinbami LJ, et al. Clinician agreement, self-efficacy, and adherence with the guidelines for the diagnosis and management of asthma. J Allergy Clin Immunol Pract 2018; 6: 886-894.e4.
8. Rance K, O’Laughlen M, Ting S. Improving asthma care for African American children by increasing national asthma guideline adherence. J Pediatr Health Care 2011; 25: 235-249.
9. Srour-Alphonse P, Cvetkovski B, Rand CS, et al. It takes a village - asthma networks utilized by parents when managing childhood asthma medications. J Asthma 2020; 57: 306-318.
10. Haggie S, Robinson PD. Paediatric asthma: update on the stepwise management approach. Respiratory Medicine Today 2020; 5(2): 6-16.
11. Bush A, Kleinert S, Pavord ID. The asthmas in 2015 and beyond: a Lancet Commission. Lancet 2015; 385: 1273-1275.
12. Ebmeier S, Thayabaran D, Braithwaite I, Bénamara C, Weatherall M, Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993-2012). Lancet 2017; 390: 935-945.
13. Fitzgerald DA, Gillis J. Asthma deaths in children in New South Wales 2004-2013: Could we have done more? J Paediatr Child Health 2015; 51: 1127-1133.
14. Levy ML. National review of asthma deaths (NRAD). Br J Gen Pract 2014; 64: 564.
15. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995; 332: 133-138.
16. Castro-Rodriguez JA, Cifuentes L, Rodríguez-Martínez CE. The asthma predictive index remains a useful tool to predict asthma in young children with recurrent wheeze in clinical practice. J Allergy Clin Immunol 2011; 127: 1082-1083.
17. Brand PL. The Asthma Predictive Index: not a useful tool in clinical practice. J Allergy Clin Immunol 2011; 127: 293-294.
18. Reyna ME, Dai R, Tran MM, et al. Development of a symptom-based tool for screening of children at high risk of preschool asthma. JAMA Netw Open 2022; 5: e2234714.
19. Marlow R, Finn A, Henderson J. Assessing the association between bronchiolitis in infancy and recurrent wheeze: a whole English birth cohort case-control study. Thorax 2019; 74: 503-505.
20. Drysdale SB, Cathie K, Flamein F, et al. Nirsevimab for prevention of hospitalizations due to RSV in infants. New Engl J Med 2023; 389: 2425-2435.
21. Rosas-Salazar C, Chirkova T, Gebretsadik T, et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. Lancet 2023; 401: 1669-1680.
21. O’Brien S, Borland ML, Cotterell E, et al. Australasian bronchiolitis guideline. J Paediatr Child Health 2019; 55: 42-53.
23. Yusuf F, Prayle AP, Yanney MP. ß2-agonists do not work in children under 2 years of age: myth or maxim? Breathe (Sheff) 2019; 15: 273-276.
24. Bialy L, Foisy M, Smith M, Fernandes RM. The Cochrane Library and the treatment of bronchiolitis in children: an overview of reviews. Evidence‐Based Child Health 2011; 6: 258-275.
25. Cai Z, Lin Y, Liang J. Efficacy of salbutamol in the treatment of infants with bronchiolitis: A meta-analysis of 13 studies. Medicine (Baltimore) 2020; 99: e18657.
26. Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med 2006; 354: 1998-2005.
27. Frey U, von Mutius E. The challenge of managing wheezing in infants. N Engl J Med 2009; 360: 2130-2133.
28. Bush A. Practice imperfect - treatment for wheezing in preschoolers. N Engl J Med 2009; 360: 409-410.
29. Royal Children’s Hospital Melbourne. Asthma acute. Last updated June 2018. Available online at: https://www.rch.org.au/clinicalguide/guideline_index/Asthma_acute/ (accessed August 2020).
30. Children’s Health Queensland Hospital and Health Service. Pre-school wheeze - emergency management in children. Available online at: https://www.childrens.health.qld.gov.au/guideline-preschool-wheeze-emergency-management-in-children/ (accessed August 2020).
31. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med 2009; 360: 329-338.
32. Foster SJ, Cooper MN, Oosterhof S, Borland ML. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2018; 6: 97-106.
33. Zorc JJ. Oral corticosteroids reduce length of hospital stay for preschool children with virus-associated wheeze. Lancet Respir Med 2018; 6: 76-77.
34. Grigg J. Role of systemic steroids in acute preschool wheeze. Arch Dis Child 2010; 95: 491-492.
35. Abrams EM, Becker AB, Szefler SJ. Use of oral corticosteroids in the wheezy toddler. J Pediatr 2018; 201: 16-20.
36. Brand PL, Baraldi E, Bisgaard H, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J 2008; 32: 1096-1110.
37. Wassall HJ, Devenny AM, Daud Khan S, Ninan TK, Russell G. A comparison of virus-associated and multi-trigger wheeze in school children. J Asthma 2005; 4 2: 737-744.
38. De Queiroz Andrade E, Sena C, de Gouveia Belinelo P, et al. In utero smoking exposure induces changes to lung clearance index and modifies risk of wheeze in infants. Pediatr Pulmonol 2024; 59: 1686-1694
39. Wang Z, May SM, Charoenlap S, et al. Effects of secondhand smoke exposure on asthma morbidity and health care utilization in children: a systematic review and meta-analysis. Ann Allergy Asthma Immunol 2015; 115: 396-401.e2.
40. Brand PL, Caudri D, Eber E, et al. Classification and pharmacological treatment of preschool wheezing: changes since 2008. Eur Respir J 2014; 43: 1172-1177.
41. Bacharier LB, Guilbert TW. Diagnosis and management of early asthma in preschool-aged children. J Allergy Clin Immunol 2012; 130: 287-298.
42. Kampschmidt JC, Brooks EG, Cherry DC, Guajardo JR, Wood PR. Feasibility of spirometry testing in preschool children. Pediatr Pulmonol 2016; 51: 258-266.
43. Kerem E, Levison H, Schuh S, et al. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr 1993; 123: 313-317.
44. Ram FS, Brocklebank DM, White J, Wright JP, Jones PW. Pressurised metered dose inhalers versus all other hand-held inhaler devices to deliver beta-2 agonist bronchodilators for non-acute asthma. Cochrane Database Syst Rev 2002; (1): CD002158.
45. Zeiger RS, Szefler SJ, Phillips BR, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol 2006; 117: 45-52.
46. Castro-Rodriguez JA, Rodrigo GJ. The role of inhaled corticosteroids and montelukast in children with mild-moderate asthma: results of a systematic review with meta-analysis. Arch Dis Child 2010; 95: 365-370.
47. Fitzpatrick AM, Jackson DJ, Mauger DT, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol 2016; 138: 1608-1618.e12.
48. Ducharme FM, Noya FJ, Allen-Ramey FC, Maiese EM, Gingras J, Blais L. Clinical effectiveness of inhaled corticosteroids versus montelukast in children with asthma: prescription patterns and patient adherence as key factors. Curr Med Res Opin. 2012; 28: 111-119.
49. Gulliver T, Morton R, Eid N. Inhaled corticosteroids in children with asthma: pharmacologic determinants of safety and efficacy and other clinical considerations. Paediatr Drugs 2007; 9: 185-194.
50. Powell H, Gibson PG. High dose versus low dose inhaled corticosteroid as initial starting dose for asthma in adults and children. Cochrane Database Syst Rev 2004; (2): CD004109.
51. Pedersen S. Do inhaled corticosteroids inhibit growth in children? Am J Respir Crit Care Med 2001; 164: 521-535.
52. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med 2012; 367: 904-912.
53. Cockcroft DW. Clinical concerns with inhaled β 2-agonists: adult asthma. Clin Rev Allergy Immunol 2006; 31: 197-207.
54. Melén E, Nwaru B, Wiklund F, et al. Short‐acting β2‐agonist use and asthma exacerbations in Swedish children: a SABINA Junior study. Pediatr Allergy Immunol 2022; 33: e13885.
55. Villaran C, O’Neill SJ, Helbling A, et al. Montelukast versus salmeterol in patients with asthma and exercise-induced bronchoconstriction. Montelukast/Salmeterol Exercise Study Group. J Allergy Clin Immunol 1999; 104(3 Pt 1): 547-553.
56. Raissy HH, Harkins M, Kelly F, Kelly HW. Pretreatment with albuterol versus montelukast for exercise-induced bronchospasm in children. Pharmacotherapy 2008; 28: 287-294.
57. Fogel RB, Rosario N, Aristizabal G, et al. Effect of montelukast or salmeterol added to inhaled fluticasone on exercise-induced bronchoconstriction in children. Ann Allergy Asthma Immunol 2010; 104: 511-517.
58. Stelmach I, Grzelewski T, Majak P, Jerzynska J, Stelmach W, Kuna P. Effect of different antiasthmatic treatments on exercise-induced bronchoconstriction in children with asthma. J Allergy Clin Immunol 2008; 121: 383-389.
59. Aldea Perona A, García-Sáiz M, Sanz Álvarez E. Psychiatric disorders and montelukast in children: a disproportionality analysis of the VigiBase(®). Drug Saf 2016; 39: 69-78.
60. Glockler-Lauf SD, Finkelstein Y, Zhu J, Feldman LY, To T. Montelukast and neuropsychiatric events in children with asthma: a nested case-control study. J Pediatr 2019; 209: 176-182.e4.
61. Benard B, Bastien V, Vinet B, Yang R, Krajinovic M, Ducharme FM. Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice. Eur Respir J 2017; 50: 1700148.
62. Arets HG, Kamps AW, Brackel HJ, Mulder PG, Vermue NA, van der Ent CK. Children with mild asthma: do they benefit from inhaled corticosteroids? Eur Respir J 2002; 20: 1470-1475.
63. Lemanske RF Jr, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010; 362: 975-985.
64. Marks G, Ampon R, Poulos L, Reddel H. Incidence and remission of asthma in Australian children: findings from a population cohort. Eur Resp J 2019; 54 Suppl 63: PA2784.
65. Kosse RC, Koster ES, Kaptein AA, de Vries TW, Bouvy ML. Asthma control and quality of life in adolescents: the role of illness perceptions, medication beliefs, and adherence. J Asthma 2020; 57: 1145-1154.
66. Netz M, Fedele DA, Sweenie R, Baker D, Light M, McQuaid EL. Asthma management responsibility, control, and quality of life among emerging adolescents. J Pediatr Psychol 2020; 45: 40-49.
67. de Benedictis D, Bush A. Asthma in adolescence: Is there any news? Pediatr Pulmonol 2017; 52: 129-138.
68. Carroll W, Clayton S, Frost S, et al. If it’s ‘only’ asthma, why are children still dying? Arch Dis Child 2020; 105: 494-498.
69. Levy ML. The national review of asthma deaths: what did we learn and what needs to change? Breathe (Sheff) 2015; 11: 14-24.
70. Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62: 591-604.
71. Lugogo N, O’Connor M, George M, et al. Expert consensus on SABA use for asthma clinical decision-making: a Delphi approach. Curr Allergy Asthma Rep 2023; 23: 621-634.
72. O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med 2018; 378: 1865-1876.
73. Bateman ED, Reddel HK, O’Byrne PM, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med 2018; 378: 1877-1887.
74. Beasley R, Holliday M, Reddel HK, et al. Novel START Study Team. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med 2019; 380: 2020-2030.
75. Hardy J, Baggott C, Fingleton J, et al. PRACTICAL study team. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet 2019; 394: 919-928
76. Reddel HK, O’Byrne PM, FitzGerald JM, et al. Efficacy and safety of as-needed budesonide-formoterol in adolescents with mild asthma. J Allergy Clin Immunol Pract 2021; 9: 3069-3077.
77. Farzan S, Ponda P. What is SMART for some may not be right for all. J Allergy Clin Immunol Pract 2021; 9: 3078-3079.
78. Shanthikumar S, Robinson PD. As-needed budesonide-formoterol for adolescents with mild asthma: Importance of lung function. J Allergy Clin Immunol Pract 2021; 9: 4178.
79. Granell R, Haider S, Deliu M, et al. Lung function trajectories from school age to adulthood and their relationship with markers of cardiovascular disease risk. Thorax 2024; 79: 770-777.
80. Vasquez MM, Zhou M, Hu C, Martinez FD, Guerra S. Low lung function in young adult life is associated with early mortality. Am J Respir Crit Care Med 2017; 195: 1399-1401.
81. Abrams EM, Shaker M, Greenhawt M, Fernandes RM, Sinha I. Treatment of mild-to-moderate asthma in childhood and adolescence in 2021. Lancet Respir Med 2021; 9: 443-445.
82. Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management. Treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J 2019; 53: 1901046.
83. Jorup C, Lythgoe D, Bisgaard H. Budesonide/formoterol maintenance and reliever therapy in adolescent patients with asthma. Eur Respir J 2018; 51: 1701688.
84. O’Byrne PM, Bisgaard H, Godard PP, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 2005; 171: 129-136.
85. Rogers L, Reibman J. Stepping down asthma treatment: how and when. Curr Opin Pulm Med 2012; 18: 70-75.
86. Espinoza-Palma T, Zamorano A, Arancibia F, et al. Effectiveness of asthma education with and without a self-management plan in hospitalized children. J Asthma 2009; 46: 906-910.
87. Kelso JM. Do written asthma action plans improve outcomes? Pediatr Allergy Immunol Pulmonol 2016; 29: 2-5.
88. Agrawal SK, Singh M, Mathew JL, Malhi P. Efficacy of an individualized written home-management plan in the control of moderate persistent asthma: a randomized, controlled trial. Acta Paediatr 2005; 94: 1742-1746.
89. Cicutto L, Conti E, Evans H, et al. Creating asthma-friendly schools: a public health approach. J Sch Health 2006; 76: 255-258.
90. Hill RA, Britton JR, Tattersfield AE. Management of asthma in schools. Arch Dis Child 1987; 62: 414-415.
91. Kew KM, Carr R, Donovan T, Gordon M. Asthma education for school staff. Cochrane Database Syst Rev 2017; 4(4): CD012255.
92. Mellis CM, Bowes G, Henry RL, et al. A national policy on asthma management for schools. The Asthma Special Interest Group, Thoracic Society of Australia and New Zealand. J Paediatr Child Health 1994; 30: 98-101.
93. Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol 2008; 122: 662-668.
94. Penza-Clyve SM, Mansell C, McQuaid EL. Why don’t children take their asthma medications? A qualitative analysis of children’s perspectives on adherence. J Asthma 2004; 41: 189-197.
95. Ezzell K. Strategies to guide medication adherence discussions with parents of children with asthma. Pediatr Nurs 2017; 43: 219-222.
96. Klok T, Kaptein AA, Brand PLP. Non-adherence in children with asthma reviewed: the need for improvement of asthma care and medical education. Pediatr Allergy Immunol 2015; 26: 197-205.
97. Jochmann A, Artusio L, Jamalzadeh A, et al. Electronic monitoring of adherence to inhaled corticosteroids: an essential tool in identifying severe asthma in children. Eur Respir J 2017; 50: 1700910.
98. Kagen S, Garland A. Asthma and allergy mobile apps in 2018. Curr Allergy Asthma Rep 2019; 19: 6.
99. Unni E, Gabriel S, Ariely R. A review of the use and effectiveness of digital health technologies in patients with asthma. Ann Allergy Asthma Immunol 2018; 121: 680-691.e1.
100. Huckvale K, Morrison C, Ouyang J, Ghaghda A, Car J. The evolution of mobile apps for asthma: an updated systematic assessment of content and tools. BMC Med 2015; 13: 58.
101. Fleming L, Murray C, Bansal AT, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J 2015; 46: 1322-1333.
102. Nordlund B, Melén E, Schultz ES, Grönlund H, Hedlin G, Kull I. Prevalence of severe childhood asthma according to the WHO. Respir Med 2014; 108: 1234-1237.
103. Pijnenburg MW, Fleming L. Advances in understanding and reducing the burden of severe asthma in children. Lancet Respir Med 2020; 8: 1032-1044.
104. Pike KC, Levy ML, Moreiras J, Fleming L. Managing problematic severe asthma: beyond the guidelines. Arch Dis Child 2018; 103: 392-397.
105. Lieu N, Krishnananthan T, Hatton L, Wong M, Ray R, Mahmood D, Koo S, Pandit C, Towns S, Milne B, Middleton A, Osland K, Kennedy B, Jeyasuriya G, Singh J, Lu M, Field P, Selvadurai H, Fitzgerald D, Marshall T, Robinson P. Impact of a paediatric multidisciplinary complex asthma service on outcomes. Respirology Volume 28, Issue S2, TO054 [Abstract].
106. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343-373.
107. Grainge CL, Maltby S, Gibson PG, Wark PA, McDonald VM. Targeted therapeutics for severe refractory asthma: monoclonal antibodies. Expert Rev Clin Pharmacol 2016; 9: 927-941.
108. Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol 2009; 124: 1210-1216.
109. Brodlie M, McKean MC, Moss S, Spencer DA. The oral corticosteroid-sparing effect of omalizumab in children with severe asthma. Arch Dis Child 2012; 97: 604-609.
110. Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med 2016; 4: 549-556.
111. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651-659.
112. FitzGerald JM, Bleecker ER, Menzies-Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med 2018; 6: 51-64.
113. Schepel IRM, Banzon TM, Phipatanakul W. Future of biologics in pediatric asthma: optimizing response, early introduction, and equitable access to treatment. Ann Allergy Asthma Immunol 2024; 132: 13-20.
114. Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350-400.