Endometriosis – unmasking the silent struggle
Endometriosis is greatly underdiagnosed in women, particularly because of its variable clinical manifestations that may mimic other conditions. Management is multidisciplinary and aimed at alleviating pain, increasing fertility and improving quality of life.
- Endometriosis may be present in adolescents from the time of menarche and, rarely, can persist after menopause.
- Early detection and referral to a gynaecologist reduces the time to diagnosis. A high-quality ultrasound can facilitate accurate diagnosis and surgical planning.
- Validation of the patient’s pain and goal setting should be initiated early. Management of endometriosis in a multidisciplinary care setting can improve the patient’s quality of life.
- Empirical medical management is appropriate even if ultrasound findings are normal and should include shared decision-making with the patient.
- Pregnancy should not be advised for the primary purpose of treating endometriosis.
- Laparoscopy should no longer be considered the gold standard for diagnosing deep infiltrating endometriosis if specialised ultrasound scans and MRI are available but should be considered if imaging results are negative or empirical treatment is ineffective or inappropriate.
Endometriosis affects one in seven women in Australia but remains a significantly under-recognised cause of pelvic pain.1 For the purposes of this article, the term ‘women’ includes those assigned female at birth. Often shrouded in diagnostic delays and misunderstood symptoms, endometriosis not only challenges the affected individuals but also tests the resilience and co-ordination of our healthcare system. As primary care providers, GPs are at the frontline of early detection, playing a pivotal role in initiating a comprehensive, multidisciplinary approach that can significantly improve long-term outcomes.
The challenges in recognising and diagnosing endometriosis are demonstrated by the average delay of five or more years between seeking medical care and receiving a diagnosis.2 For those with endometriosis, the consequences can be far reaching, with impacts on physical and sexual function, education and work, and mental and emotional wellbeing leading to a reduced quality of life.3
Around half of all women presenting with persistent pelvic pain will have endometriosis, making it the most common cause of this presentation.4 The initial approach of validation, investigation and early treatment of pain is important for these overlapping conditions; however, the management of persistent pelvic pain is beyond the scope of this article. This article provides an overview of the diagnosis and management of endometriosis.
Aetiology of endometriosis
Endometriosis is a chronic inflammatory disease characterised by the presence of endometrium-like tissue outside the uterus.5 Although the lesions are more commonly found on the ovaries, fallopian tubes, uterosacral ligaments and pelvic peritoneum, endometriosis can also affect the bowel (5–25%), bladder and ureters (1–5%), or the diaphragm (<2%).6-8 Endometriotic lesions can be superficial or can invade the underlying organs or structures as deep infiltrating endometriosis.
Although its precise aetiology remains uncertain, current evidence suggests a multifactorial origin. This includes the transportation of endometrial tissue outside the uterine cavity, likely through retrograde menstruation, combined with a genetic predisposition and altered immune responses that allow the tissue to persist. As the endometrial lining sheds during menstruation, so too does the ectopic tissue, triggering pain, inflammation and the formation of adhesions. Over time, these processes can lead to structural abnormalities, invasion into adjacent organs and remodelling of pain pathways, often resulting in persistent pelvic pain.9
Who is affected by endometriosis?
One in seven women have endometriosis. As endometriotic tissue is oestrogen dependent, it primarily affects women during the reproductive years. Symptoms can begin as early as menarche and, rarely, can persist after menopause.
Endometriosis can affect any woman, but some risk factors have been established. These include a family history of endometriosis, early menarche (before the age of 11–13 years), short menstrual cycles (<27 days) and heavy menstrual bleeding.10,11 Conditions that obstruct menstrual bleeding, such as cervical stenosis or Müllerian anomalies, are also associated with an increased risk of endometriosis, possibly because of retrograde menstruation seeding endometrial tissue.12 Nulliparity is associated with an increased risk of endometriosis, whereas multiparity appears to be protective.13
Clinical presentation
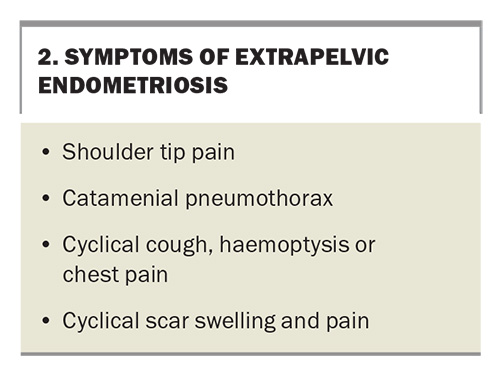
Symptoms of endometriosis can be diverse and can frequently be misunderstood or confused for other conditions. The most common symptoms of pelvic and extra pelvic endometriosis are summarised in Box 1 and Box 2. These symptoms may be cyclical or noncyclical. The chance of having endometriosis increases with an increasing number of symptoms reported.14
Endometriosis should be suspected in people who have dysmenorrhoea that:
- does not respond to simple analgesia
- is severe
- affects daily activities
- is associated with other symptoms listed in Box 1 and Box 2.
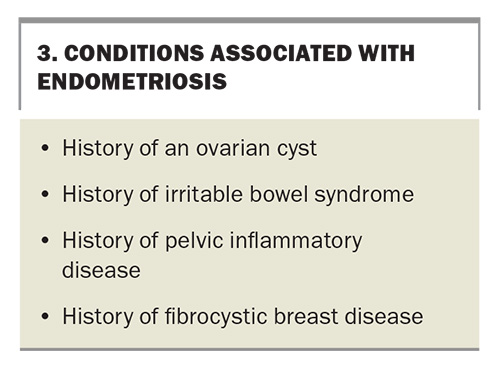
Around 50% of women presenting with infertility will have endometriosis.12 The condition is more likely to present in women who see their GP more frequently and is associated with a number of other conditions, as listed in Box 3.14
Examination findings
Clinical examination is an important initial step in assessing women with suspected endometriosis, although normal examination findings do not exclude the diagnosis. Where appropriate, abdominopelvic and speculum examinations should be considered.
Bimanual examination may reveal an immobile uterus, adnexal tenderness or masses (endometriomas). Speculum examination may reveal vaginal or cervical endometriosis nodules, and can provide a chance for opportunistic investigations, such as cervical screening and testing for sexually transmitted infections. For deep endometriosis, vaginal examination can facilitate the detection of infiltration or nodules of the vagina, uterosacral ligaments, or pouch of Douglas. Concurrent pelvic floor dysfunction, vaginismus and vulvodynia are common in women presenting with pelvic pain and may make these examinations inappropriate or impossible. If endometriosis is suspected based on history, further investigation with imaging is recommended, even if clinical examination is normal or not possible.
Diagnosis of endometriosis
There are no biomarkers that show sufficient sensitivity or specificity to warrant use in a clinical setting and should, therefore, not be used in the diagnostic workup of endometriosis.5
Imaging modalities
Ultrasound
Ultrasound is a useful noninvasive tool when assessing women with suspected disease and should be the first-line imaging investigation. Although only 65% of cases are detected using ultrasound, it is more sensitive for diagnosing cysts of endometriosis within the ovaries (endometriomas) (93%) and deep infiltrating endometriosis (79%).15 Despite advances in ultrasound detection of endometriosis, a negative ultrasound result does not exclude the disease.
The quality of the ultrasound is important when assessing these patients, as an ultrasound performed by a skilled provider will improve diagnostic accuracy and assist surgical planning and resource allocation (e.g. allocating an appropriately trained surgeon and adequate time for complex surgery). Furthermore, having a high-quality ultrasound prior to a specialist appointment prevents diagnostic and treatment delays, as many clinicians and departments will require a detailed ultrasound scan prior to planning surgery for a patient with suspected endometriosis.
A ‘high-quality’ pelvic ultrasound for the investigation of endometriosis is ideally a transvaginal scan that goes beyond basic assessment of the uterus and ovaries. Such a scan includes an assessment of:
- ovarian mobility and tenderness
- adhesions or obliteration of the pouch of Douglas
- nodules on the uterosacral ligaments, bladder and ureters
- the mobility of pelvic organs.
Features of adenomyosis may be suggestive of coexisting endometriosis and are associated with severe disease. One study found that 42% of women with adenomyosis at the time of hysterectomy also had endometriosis, and another found that 41% of women with stage IV endometriosis had ultrasound features of adenomyosis.16-18 Practitioners with a Certificate in Obstetric and Gynaecology Ultrasound or a Diploma of Diagnostic Ultrasound Obstetrics and Gynaecology are preferred providers for these ultrasounds if they are available.
Ultrasound can also be used to monitor the progression of stage 3 and 4 disease by measuring the number of endometrial nodules and size of endometriomas.19 For adolescents or women in whom a transvaginal ultrasound may not be appropriate, a transabdominal ultrasound can provide additional information and be useful to exclude alternative diagnoses.20 Some guidelines also recommend MRI in these women.5
MRI
In Australia, MRI is not recommended as the primary investigation to diagnose endometriosis, except for women in whom an ultrasound may not be appropriate.20 Its use lies primarily in surgical planning of deep infiltrating disease, and it is only covered by the Medicare Benefits Schedule when ordered by a specialist for this indication. MRI has been shown to be sensitive at detecting deep endometriosis and can help to assess the depth of involvement of the bowel, ureters and bladder. Endometriosis guidelines recommend that MRI findings be interpreted by specialists in gynaecological imaging.5,20,21
Diagnostic laparoscopy
Normal ultrasound or MRI findings do not exclude the diagnosis of endometriosis. Superficial peritoneal disease is usually not visible using these imaging modalities. In women with symptoms suggestive of endometriosis, empirical treatment is still recommended. If this is unsuccessful or not suitable, referral to a gynaecologist is recommended, and laparoscopy may be considered.5,20
Staging of endometriosis
Endometriosis staging is usually classified into four stages based on the extent and location of the disease; however, staging is more useful for anticipating surgical complexity and does not necessarily correlate well with the severity of symptoms.22 There is no single universally accepted staging system, but the revised American Society of Reproductive Medicine and, more recently, the American Association of Gynecologic Laparoscopists classification systems are the most widely accepted. Each of these uses a points-based system following laparoscopic assessment of endometriotic lesions. The stages are broadly summarised in Box 4.23,24
Cancer risk and surveillance
Endometriosis may be associated with a small increased risk of ovarian (relative risk [RR], 1.93), breast (RR, 1.04) and thyroid (RR, 1.39) cancers, although the data are inconclusive and the overall risk of cancer remains low.5 The risk of cervical cancer appears to be reduced in these patients (RR, 0.68).5 Patients should be reassured that they are not at an increased risk of developing cancer overall. Increased cancer surveillance is not recommended in patients with endometriosis.25
Management of endometriosis
Nonhormonal analgesia
NSAIDs alone or in combination with paracetamol are a reasonable first-line approach to endometriosis-associated pain.20,21 NSAIDs may work by reducing inflammation and prostaglandin-mediated pain in endometriosis.26 These can be used alone or in combination with hormonal therapy.
Hormonal therapy
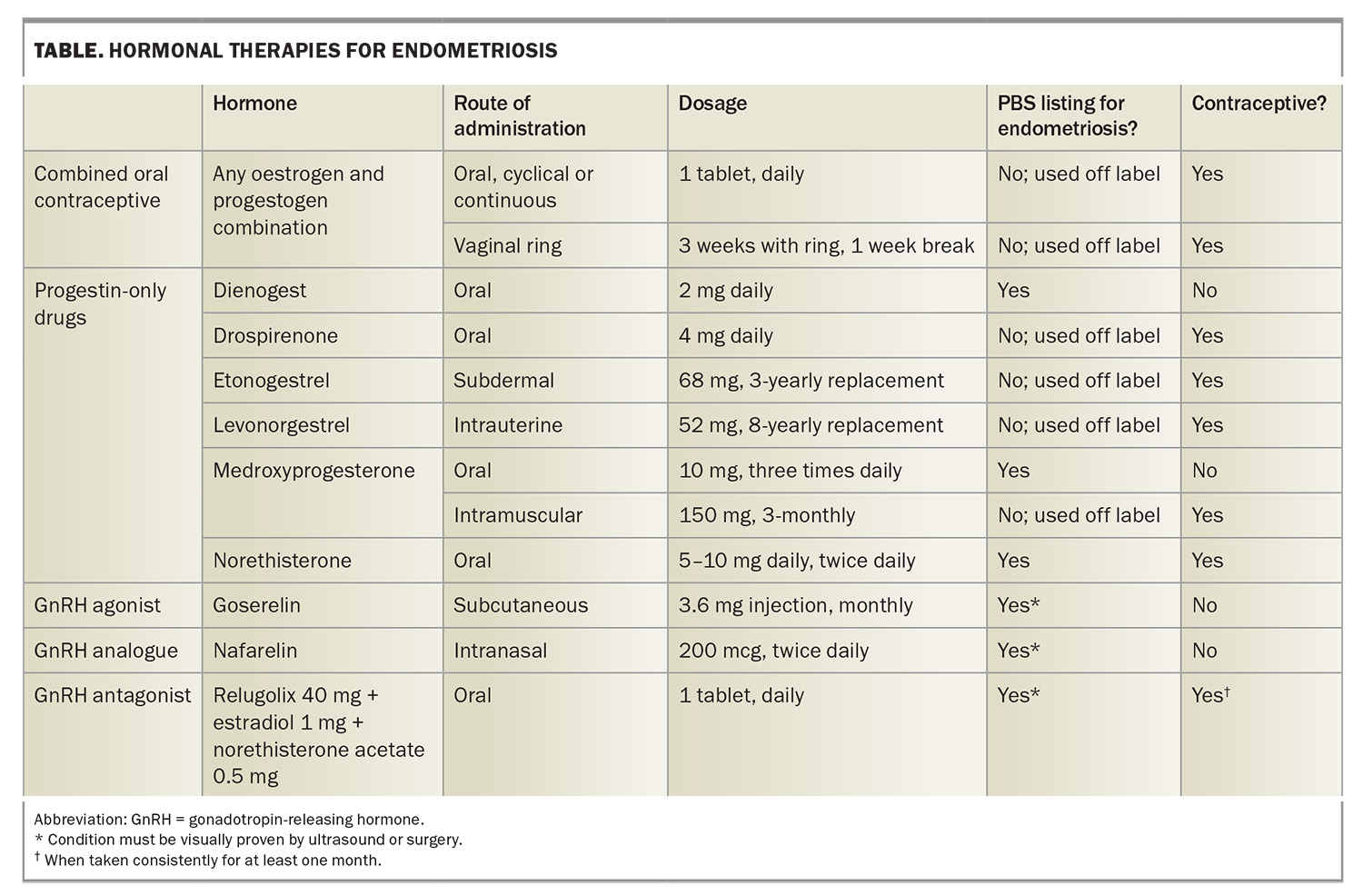
Hormonal treatments aim to reduce pain by suppressing ovarian function and inhibiting the proliferation of endometrial tissue.5 Combined oral contraceptive pills, progestogen-only pills, implants, depot injections and intrauterine devices have all been shown to be effective in managing endometriosis-associated pain, with some being PBS listed as well (Table).5,21 Hormone therapy is recommended to reduce pain in people with suspected or confirmed endometriosis who are not seeking fertility. There is no evidence that one hormonal therapy or oral progestogen is more efficacious than another, and side effects vary considerably between patients.5,20,27 Hormonal management should be a shared decision-making process with the patient, and arriving at the optimal therapy often requires a trial-and-error approach to optimise symptom control and the side effect profile.
For people with dysmenorrhoea, continuous use of combined oral contraceptives is more effective than cyclical use.5 Second-line treatments, such as gonadotropin-releasing hormone agonists and antagonists, work by inducing a hypo-oestrogenic state to shrink endometriotic lesions.28 Add-back hormone replacement therapy is important to offset the menopausal symptoms and reduced bone mineral density caused by severe hypo-oestrogenism and does not reduce the efficacy of treatment.29 Evidence is limited regarding the dosing and duration of treatment and monitoring of bone mineral density. Bone mineral density monitoring is recommended at baseline and after one year of treatment with relugolix combination therapy, and treatment is currently limited to two years.
Surgery
Surgery may be indicated when medical management fails or is inappropriate, or in shared decision-making regarding goals of care. Minimally invasive (laparoscopic or robotic) surgery to remove endometriosis lesions, excise endometriomas and remove adhesions aims to improve pain by removing the source of inflammation, and to restore normal anatomy. Surgery has been shown to alleviate pain and improve the quality of life in people with endometriosis and improve fertility outcomes for those with infertility and stage 1 or 2 disease.5,30,31
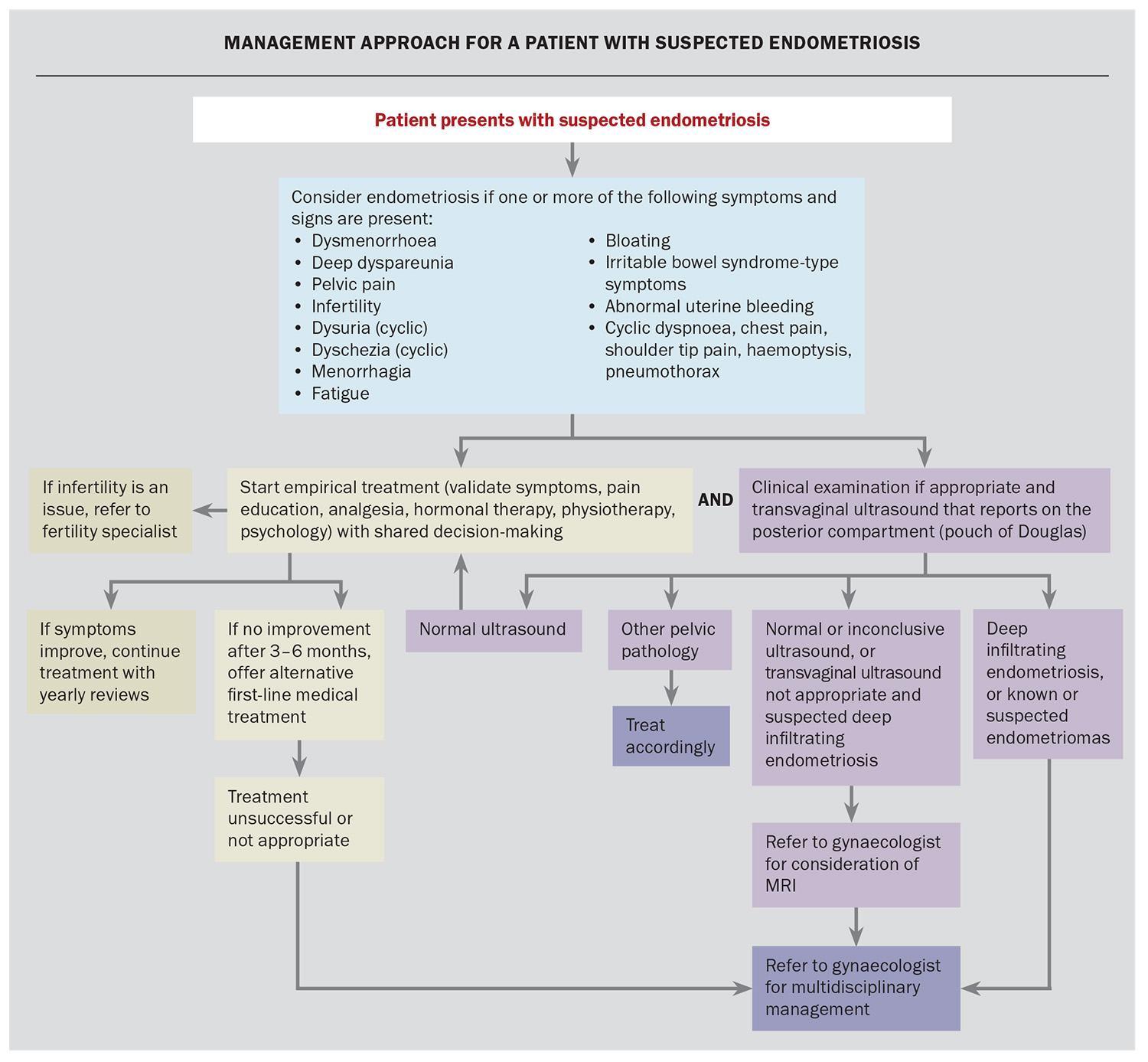
In patients with deep infiltrating endometriosis where other organs such as the bowel or ureter are involved, referral to a gynaecologist with advanced training in endometriosis surgery is beneficial. Such patients may require surgical intervention with a trained gynaecologist and a colorectal surgeon, urologist or thoracic surgeon with experience in endometriosis. Postoperative hormonal medical therapies can prolong the time to pain recurrence and can provide secondary prevention of endometriosis-associated pain.5 A management approach for patients with suspected endometriosis is illustrated in the Flowchart.
Nonpharmacological and multidisciplinary management
Although medical and surgical treatment can alleviate pain, increase fertility and improve quality of life for patients with endometriosis, it is an inherently chronic disease. As such, involvement of a multidisciplinary team is essential to provide holistic care and optimise long-term outcomes.32 This approach takes into consideration the biopsychosocial influences on a patient’s experience of pain and aims to provide individualised, comprehensive and co-ordinated care by a range of practitioners with knowledge and skills in the management of endometriosis.32
Multidisciplinary care of endometriosis as a chronic health condition is important for management of this disease. Although an endometriosis centre of excellence with a multidisciplinary team support would be ideal, management is not reliant on these centres, and GPs and gynaecologists are essential in co-ordinating holistic care for their patients. Involvement of nonpharmacological approaches, specialists, allied health professionals and complementary and alternative therapies may be selected depending on the symptoms, priorities and goals of the patient. Features that should prompt a patient’s referral to a gynaecologist are listed in Box 5.
Nonpharmacological approaches
Patient education, dietary guidance and increased physical activity are some nonpharmacological approaches that may be considered for patients with endometriosis. Accurate and consistent information about their condition empowers patients to take control of their care, allows for true shared decision-making and can improve patient self-efficacy around pain.32
A diet low in fermentable oligo-, di- and monosaccharides and polyols (FODMAP) and an ‘endometriosis diet’ may improve pain, fatigue and quality of life, particularly for those with irritable bowel syndrome symptoms and endometriosis. An endometriosis diet involves avoiding foods that aggravate the patient’s endometriosis symptoms, which commonly include red meat, gluten, cow’s milk, sugars, foods high in phyto-oestrogens (e.g. soy) and caffeine.33-35 Many patients find an anti-inflammatory diet beneficial.
Aside from the general health benefits, physical exercise for 45 to 60 minutes, three times a week, has been shown to improve dysmenorrhoea, although not specifically in women with endometriosis.36
Specialists and allied health
Pain specialists focus on holistic pain management and tailor pain management plans, considering the use of neuromodulators, such as gabapentin, amitriptyline and duloxetine, for chronic pain. They also implement interventional pain techniques, ketamine protocols and containment strategies in opioid management.
Pelvic floor physiotherapists can assist with goal setting, pain education and management when pelvic floor dysfunction and dyspareunia are present. They can provide education about stretches and at-home exercises that may help relieve pain. They can also help provide strategies to manage bladder and bowel symptoms.
Psychologists implement interventions such as pain education, cognitive behavioural therapy, mindfulness and somatosensory stimulation, which can improve pain, depression and anxiety and quality of life.37 Pain psychologists can assist those with persistent pelvic pain. Fertility psychology services may be used for patients needing or wishing for a hysterectomy as part of their surgical therapy for endometriosis, or those with infertility.
Endometriosis nurse co-ordinators can provide education, validation and empathy; ascertain care goals; co-ordinate care; and refer patients to other members of the multidisciplinary team.38
Complementary and alternative therapies
Interventions such as the use of heat packs, transcutaneous electrical nerve stimulation and acupuncture currently lack evidence and should not replace medical and surgical therapy. However, some patients may find them useful adjuncts.5 The use of Chinese herbal medicines and supplements for women with endometriosis is not supported by evidence and may be associated with potential side effects.20
Pregnancy does not always lead to symptom improvement and is not advised for the primary purpose of treating endometriosis.5
Conclusion
Endometriosis is a chronic inflammatory disease that often presents as a silent struggle, with invisible symptoms yet a profound impact. A high index of suspicion, early recognition and prompt initiation of empirical management are crucial to unmasking this often-misunderstood condition and reducing the burden of diagnostic delay. Through a holistic, multidisciplinary and shared decision-making approach, we can improve pain, fertility and quality of life in those affected. Some resources for GPs are provided in Box 6. MT
COMPETING INTERESTS: Dr Gwata is an Ordinary Director Board Member of the Australasian Gynaecological Endoscopy and Surgery (AGES) Society and is an affiliated gynaecologist of the Epworth Endometriosis Centre. Dr Hunt: None.
References
1. Australian Institute of Health and Welfare (AIHW). Endometriosis. Canberra: AIHW; 2025. Available online at: https://pp.aihw.gov.au/reports/chronic-disease/endometriosis/contents/about (accessed May 2025).
2. Armour M, Sinclair J, Ng CHM, et al. Endometriosis and chronic pelvic pain have similar impact on women, but time to diagnosis is decreasing: an Australian survey. Sci Rep 2020; 10: 16253.
3. Culley L, Law C, Hudson N, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update 2013; 19: 625-639.
4. Karp BI, Stratton P. Endometriosis-associated chronic pelvic pain. Med 2023; 4: 143-1146.
5. Becker CM, Bokor A, Heikinheimo O, et al. ESHRE guideline: endometriosis. Hum Reprod Open 2022; 2022(2): hoac009.
6. Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol 2023; 30: 329-334.
7. Barra F, Scala C, Biscaldi E, et al. Ureteral endometriosis: a systematic review of epidemiology, pathogenesis, diagnosis, treatment, risk of malignant transformation and fertility. Hum Reprod Update 2018; 24: 710-730.
8. Abrão MS, Dias JA, Rodini GP, Podgaec S, Bassi MA, Averbach M. Endometriosis at several sites, cyclic bowel symptoms, and the likelihood of the appendix being affected. Fertil Steril 2010; 94: 1099-1101.
9. Maddern J, Grundy L, Castro J, Brierley SM. Pain in endometriosis. Front Cell Neurosci 2020; 14: 590823.
10. Treloar SA, Bell TA, Nagle CM, Purdie DM, Green AC. Early menstrual characteristics associated with subsequent diagnosis of endometriosis. Am J Obstet Gynecol 2010; 202: 534.e1-6.
11. Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol 2017; 209: 3-7.
12. Giudice LC. Endometriosis. N Engl J Med 2010; 362: 2389-2398.
13. Missmer SA, Hankinson SE, Spiegelman D, et al. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol 2004; 104: 965-974.
14. Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG Int J Obstet Gynaecol 2008; 115: 1382-1391.
15. Nisenblat V, Bossuyt PMM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016; 2(2): CD009591.
16. Eisenberg VH, Arbib N, Schiff E, Goldenberg M, Seidman DS, Soriano D. Sonographic signs of adenomyosis are prevalent in women undergoing surgery for endometriosis and may suggest a higher risk of infertility. BioMed Res Int 2017; 2017: 8967803.
17. Facadio Antero M, O’Sullivan D, Mandavilli S, Mullins J. High prevalence of endometriosis in patients with histologically proven adenomyosis. Fertil Steril 2017; 107: e46.
18. Dior UP, Nisbet D, Fung JN, et al. The association of sonographic evidence of adenomyosis with severe endometriosis and gene expression in eutopic endometrium. J Minim Invasive Gynecol 2019; 26: 941-948.
19. Gwata N, Hui A, Wong L, Thee LJ, Tsaltas J, Mol B. A 24-months follow-up study of individuals with endometriosis using transvaginal ultrasound. J Minim Invasive Gynecol 2024; 31: 695-703.
20. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). Australian Living Evidence Guideline: Endometriosis. Melbourne: RANZCOG; 2025. Available online at: https://ranzcog.edu.au/resources/endometriosis-clinical-practice-guideline/ (accessed May 2025).
21. National Institute for Health and Care Excellence (NICE). Endometriosis: diagnosis and management. London: NICE; 2024. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK604070/ (accessed May 2025).
22. Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod Oxf Engl 2007; 22: 266-271.
23. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997; 67: 817-821.
24. Abrao MS, Andres MP, Miller CE, et al. AAGL 2021 endometriosis classification: an anatomy-based surgical complexity score. J Minim Invasive Gynecol 2021; 28: 1941-1950.e1.
25. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). Australian Living Evidence Guideline: Endometriosis. Melbourne: RANZCOG; 2025. Available online at: https://ranzcog.edu.au/resources/endometriosis-clinical-practice-guideline/ (accessed May 2025).
26. Brown J, Crawford TJ, Allen C, Hopewell S, Prentice A. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev 2017; 1(1): CD004753.
27. Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev 2012; 2012(3): CD002122.
28. Veth VB, van de Kar MM, Duffy JM, van Wely M, Mijatovic V, Maas JW. Gonadotropin-releasing hormone analogues for endometriosis. Cochrane Database Syst Rev 2023; 6(6): CD014788.
29. Wu D, Hu M, Hong L, et al. Clinical efficacy of add-back therapy in treatment of endometriosis: a meta-analysis. Arch Gynecol Obstet 2014; 290: 513-523.
30. Arcoverde FVL, Andres M de P, Borrelli GM, Barbosa P de A, Abrão MS, Kho RM. Surgery for endometriosis improves major domains of quality of life: a systematic review and meta-analysis. J Minim Invasive Gynecol 2019; 26: 266-278.
31. Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JM. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev 2020; 10: CD011031.
32. Fang QY, Campbell N, Mooney SS, Holdsworth‐Carson SJ, Tyson K. Evidence for the role of multidisciplinary team care in people with pelvic pain and endometriosis: a systematic review. Aust N Z J Obstet Gynaecol 2024; 64: 181-192.
33. Van Haaps AP, Wijbers JV, Schreurs AMF, et al. The effect of dietary interventions on pain and quality of life in women diagnosed with endometriosis: a prospective study with control group. Hum Reprod 2023; 38: 2433-2446.
34. Vennberg Karlsson J, Patel H, Premberg A. Experiences of health after dietary changes in endometriosis: a qualitative interview study. BMJ Open 2020; 10: e032321.
35. Schink M, Konturek PC, Herbert SL, et al. Different nutrient intake and prevalence of gastrointestinal comorbidities in women with endometriosis. J Physiol Pharmacol Off J Pol Physiol Soc 2019; 70(2): e-pub (https://doi.org/10.26402/jpp.2019.2.09).
36. Armour M, Ee CC, Naidoo D, et al. Exercise for dysmenorrhoea. Cochrane Database Syst Rev 2019; 9(9): CD004142.
37. Del Pino-Sedeño T, Cabrera-Maroto M, Abrante-Luis A, González-Hernández Y, Ortíz Herrera MC. Effectiveness of psychological interventions in endometriosis: a systematic review with meta-analysis. Front Psychol 2024; 15: 1457842.
38. Tyson K, Campbell N, Mooney SS, Holdsworth-Carson SJ. The endometriosis nurse coordinator – a new paradigm for endometriosis multidisciplinary care: a commentary. J Endometr Uterine Disord 2024; 8: 100086.