COPD - reducing hospitalisations this winter

Effective and appropriate management of patients with chronic obstructive pulmonary disease (COPD) includes immunising against influenza and pneumococcus, encouraging smoking cessation, regular exercise and a healthy diet, and treating exacerbations early. These measures can help prevent hospitalisations due to COPD exacerbations.
- Review management of patients with chronic obstructive pulmonary disease (COPD) before winter, ensuring an appropriate management plan is in place, including pharmacological and nonpharmacological therapies.
- Pulmonary rehabilitation is an effective intervention in COPD and can improve quality of life, fitness and self-confidence, and reduce hospitalisations.
- Check that patients’ vaccinations, including influenza and pneumococcal vaccinations, are up to date. Consider a COVID-19 booster and respiratory syncytial virus vaccination.
- Assess and manage comorbidities.
- Patients should be encouraged to have a self-management plan for COPD.
- Patients should be reviewed early and regularly after an exacerbation, whether they are treated at home or in hospital; readmission risk is highest within three months of discharge.
- The involvement of outreach and community home services in the management of patients with COPD should be considered.
- Early treatment of patients with exacerbations of COPD may reduce hospitalisations.
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in Australia in those aged 65 to 74 years, with many deaths occurring as a result of an exacerbation.1 COPD is a common clinical problem encountered in general practice, with about one million Australians being significantly affected by long-term lung conditions characterised by shortness of breath, such as chronic bronchitis and emphysema.2 Exacerbations of COPD can significantly impair a patient’s quality of life, contribute to progressive decline in lung function and are frequently under-recognised by both the patient and medical staff. A considerable increase in the number of COPD exacerbations and hospital admissions is seen during the winter months, and deaths from COPD tend to be highest in the late winter months (July to August).3
This article reviews current recommendations for the care of patients with COPD and the management of exacerbations in the general practice setting, with the aim of reducing the number of exacerbations and hospitalisations this winter. Advanced treatments, including surgical and endoscopic treatments for COPD, are beyond the scope of this review and are not discussed in detail.
What is COPD in 2025?
Our understanding of COPD has evolved dramatically over the past two decades, with the past 15 years in particular seeing an exponential increase in research in COPD. Successful new options for treatment have been developed and new evidence has informed the use of older drugs in certain types of patients with COPD. There has been a shift from an airflow limitation (forced expiratory volume in one second [FEV1]) and ‘one size fits all’ approach to diagnosis and management towards recognition of COPD as a very complex and heterogeneous condition. This recognition is leading to increased individualisation of COPD management.4
International guidelines from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that COPD be considered as a whole condition, not only airflow obstruction.5 Severity of airflow limitation (based on postbronchodilator FEV1) remains a core feature, but symptoms experienced by the patient and history of moderate or severe exacerbations should now be included in the assessment. Dyspnoea is a better prognostic indicator of mortality in COPD than FEV1, and previous history of exacerbations is the best surrogate marker of the risk of future exacerbations.
Previously, limited options for pharmacological treatment made it unnecessary to clinically identify different types of patients. However, the number of treatments now available for COPD treatment has increased considerably over the past decades. Phenotyping can help clinicians identify patients who share clinical characteristics and outcomes and, more importantly, similar responses to existing treatments. It has become increasingly evident that not all patients respond equally to all drugs, and the need to identify ‘responders’ is crucial.
There is no consensus on the definition and number of different COPD phenotypes, which may be anywhere from two to 328 million (estimated worldwide number of patients with COPD in 2010).6 Some clinically relevant COPD phenotypes include:6
- ‘frequent exacerbators’ with two or more exacerbations per year, who may benefit from anti-inflammatory treatment added to bronchodilators
- ‘overlap COPD-asthma’ who have an enhanced response to inhaled corticosteroids
- ‘infrequent exacerbators’ whose treatment may be based on long-acting bronchodilators, either alone or in combination
- high rates of ‘comorbidities’, particularly cardiovascular disease and metabolic syndrome, who may benefit from aggressive risk-factor management.
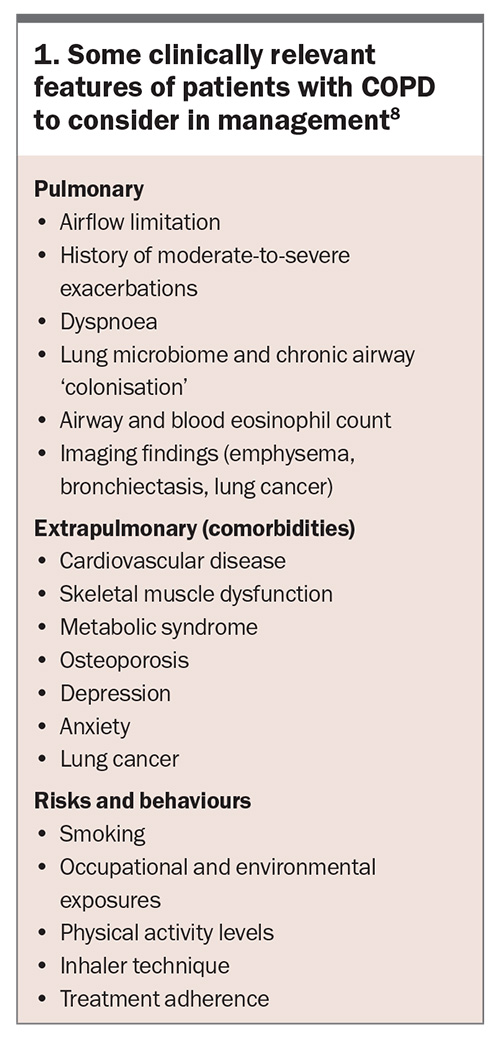
Treatable traits is a proposed treatment approach for management of patients with airways diseases. Patients are assessed by detailed clinical assessment and identification of airways risk factors, including smoking, asthma, occupational exposures, allergy, family history and early life respiratory disease; spirometry; blood eosinophil levels and assessment of comorbidities.7 A chest x-ray is not useful to establish a diagnosis of COPD but is of value in excluding alternative diagnoses and assessing for significant comorbidities, such as additional respiratory (pulmonary fibrosis, pleural disease, bronchiectasis), cardiac (cardiomegaly) and skeletal (kyphoscoliosis) diseases.5
Traits are grouped into three domains: pulmonary, extrapulmonary and behaviours/risks. Treatment of each individual trait has been found in a systemic review to lead to improved health-related quality of life, and small-to-moderate improvements in reductions in hospitalisations and one-year mortality (Box 1).8
Who is at risk of COPD?
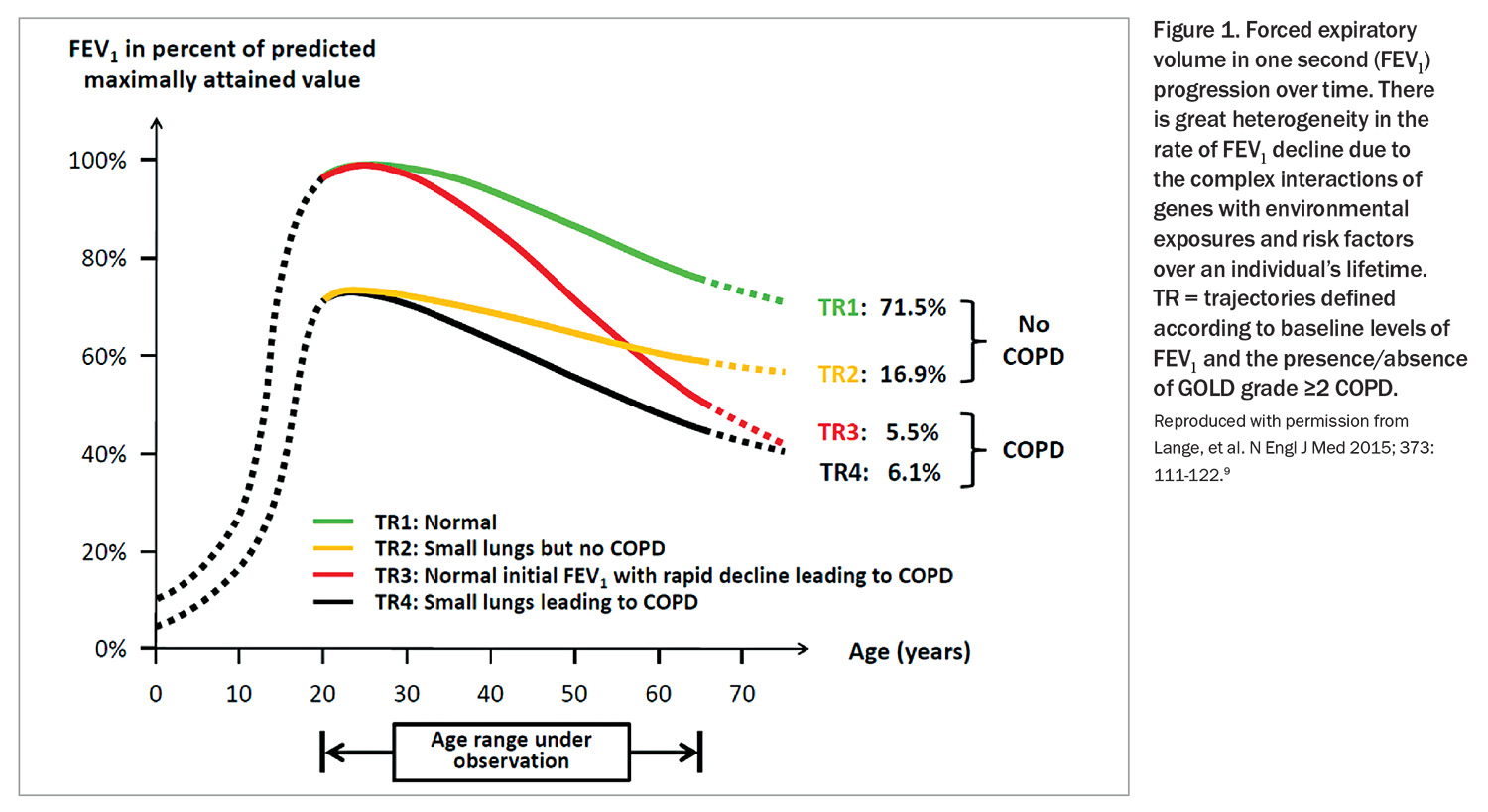
Smoking remains the major risk factor for COPD. However, even among heavy smokers, fewer than 50% develop clinically significant COPD, and some genuinely light smokers or nonsmokers develop chronic airflow limitation.5 Genetics, lung growth and development, asthma and other environmental exposures are some of the factors that can lead to development of COPD in later life. COPD is considered to result from an accelerated decline in FEV1 over time, but in some patients it may be related to reaching early adulthood with a low FEV1 due to impaired lung development during neonatal, childhood or adolescent periods (Figure 1).9
COPD should be considered in any patient who has dyspnoea, chronic cough or sputum production, and a history of exposure to risk factors for the disease, mainly smoking (usually more than 10 to 15 pack-years). The measurement of FEV1 by spirometry remains the diagnostic test for COPD and should be performed in all patients with suspected COPD. COPD is defined as a postbronchodilator FEV1 to forced vital capacity (FVC) ratio of below 0.7.7 If the airflow obstruction is fully reversible, the patient should be treated as for asthma.7
Underdiagnosis of COPD remains prevalent. However, patients with a history of smoking and symptoms suggestive of COPD need thorough assessment; the problem may be COPD alone, but often symptoms are due to a combination of cardiovascular disease, deconditioning and other issues, as well as airways disease.
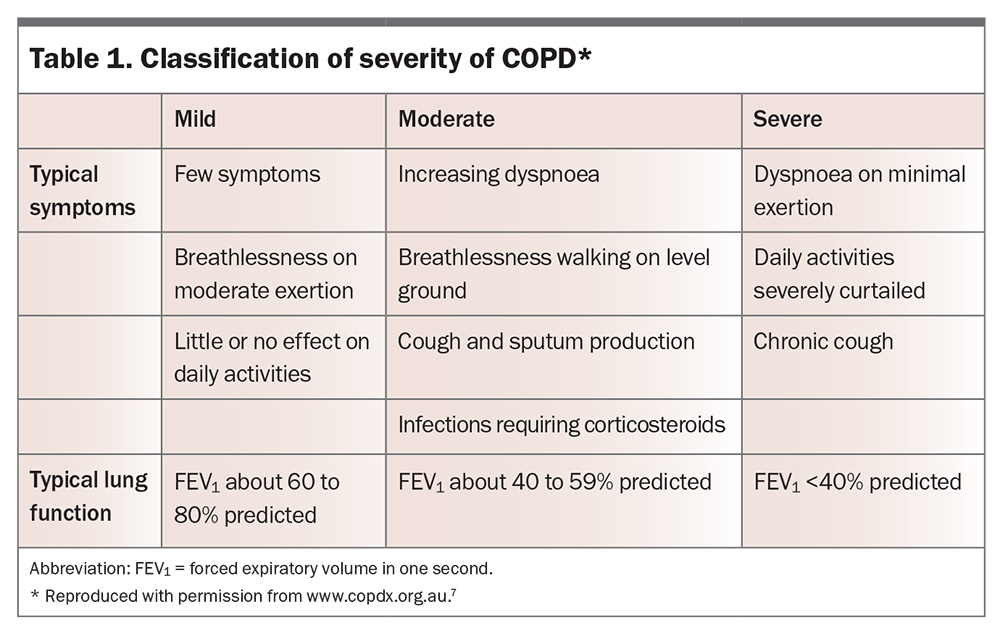
There is a continuum of COPD from mild to severe disease; severity is not solely related to reduction in FEV1, but to symptoms, frequency of exacerbations, presence of complications and extrapulmonary effects. One classification, from the COPD-X guidelines, is shown in Table 1.7
Lung cancer screening by low-dose computed tomography (LDCT) will be available in Australia from July 2025. Eligibility criteria are: age between 50 and 70 years, asymptomatic for lung cancer, have an at least 30 pack-year smoking history and are either currently smoking or ceased in the past 10 years.10 Emphysema is a hallmark of COPD and is detectable on LDCT, as are other features that may indicate COPD, such as airway wall thickening and mucus plugging.5 Findings of these abnormalities will provide an opportunity for detailed COPD assessment in this high-risk patient group.
Who is at risk of a COPD exacerbation?
All patients with COPD may develop exacerbations, and even those with underlying mild disease may experience a severe exacerbation, particularly in the winter months. Those with severe COPD are more likely to have a serious outcome even with a mild exacerbation. The single best predictor of exacerbations is previous exacerbations, across all levels of COPD severity. However, exacerbations also become more frequent as COPD severity worsens.
The lung ‘microbiome’ is likely to be one of the factors involved in exacerbation risk; the more pathogens present in the lower airways, the worse the COPD outcome. Patients with COPD may have lower airways colonised by bacteria including Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis. Pseudomonas aeruginosa and Staphylococcus aureus may colonise airways of patients with severe airflow obstruction, and appear associated with more frequent exacerbations and worse outcomes. Identification of these patients may highlight a higher exacerbation risk (and also direct antibiotic management when needed). Other differential diagnoses of increased respiration symptoms include left ventricular failure, pulmonary embolus and pneumonia.
Exacerbations are often triggered by respiratory tract infections, either viral or bacterial. In a retrospective study in Australian hospitals, the most common viruses isolated in patients presenting with COPD exacerbations were influenza virus, rhinovirus and respiratory syncytial virus A/B.11
Optimising baseline COPD management
Ensuring that each patient’s usual COPD management is effective and appropriate will help in reducing both exacerbations and the impact of exacerbations. The Australian and New Zealand COPD guidelines are regularly updated. The current version is available online through the Lung Foundation Australia website (https://copdx.org.au).7 These guidelines are known as the COPD-X plan, from:
C – confirm diagnosis
O – optimise function
P – prevent deterioration
D – develop a self-management plan and manage
X – exacerbations.
An approach to the management of patients with COPD based on these guidelines is discussed in this article.
Referral of patients
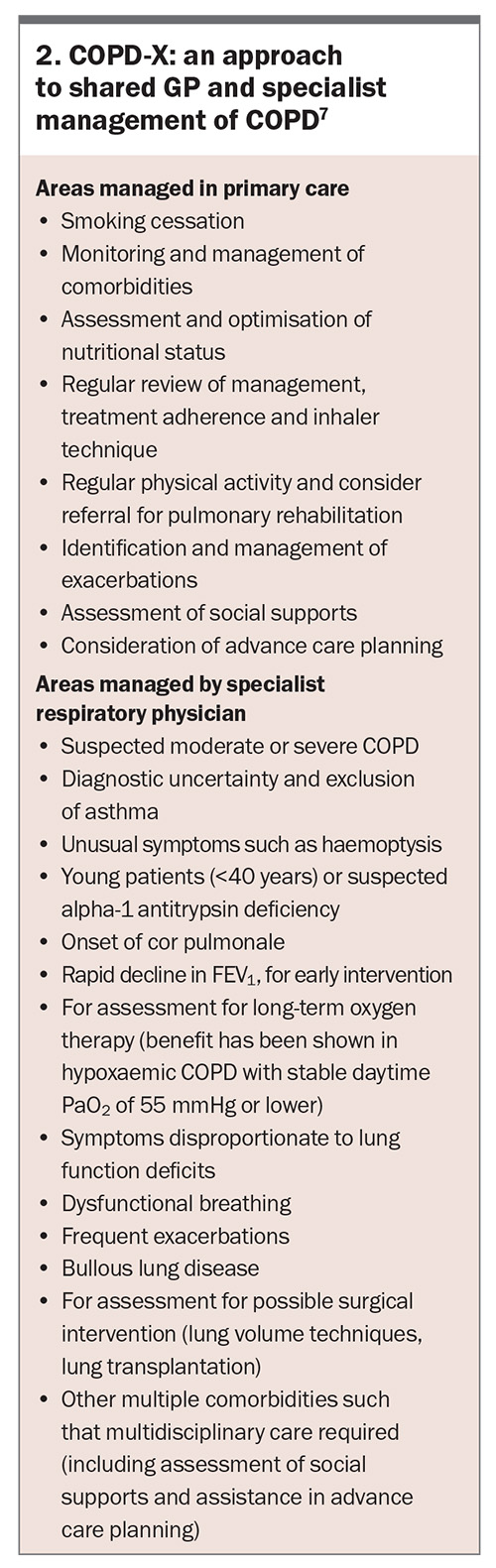
Referral of patients to a respiratory physician should be considered if their COPD is moderate to severe, the diagnosis is unclear or complications such as cor pulmonale are present (Box 2).7
Treatment
Management strategies in patients with COPD focus on relief of symptoms, prevention of disease progression, and prevention and treatment of exacerbations and complications, with the aims of improving exercise tolerance and health status and reducing mortality. The extents to which these goals can be realised vary with each patient, and some treatments will produce benefits in more than one area. Treatments include both pharmacological and nonpharmacological therapies.
COPD is linked to a range of comorbid diseases, including cardiovascular diseases, metabolic syndrome, gastro-oesophageal reflux, osteoporosis, depression and anxiety, likely in relation to shared risk factors. COPD is also associated with an increased risk of lung cancer, due to the common risk factor of smoking.5,12 COPD can also have significant extrapulmonary effects, including weight loss and abnormal skeletal muscle dysfunction. Multimorbidity influences mortality and hospitalisations, independent of airflow obstruction, and should be routinely assessed and treated.
The predominant cause of death in patients with COPD changes with increasing COPD severity. In patients with mild to moderate COPD, deaths are mostly due to cancer and cardiovascular diseases. As COPD severity increases, deaths due to respiratory diseases are increasingly common.13 Many predictors of mortality in COPD have been identified, including reduction in FEV1, dyspnoea, body mass index (BMI), exercise capacity and frequency of exacerbations. However, prediction of survival in an individual patient with COPD is recognised to remain very challenging, compared with other diseases such as cancer or severe heart failure.
Pharmacological therapy
There has been an increase in the types and numbers of inhaler devices and medications available for COPD maintenance therapy. However, the classes of inhaled medications have not changed:
- short-acting beta2-agonists (SABA)
- long-acting beta2-agonists (LABA)
- short-acting muscarinic antagonists (SAMA)
- long-acting muscarinic antagonists (LAMA)
- inhaled corticosteroids (ICS).
Meta-analyses to date have not shown any statistically significant differences among LAMAs in preventing moderate-to-severe exacerbations of COPD.7 Comparisons within other classes appear limited at present.
When considering treatment for an individual patient, factors to consider include the patient’s symptoms, COPD severity and comorbidities, and the type of inhaler device to use. Combining two medications of the same class, such as a LABA/LAMA or LABA/ICS combination and an additional LABA is not advised; the risk of side effects is increased for no added symptomatic improvement.
Inhaler technique is often suboptimal, including in those who are long-term inhaler users. Use of multiple inhaler device types is associated with an increase in errors and may be associated with a poorer outcome in patients with COPD. Therefore, frequent review of inhaler technique and rationalisation of device type is beneficial.14,15
SABA and SAMA
Short-acting bronchodilators, such as the SABA salbutamol or the SAMA ipratropium bromide, can be used as as-needed therapy for patients with only occasional dyspnoea. SABAs can be given for immediate relief of symptoms in patients already using a long-acting bronchodilator for maintenance therapy. Side effects are generally minor; however, a meta-analysis of randomised controlled trials, and a later cohort study, found an increased risk of adverse cardiovascular events with ipratropium bromide.16 This has not been seen with tiotropium.17,18
LAMA, LABA and LAMA/LABA combinations
Most patients will need more than occasional use of short-acting bronchodilators and can be commenced on long-acting bronchodilators, either a LAMA or LABA or combination LAMA/LABA. A meta-analysis comparing LABAs with LAMAs assessed 16 randomised double-blinded controlled trials of patients with moderate-to-very-severe COPD.19 It found that LAMAs were associated with a lower risk of acute exacerbations and lower incidence of adverse events, compared with LABAs. No significant differences between LAMAs and LABAs were found in terms of changes in lung function, symptoms or health status. LAMAs may be preferable to LABAs in patients with stable COPD, especially those at risk of frequent exacerbations.19
In patients with persistent dyspnoea on one bronchodilator treatment, a second bronchodilator should be added.5 Several LAMA/LABA fixed-dose combinations delivered in a single inhaler are available in Australia, via a range of devices. A network meta-analysis of LAMA/LABA combinations compared with the individual monotherapies found that the fixed-dose combinations provided benefits in lung function and quality of life, with no increase in adverse outcomes.20 Combination therapy reduced moderate-to-severe exacerbations compared with a LABA alone but not compared with a LAMA alone. Effects on severe exacerbations were similar with both combination and monotherapies. Other network meta-analyses have also found benefits for LAMA/LABA fixed-dose combinations, compared with their monocomponents.21 PBS eligibility for these medications should be reviewed.
Inhaled corticosteroids
The potential benefits of ICS must be balanced against the potential risks including local oropharyngeal adverse effects and pneumonia. A meta-analysis of 43 studies of COPD showed an increased risk of pneumonia with ICS use, but this was balanced against the benefit of reduced exacerbations.22 Their main impact is to reduce the risk of exacerbations; in contrast to long-acting bronchodilators, their effects on symptoms and lung function are small and often insufficient to use as a guide to treatment efficacy. Lower doses of ICS should be used in patients with COPD whenever possible. Treatment of people with COPD, especially with high-dose ICS, has been associated with a higher risk of bone fractures and osteoporosis, and there is some evidence of an association with a higher risk of mycobacterial infection and blood glucose levels.7
Treatment with ICS is directed at patients deemed to be at risk of exacerbations because of a past history of exacerbations and/or poor lung function.23 There is emerging evidence that blood eosinophil counts may be a useful biomarker of ICS response in patients with COPD.24 Those with blood eosinophil counts less than 100 cells/mcL appear less likely to benefit from ICS, and those with a count of more than 300 cells/mcL are more likely to benefit. ICS alone are not indicated as sole inhaler therapy for COPD. The risk of pneumonia is higher in patients of older age, with a lower BMI, with greater general fragility and who are receiving higher ICS doses, and possibly in those with blood eosinophil counts less than 100 cells/mcL.24
A reasonable approach based on current evidence is to consider the addition of ICS to long-term maintenance bronchodilator LAMA/LABA therapy in patients with COPD and a history of multiple or severe exacerbations and poor lung function (FEV1 <50% predicted), particularly if blood eosinophil counts are more than 300 cells/mcL, and in those with coexistent COPD and asthma. ‘Triple therapy’ with ICS/LAMA/LABA can be a single inhaler or an additional inhaler. Different formulations of single inhaler triple therapy have similar efficacy in exacerbation reduction.25,26
In general, the use of drugs in COPD does not involve back-titration. The exception is when oral corticosteroids have been given for an exacerbation. Additionally, in light of recent trials, in patients with COPD with no evidence of asthma and with infrequent exacerbations, ICS withdrawal can be considered.7 Close monitoring is advised after withdrawal, and withdrawal should be considered cautiously in those with elevated blood eosinophil counts and/or poor lung function.
Other and emerging therapies
Long-term use of systemic corticosteroids is not recommended in patients with COPD due to an unfavourable risk-benefit ratio.7 However, short-term use to treat exacerbations is supported by good-quality evidence, with reduction in the severity of exacerbations, shortened recovery times and reduced hospital admissions and readmissions being noted.7
Several recent trials of biologic therapies targeting interleukin-5, interleukin-5 receptors or interleukin-4 and -13 receptors to reduce eosinophil activity and type 2 inflammation have shown promise in select patient groups. Patients with COPD, eosinophilia and frequent exacerbations despite triple inhaler therapy may show a reduction in exacerbation rates and improvement in FEV1.27,28 Dupilumab, a monoclonal antibody blocking interleukin-4 and interleukin-13 receptor signalling, has recently been TGA approved for use in adults as add-on maintenance treatment for uncontrolled COPD characterised by raised blood eosinophils on a stable combination of an ICS, a LABA, and a LAMA, or on a combination of a LABA and a LAMA if ICS is not appropriate. Specialist review of patients is advised.
Several trials have suggested that in patients with moderate-to-severe COPD and frequent exacerbations, long-term treatment with oral macrolides may reduce the frequency of exacerbations. However, owing to the potential significant adverse effects including cardiac toxicity, ototoxicity, diarrhoea and antibiotic resistance it is recommended that specialist advice be sought if this therapy is being considered.29 There is no evidence base to support long-term use of other antibiotics.
Theophylline has a modest bronchodilator effect, but is not currently recommended in Australia due to its narrow therapeutic index, its potential for significant side effects and the lack of demonstration of a reduction in exacerbation rates in patients who are on adequate inhaled therapy.30 Phosphodiesterase type-4 inhibitors are potential candidates for the treatment of COPD, but this class of medications is currently not available in Australia.
Nonpharmacological therapies
Smoking cessation
Smoking cessation remains the single most effective intervention to slow the progression of COPD. Short-term benefits on lung function and quality of life are also seen. GPs should aim to identify all current, or relapsed, smokers at every consultation as each brief counselling intervention increases the chance of successful cessation by 5 to 10%.31 No single cessation plan works for all; a discussion is needed with each patient to find the best technique. As few smokers are successful in their first attempt at quitting, persistence by everyone is important. Smoking cessation is usually a long-term process rather than a single event, with episodes of relapse before long-term success is achieved. The five As can be used as a framework for helping patients to quit smoking (Box 3 and Box 4).32
Nicotine dependence is most effectively treated with a combination of nicotine replacement therapy (NRT), behavioural support and pharmacotherapy. NRT (available as a patch, gum, lozenge, sublingual tablet and inhaler) is widely available, and more than one form of NRT can be used concurrently with increased success rates and no safety risks. Those who are not willing to quit can be advised to progressively substitute their cigarette intake with NRT. This use of NRT can double the odds of smoking cessation.32 Some patients may be eligible for PBS NRT; this can be checked via the PBS.32
Varenicline is a nicotinic receptor partial agonist that more than doubles the chances of quitting compared with placebo. Adverse effects include unusual mood change, depression, behaviour disturbance and suicidal thoughts. A Cochrane review found that varenicline helped about 50% more people to quit than nicotine patches and ‘other’ forms of NRT (tablets, sprays, lozenges and inhalers), and about 70% more people than nicotine gum.33 Combining two types of NRT was as effective as using varenicline, and helped more people to quit than single types of NRT.
Bupropion, a non-nicotine oral therapy, significantly increases cessation rates compared with placebo. It has been shown to be effective for smokers with depression, cardiac disease and respiratory diseases, including COPD. A Cochrane review found evidence that smokers with COPD who received a combination of high-intensity behavioural support and medication were more than twice as likely to quit as people who received behavioural support alone. It found no clear evidence that one particular form of behavioural support or medication is better than another.34
Immunisations
Influenza immunisation can reduce the incidence of serious illness and death in patients with COPD, and a significant reduction in the number of exacerbations has been seen in immunised patients.5,7 All patients with COPD should be offered annual influenza vaccination. Development of an immune response takes at least two weeks. One multicentre study suggested that influenza vaccine efficacy decreases in older adults as frailty increases.35 Despite this, a recent meta-analysis suggested that older adults receiving influenza vaccination may have a lower risk of influenza and lower respiratory tract infections than those not vaccinated.36 Repeat vaccination later in the influenza season may also be considered in the elderly and in those with underlying severe airways disease. People with COPD, particularly the elderly, may have a decreased risk of ischaemic heart disease when they have received influenza vaccination over many years.5
Previously, a history of anaphylaxis or a serious allergic reaction to eggs was a contraindication to influenza vaccination. However, based on prospective and retrospective studies of influenza vaccination in those with and without egg allergy (including egg anaphylaxis), people with egg allergy can safely receive influenza vaccines that contain less than 1 mcg of ovalbumin per dose. Vaccination may be administered in community vaccination clinics (which may or may not have direct medical practitioner supervision) as a single dose followed by the recommended waiting period of 15 minutes (in Australia) or 20 minutes (in New Zealand). A longer waiting period of 30 minutes can be considered in those with past egg anaphylaxis or significant patient or healthcare provider anxiety. The immediate availability of medical practitioner care is recommended and staff should be familiar with the recognition and treatment of anaphylaxis.37 Influenza vaccine should not be given to patients with a history of anaphylaxis to influenza vaccine, current febrile illness or history of Guillain-Barré syndrome.
Pneumococcal immunisation is recommended for all patients with COPD. People with COPD vaccinated with injectable polyvalent pneumococcal vaccines are less likely to experience an exacerbation of COPD or episode of community-acquired pneumonia, with numbers needed to treat of 8 and 21, respectively.38 Immunisation with 13-valent pneumococcal conjugate vaccine (13vPCV) is highly effective in preventing community-acquired pneumococcal pneumonia in older adults.39 23-valent pneumococcal conjugate vaccine (23vPPV) is less effective in elderly or immunosuppressed patients.7 For those with newly diagnosed COPD, not previously vaccinated again pneumococcus, current recommendation is for 13vPCV followed by first dose of 23vPPV 12 months later, then a second dose of 23vPPV at least five years later.40 In the current National Immunisation Program, non-First Nations patients under the age of 70 years with COPD and chronic emphysema are not included in the risk conditions for funded pneumococcal vaccination. Thus, they are not eligible for reimbursement.
Respiratory syncytial virus (RSV) can cause severe infection in the elderly and in those with significant underlying illnesses. An effective RSV vaccine has been available in Australia since 2024. Among the groups in whom it is recommended are First Nations people aged 60 years and over, people aged 60 years and over with medical conditions that increase their risk of severe RSV disease, such as COPD, and in people aged 75 years and over.41 However, it is not funded for these groups and must be purchased privately.
Patients with COPD are at increased risk of pertussis.5,42 In the National Immunisation Program adults aged 65 years are recommended to consider having a pertussis-containing vaccine if their last dose was more than 10 years ago. This is due to waning immunity over time and increased pertussis morbidity in older people; however, COPD is not included in the risk conditions for funded vaccination. Patients should also consider herpes zoster vaccination; shingles is associated with significant morbidity.
Physical activity and reducing sedentary behaviour
On average, people with COPD participate in 57% of the total duration of physical activity undertaken by healthy controls.43 Reductions in physical activity commence early in COPD and, over time, levels of physical activity substantially decline across all severities of COPD. This decline is accompanied by a deterioration in lung function and health status.44 Levels of physical activity are reduced further during hospitalisation for a COPD exacerbation. Return to previous levels of activity often does not occur.
Low levels of physical activity are associated with increased mortality and exacerbations in people with COPD.45 People with COPD should be encouraged to be physically active and participate in activities of daily living that require the use of muscle strength, such as lifting or gardening as well as doing physical activities they enjoy, such as bowls, golf or swimming.7
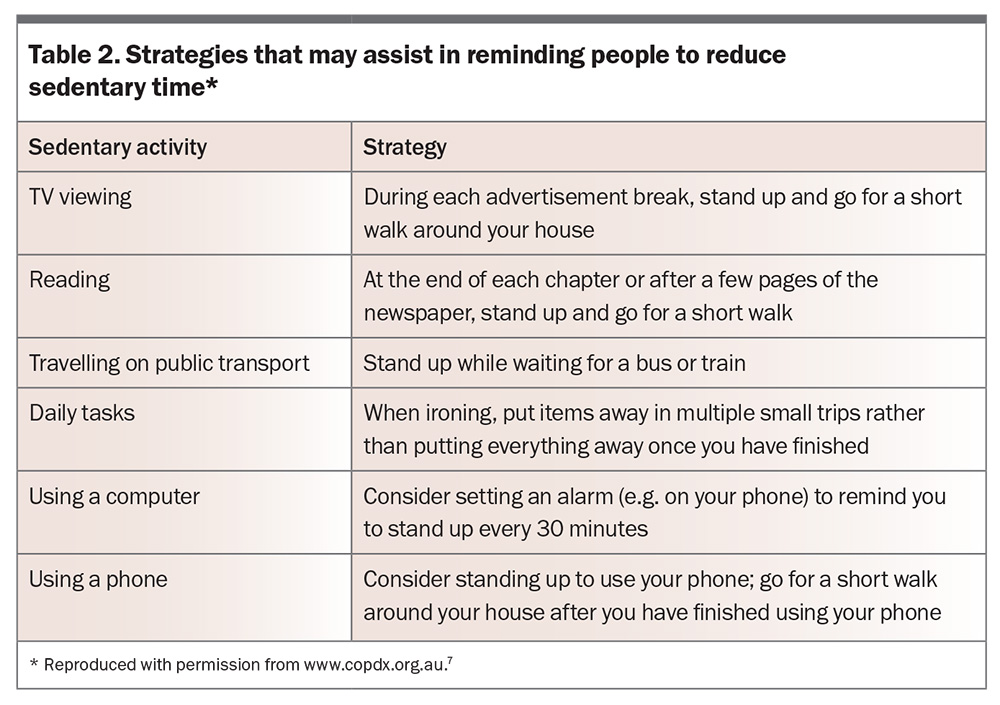
There is growing recognition that people with COPD spend many of their waking hours in sedentary behaviours, defined as those behaviours that are undertaken in a sitting or reclined posture and have low energy requirements, such as watching television, reading and sitting at a computer. People with COPD with the greatest sedentary time during daily life are characterised by more frequent exacerbations, lower exercise capacity, long-term oxygen use, lower motivation for exercise and the presence of physical comorbidities including obesity and arthritis. Compared with the goal of increasing moderate- or high-intensity physical activity, the goal of reducing sedentary time by increasing light-intensity physical activity is likely to be more feasible in some patients with COPD. Of note, in people with COPD, greater participation in light-intensity physical activity has been reported to reduce the risk of respiratory-related hospitalisations.46Table 2 provides some strategies aimed at avoiding prolonged sedentary time.7
Pulmonary rehabilitation
The benefits of pulmonary rehabilitation in improving dyspnoea, quality of life, exercise capacity, anxiety and depression, fatigue and emotional function are well established. Evidence also suggests that pulmonary rehabilitation is safe and highly effective in reducing hospital admissions and mortality and improving health-related quality of life in COPD patients after exacerbations.7
Pulmonary rehabilitation programs consist of general assessment of the patient and specific assessment of exercise capacity and quality of life, followed by an exercise program and education sessions. Pulmonary rehabilitation programs are available at many community centres and hospitals, and usually welcome referrals from GPs.
Nutrition
Both obesity and low BMI are associated with increased morbidity in patients with COPD. Obesity increases the work of breathing and is associated with sleep apnoea, hypoventilation and cor pulmonale, and metabolic complications. Malnutrition is an independent predictor of mortality and use of healthcare services in patients with COPD. Energy intake is often reduced due to dyspnoea, medications and lung hyperinflation whereas expenditure is increased due to the metabolic demands of breathing, infections and systemic inflammation. Low BMI and low fat-free mass are inversely associated with respiratory and peripheral muscle function, exercise capacity and health status. Importantly, those with poor nutrition are most likely to benefit from nutrition therapy before an undernutrition state is established.47 Nutritional supplementation in malnourished patients can improve walking distance and respiratory muscle strength. High-calorie nutritional supplements should be considered in patients with COPD and a low BMI, particularly those who are malnourished and/or have severe disease.
Self-management and action plans
COPD self-management programs may lead to improved health-related quality of life, with reduced exacerbations being a positive outcome of some studies. Other studies have not shown benefit. Trials to date have used a wide range of study designs and interventions, thus no recommendations as to the essential elements of a COPD self-management program can be made.7
Interventions targeting mental health, an active lifestyle, relaxation therapy, use of written action plans, correct medication use and facilitated access to services have been found to reduce exacerbations and visits to the emergency department. Action plans should be completed by the clinician and patient together, with the aim of assisting the patient to identify symptoms of an exacerbation and know what actions they should take. A sample action plan is shown in Figure 2.
Anxiety and depression are common in patients with COPD and are associated with reduced quality of life, poor self-management and medical symptoms. There is also some evidence that mood disorders are independent risk factors for exacerbations and hospitalisations. Elderly patients with COPD prescribed benzodiazepines may be at increased risk of exacerbations; caution with use of these medications, or avoidance, is warranted in all patients with COPD due to their potential for depression of respiratory drive. Behavioural therapy and selective serotonin receptor inhibitors may be better management options, along with referral to clinical psychologists and psychiatrists.
In patients with debilitating breathlessness despite optimal COPD management, referral for specialist advice, consideration of judicious use of low-dose opiates and palliative care involvement can be considered. Use of a handheld fan can also be of considerable help.
Additional therapies
Use of long-term continuous (15 or more hours/day) domiciliary oxygen therapy in patients with severe COPD and chronic hypoxaemia prolongs survival. Long-term domiciliary noninvasive ventilation can be considered in select patient with severe stable COPD and chronic hypercapnia. Specialist advice should be sought.7
Outreach teams
Many regions now have specialist multidisciplinary outreach teams to assist in the co-ordination of home care. For example, in Victoria, the Hospital Admission Risk Program aims to reduce avoidable hospital admissions and emergency department presentations.
Services provided by these teams may include outreach services with rapid response such as a mobile assessment and treatment service (assessment by medical practitioner and outreach nurse) and home visit assessment service. Other home services, such as physiotherapy and pharmacy, may also be accessible. The evidence is not yet available for the overall patient and economic benefits of home care, but a systematic review of seven studies found no significant differences in readmission rates or mortality, and ‘Hospital at Home’ schemes were preferred by patients and carers.48 Some patients may need initial hospital assessment, and may then be able to return to their own homes with increased social support and a supervised medical care package.
Prompt treatment of exacerbations
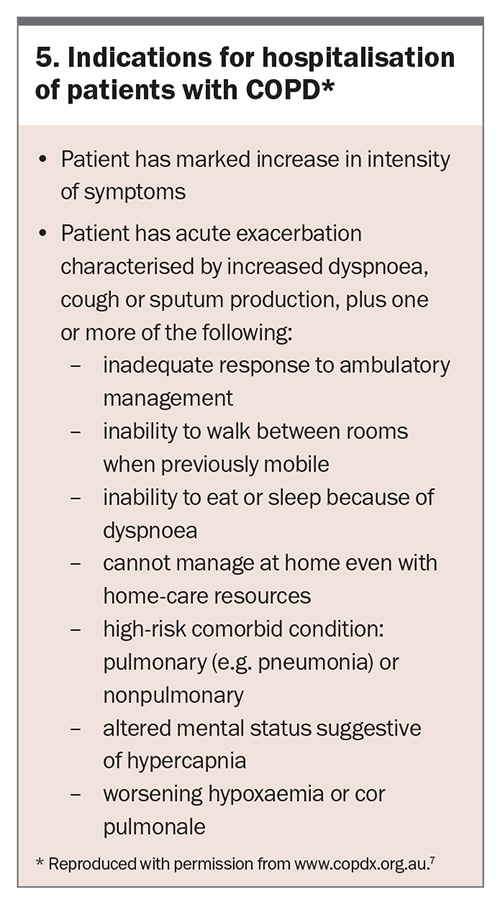
Early identification of a COPD exacerbation and early primary care management may reduce the need for hospitalisation. Initial management includes use of short-acting bronchodilators, oral corticosteroids and/or antibiotics. Indications for hospitalisation of patients with a COPD exacerbation are shown in Box 5.7
Follow up after hospitalisation
All patients discharged from hospital after an exacerbation of COPD should have an early (preferably within one week) follow-up consultation with their GP. The risk of readmission is highest within three months of discharge, and more than half of patients are readmitted within 12 months. All the preventive strategies for COPD exacerbations discussed above should be revisited during this consultation, including revising the patient’s COPD self-management or action plan. Pulmonary rehabilitation has been shown to reduce readmissions if provided within one week.49
Consider advance care planning in all patients with COPD, especially those with moderate-severe disease and/or prior hospitalisation. Post-hospital admission is an opportune time to discuss advance care directives with patients and, if appropriate, their family and carers. End-of-life issues are relevant for patients with severe and moderate COPD. Most patients with end-stage COPD wish to participate in end-of-life management decisions and would prefer to do so in a nonacute setting. For some patients, palliative care team involvement can be helpful.
Conclusion
To help reduce the number of hospitalisations due to COPD exacerbations in the colder winter months, GPs should ensure that their patient’s usual COPD management is effective and appropriate. They can also encourage their patients to be vaccinated against influenza and pneumococcus, avoid exposure to cigarette smoking, participate in pulmonary rehabilitation, exercise regularly and have a healthy diet and good nutritional state. Educating patients with COPD to pay particular attention to their respiratory symptoms, follow their self-management plan, seek early treatment for any decline in their condition and avoid exposure to other people with coughs and colds will also help reduce their risk of a severe exacerbation.
A checklist of the strategies recommended when reviewing patients with COPD is given in Box 6.7 RMT
COMPETING INTERESTS: Associate Professor Miller has received support from AstraZeneca, Boehringer Ingelheim and GlaxoSmithKline for scientific meeting and clinical advisory group attendances, and has been principal investigator in an AstraZeneca sponsored drug trial.
References
1. Australian Institute of Health and Welfare (AIHW). Chronic respiratory conditions. Canberra: AIHW; 2022. Available online at: https://www.aihw.gov.au/reports-data/health-conditions-disability-deaths/chronic-respiratory-conditions/overview (accessed March 2025 : around AIHW.
2. Access Economics. Economic impact of COPD and cost effective solutions. Report for the Australian Lung Foundation. Sydney: Access Economics Pty Ltd; October 2008.
3. Australian Institute of Health and Welfare (AIHW). Mortality from asthma and COPD in Australia. Cat. no. ACM 30. Canberra: AIHW; 2014.
4. Agusti A. The path to personalised medicine in COPD. Thorax 2014; 69: 857-864.
5. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD: 2025 report. Available online at: https://goldcopd.org/2025-gold-report (accessed March 2025).
6. Miravitlles M, Soler-Cataluña JJ, Calle M, Soriano JB. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur Respir J 2013; 41: 1252-1256.
7. Yang IA, George J, McDonald CF, et al. The COPD-X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2024. Version 2.76, September 2024. Published online 16 November 2024. https://copdx.org.au/copd-x-plan/ (accessed March 2025).
8. Sarwar MR, McDonald VM, Abramson MJ, et al. Effectiveness of interventions targeting treatable traits for the management of obstructive airway diseases: a systematic review and meta-analysis. J Allergy Clin Imunol Pract 2022; 10: 2333-2345e21.
9. Lange P, Cell B, Agusti A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med 2015; 373: 111-122.
10. Australian Government Department of Health and Aged Care. National Lung Cancer Screening Program [website]. Last updated 13 January 2025. Available online at: https://www.health.gov.au/our-work/nlcsp (accessed March 2025).
11. Biancardi E, Fennell M, Rawlinson W, Thomas PS. Viruses are frequently present as the infecting agent in acute exacerbations of chronic obstructive pulmonary disease in patients presenting to hospital. Intern Med J 2016; 46: 1160-1165.
12. Vespasiani-Gentilucci U, Pedone C, Muley-Vilamu M, Antonelli-Incalzi R. The pharmacological treatment of chronic comorbidities in COPD: mind the gap! Pulm Pharmacol Ther 2018; 51: 48.
13. Berry CE, Wise RA. Mortality in COPD: causes, risk factors and prevention. COPD 2010; 7: 375-382.
14. Bosnic-Anticevich S, Chrystyn H, Costello RW, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulm Dis 2017; 12: 59-71.
15. Chrystyn H, Van Der Palen J, Sharma J, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med 2017; 27: 22
16. Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008; 300: 1439-1450.
17. Michele TM, Pinheiro S, Iyasu S. The safety of tiotropium – the FDA’s conclusions. N Engl J Med 2010; 363: 1097-1099.
18. Ogale SS, Lee TA, Au DH, Boudreau DM, Sullivan SD. Cardiovascular events associated with ipratropium bromide in COPD. Chest 2010; 137: 13-19.
19. Chen WC, Huang CH, Sheu CC, et al. Long-acting beta2-agonists versus long-acting muscarinic antagonists in patients with stable COPD: a systematic review and meta-analysis of randomized controlled trials. Respirology 2017; 22: 1313-1319.
20. Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax 2016; 71: 15-25.
21. Calzetta L, Rogliani P, Ora J, Puxeddu E, Cazzola M, Matera MG. LABA/LAMA combination in COPD: a meta-analysis on the duration of treatment. Eur Respir Rev 2017; 26: 160043.
22. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; (3): CD010115.
23. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J 2018; 52: 1801219.
24. Pavord I, Agusti A. Blood eosinophil count: a biomarker of an important treatable trait in patients with airway disease. Eur Respir J 2016; 47: 1299-1303.
25. Bourdin A, Molinari N, Ferguson GT, et al. Efficacy and safety of budesonide/glycopyrronium/formoterol fumarate versus other triple combinations in COPD: a systematic literature review and network meta-analysis. Adv Ther 2021; 7: 375-382.
26. Lee HW, Kim HJ, Jang EJ, Lee CH. Comparisons of efficacy and safety between triple (inhaled corticosteroid/long-acting muscarinic antagonist/long-acting beta-agonist) therapies in chronic obstructive pulmonary disease: systematic review and Bayesian network meta-analysis. Respiration 2021; 100: 631-643.
27. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613-1629.
28. Bhatt SP, Rabe K, Haninia NA, et al. Dupilumab for COPD with blood eosinophil evidence of type 2 inflammation. N Engl J Med 2024; 390: 2274-2283.
29. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2013; (11): CD009764.
30. Devereux G, Cotton S, Fielding S, et al. Effect of theophylline as adjunct to inhaled corticosteroids on exacerbations in patients with COPD: a randomized clinical trial. JAMA 2018; 320: 1548-1559.
31. Wilson DH, Wakefield MA, Steven ID, Rohrsheim RA, Esterman AJ, Graham NM. ‘Sick of smoking’: evaluation of a targeted minimal smoking cessation intervention in general practice. Med J Aust 1990; 152: 518-521.
32. Royal Australian College of General Practitioners (RACGP). Supporting smoking cessation: a guide for health professionals. Melbourne: RACGP; 2024. Available online at: https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/supporting-smoking-cessation (accessed March 2025).
33. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; (5): CD009329.
34. Van Eerd EA, Van Der Meer RM, Van Schayck OC, Kotz D. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016; (8): CD010744.
35. Andrew MK, Shinde V, Ye L, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216: 405-414.
36. Demicheli V, Jefferson T, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2018; (2): CD004876.
37. Australasian Society of Clinical Immunology and Allergy (ASCIA). ASCIA guidelines – vaccination of the egg-allergic individual. Sydney: ASCIA; 2022. Available online at: https://www.allergy.org.au/hp/papers/vaccination-of-the-egg-allergic-individual (accessed March 2025).
38. Walters JA, Tang JN, Poole P, Wood-Baker R. Pneumococcal vaccines for preventing pneumonia in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; (1): CD001390.
39. Bonten MJ, Huijts S, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372: 1114-1125.
40. Australian Government Department of Health and Aged Care. National Immunisation Program Schedule [website]. Updated 19 September 2024. Available online at: https://www.health.gov.au/topics/immunisation/when-to-get-vaccinated/national-immunisation-program-schedule (accessed March 2025).
41. Australian Government Department of Health and Aged Care. Respiratory syncytial virus (RSV) vaccine [website]. Updated 5 February 2025. Available online at: https://www.health.gov.au/respiratory-syncytial-virus-rsv-vaccine (accessed March 2025).
42. Naeger S, Pool V, Macina D. Increased burden of pertussis among adolescents and adults with asthma or COPD in the United States, 2007 to 2019. Chest 2024; 165: 1352-1361.
43. Vorrink S, Kort HS, Troosters T, Lammers JW. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res 2011; 12: 33.
44. Waschki B, Kirsten AM, Holz O, et al. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 295-306.
45. Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61: 772-778.
46. Donaire-Gongzalez D, Gimeno-Santos E, Balcells E, et al. Benefits of physical activity on COPD hospitalisation depend on intensity. Eur Respir J 2015; 46: 1281-1289.
47. Akner G, Larsson K. Undernutrition state in patients with chronic obstructive pulmonary disease. A critical appraisal on diagnostics and treatment. Respir Med 2016; 117: 81-91.
48. Ram FSF, Wedzicha JA, Wright JJ, Greenstone M. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003; (4): CD003573.
49. Criner GJ, Bourbeau J, Diekemper RL, et al. Executive summary: prevention of acute exacerbation of COPD: American College of Chest Physicians and Canadian Thoracic Society Guidelines. Chest 2015; 147: 883-893.