Early COPD: how to identify it and is it worth treating?
Case finding of airflow limitation and COPD is an important step to be undertaken by GPs in individuals at risk to help address the increasing burden of this condition in the community.
- Detecting airflow limitation when the patient has no symptoms and confirming the diagnosis of chronic obstructive pulmonary disease (COPD) by spirometry identifies individuals at risk of future symptoms, increased decline in FEV1 and complications of the condition.
- Better stratification of future risk by actively diagnosing COPD (case finding) may alter management in those with additional risk factors for COPD progression and cardiovascular risk.
- Performing spirometry in current smokers may increase smoking cessation.
- An asymptomatic patient with a new diagnosis of airflow limitation should be monitored for decline in FEV1, onset of COPD symptoms and occurrence of exacerbations.
- Confirmation or exclusion of COPD allows appropriate drug prescription and helps avoid diagnostic confusion.
- Management of COPD continues to evolve as understanding of the disease increases, particularly appreciation of its heterogeneity.
Chronic obstructive pulmonary disease (COPD) is an important disease globally because of its massive societal, economic and personal burden. It is defined by airflow limitation (usually measured by spirometry) that does not normalise after administration of a short-acting bronchodilator and by typical symptoms of breathlessness on exertion and cough productive of sputum.1
An overview of COPD
Two important guidelines on COPD are the Australian COPD-X plan and the international Global Initiative for Chronic Obstructive Lung Disease (GOLD)’s strategy document; these contain practical information that is regularly updated.1,2 Both documents describe the severity of COPD based on impairment of forced expiratory volume in one second (FEV1). The GOLD document also incorporates symptoms and exacerbations to assign COPD severity.
COPD was the fifth leading cause of death in 2020 but is now the fourth leading cause of death worldwide and the eighth leading cause of disability.3 Importantly, it is estimated that tobacco smoking only accounts for 50 to 70% of cases.4 Therefore, in addition to measures aimed at preventing smoking and helping patients quit smoking, there has been a drive to find strategies to identify people at risk of COPD and to reduce their risk of the condition developing as a result of a sustained excess decline in lung function.5 Similarly, there is interest in diagnosing COPD in its earlier stages in the hope that the course of the disease can be altered if the pathophysiological changes are not advanced. There is also a greater appreciation of the heterogeneity in disease expression and considerable effort is being made to better characterise patients with established COPD, with the long-term goal of targeting treatment.
A deeper understanding of COPD and its underlying processes is therefore needed to enable advances in its management.
Spirometry for diagnosis of COPD
The prevalence of airflow limitation in the population varies between countries. The most comprehensive study to date on the prevalence of airway obstruction is the Burden of Obstructive Lung Disease (BOLD) study, which involved 12 sites in 12 different countries and 9425 subjects. The investigators reported the presence of moderate or greater airway obstruction (ratio of FEV1 to forced vital capacity [FVC] less than 0.70 and FEV1 less than 80% of predicted on post-bronchodilator spirometry testing) in 6 to 20% of the population over 40 years of age.6 The reported prevalence is a surprisingly large proportion of the population. The study did not include any developing countries where environmental pollutant exposure and tobacco consumption are high and, therefore, where COPD could be even more prevalent. In Australia, the prevalence of COPD in women and men aged 40 years or older was found to be 7.5%.7
Other studies have reported that when the largest at-risk population in western societies – current and former smokers of 10 pack-years or more and 40 years of age and older – is screened, between one in seven and one in three people have COPD.8-10 The proportion varies depending on the prevalence of COPD in the population being tested. Considering the relation between pack-years and severity of airway obstruction, the likelihood of finding airway obstruction will be even higher if individuals who have smoking histories exceeding 20 pack-years are targeted.
Is spirometry really necessary for diagnosis?
The diagnosis of COPD needs confirmation in individuals who have symptoms. As the symptoms associated with COPD are nonspecific, such as productive cough that could be due to bronchitis without COPD, bronchiectasis or postnasal drip, diagnosis by clinical symptoms and signs alone is highly inaccurate. The implications of misdiagnosis are significant: treating a patient with drugs for an erroneous diagnosis is wasteful of resources, needlessly exposes patients to potential drug side effects and may delay the correct diagnosis and appropriate management. Spirometry remains the mainstay of measuring airway function in primary care. Airway function can be assessed using a variety of other modalities in tertiary care, including the measuring of gas trapping and respiratory mechanics using oscillometry.
Arguably spirometry is mandatory in any patient who presents with worsening breathlessness or wheeze during a respiratory tract infection because such a scenario constitutes an exacerbation, which in itself has significant clinical connotations. An exacerbation of COPD is commonly defined as worsening symptoms (cough, sputum production or breathlessness) for three or more days. Apart from the short-term consequences, exacerbations are associated with increased rate of decline in lung function, further exacerbations, increased risk of death, reduced quality of life and increased health care utilisation.11
Airflow limitation
Although the most common cause of COPD is cigarette smoking, it is not the sole cause. Other causes include domestic and occupational inhalants and asthma. Although long-standing asthma can cause airflow limitation that is incompletely reversible by acute bronchodilator inhalation, the pathology of long-standing asthma is very different from that of COPD and the clinical features frequently differ. In COPD, neutrophilic inflammation in the large and small airways, including the respiratory and terminal bronchioles, is characteristic and leads to tissue destruction that also involves the lung parenchyma, resulting in emphysema.12 Even after smoking cessation, inflammation persists when COPD is established and severe.13 In asthma, however, inflammation is commonly eosinophilic, although neutrophilic inflammation becomes more common with more long-standing asthma.14
The combination of smoking and asthma results in additive effects on decline in lung function.15 If asthma is severe and smoking exposure has been heavy, the chances of having incompletely reversible airflow limitation are increased, and all such patients should have spirometry performed. The value of making a diagnosis of asthma versus a diagnosis of COPD is open to debate. The criteria on which such diagnostic splitting is based are also a matter of opinion. Whether such diagnostic labelling should alter management or affect outcomes is even more complex and will probably be influenced by greater understanding of different clinical subtypes, or phenotypes, of obstructive airways disease.
Case finding in COPD
The practical aspects of case finding in COPD have been discussed in the article ‘COPD: practical aspects of case finding, diagnosing and monitoring’, published in a previous issue of Medicine Today.16 COPD should be actively sought in all current or former smokers, and in particular in those who have respiratory symptoms (typically cough, wheeze or breathlessness) as they may have more severe disease than asymptomatic smokers. The German research team who were part of the BOLD study of COPD prevalence, together with primary care physicians, found that a new COPD case would be identified in one of every two individuals if they screened all smokers older than 40 years of age who also had symptoms of cough or breathlessness.17
There is good evidence that screening with spirometry is helpful for successful smoking cessation. In a study performed in a primary care setting in the UK, smoking cessation rates in those aged over 35 years were increased by telling individuals their estimated lung age (the age of the average healthy individual who would have similar spirometry to them), independently of whether the results were normal or abnormal.18 In a recent Canadian study in which nearly 40,000 people were screened for undiagnosed airways disease (irrespectively of diagnostic labels of asthma or COPD), 508 individuals were found to have undiagnosed asthma or COPD and were randomised to specialist clinic care or referred to their usual general practitioner.19 Improved FEV1, less healthcare utilisation and better quality of life were found in the specialist clinic patients. Such evidence may be sufficient justification for mass screening with spirometry in all smokers for some healthcare givers. However, the US Preventive Services Task Force does not recommend mass screening of asymptomatic adults for COPD with spirometry because of the scarcity of comparative studies in terms of the overall cost–benefit ratio.20 Nevertheless, we believe there is sufficient evidence to support case finding of COPD with spirometry in high-risk populations, including current and ex-smokers older than 35 years of age. The Box lists the target population for COPD case finding.2
Successful smoking cessation before there is loss of lung function will have larger potential benefits in preserving lung function. In early COPD, lifestyle changes (optimisation of weight, exercise, dietary changes) should be instituted as early as possible, with or without pharmacological treatment, depending on the presence of symptoms and exacerbations. The diagnosis of COPD should also alert GPs and other physicians to the increased risk of mortality from any cause, importantly cardiovascular disease, respiratory failure, cerebrovascular disease and cancer, which may have implications for patient management in relation to risk modification.21
Potential to improve clinical outcomes
The aim of early detection of airflow limitation is to allow early intervention and, as a result, to improve outcomes. The benefits of early diagnosis of COPD and airflow limitation are poorly studied but the natural history of COPD strongly suggests that intervention should be as early as possible. The earlier the intervention, the greater the potential benefits in terms of improved life expectancy and health outcomes; therefore, the earlier patients can quit smoking, the greater the benefits in terms of preserving lung function.22 Furthermore, loss of small airways occurs early in the disease, yet symptoms usually do not occur until there has been about a 50% loss of FEV1.4 Hence, early diagnosis of COPD clearly mandates case-finding: that is, performing spirometry in smokers.
All smokers should be strongly encouraged to quit smoking and, therefore, the presence of COPD should not influence management in terms of smoking cessation. However, there is evidence that smoking cessation is more likely if the subject has airway obstruction. In a smoking cessation program in Poland involving 100,000 people, about 4500 individuals with a history of at least 10 pack-years of smoking were invited to attend a smoking cessation session.23 More than two-thirds of subjects attended the sessions where spirometry was used as a tool to help quitting. The presence of airway obstruction was associated with higher quit rates at one year (verified by exhaled carbon monoxide level), with the difference being highest in those with severe airflow limitation (16.3% vs 12% in those with normal spirometry results).
After airflow limitation is detected with spirometry, it should be interpreted in the context of the individual patient, as for any test result. Patients are concerned about the consequences to them, in terms of current or future impairment and disability, and possible treatment requirements. Although there is a sound evidence base to inform treatment in some instances, given the heterogeneity of COPD, there are many instances where there is little evidence to inform treatment strategy. Examples include people with asthma who have smoked and those with asthma who have not smoked but have fixed airway obstruction. These people are usually excluded from both asthma studies and COPD studies, so the evidence from studies may not be generalisable to these populations. However, management that is based on identifying and treating clinical problems such as frequent exacerbations, breathlessness, obesity and anxiety in patients who have airways disease may result in greater clinical benefits than the more narrow approach of prescribing an inhaler as specific treatment for the airways.24 This approach seems logical as the quality of life in patients with airways disease is impaired in proportion to the number of identifiable comorbidities.25
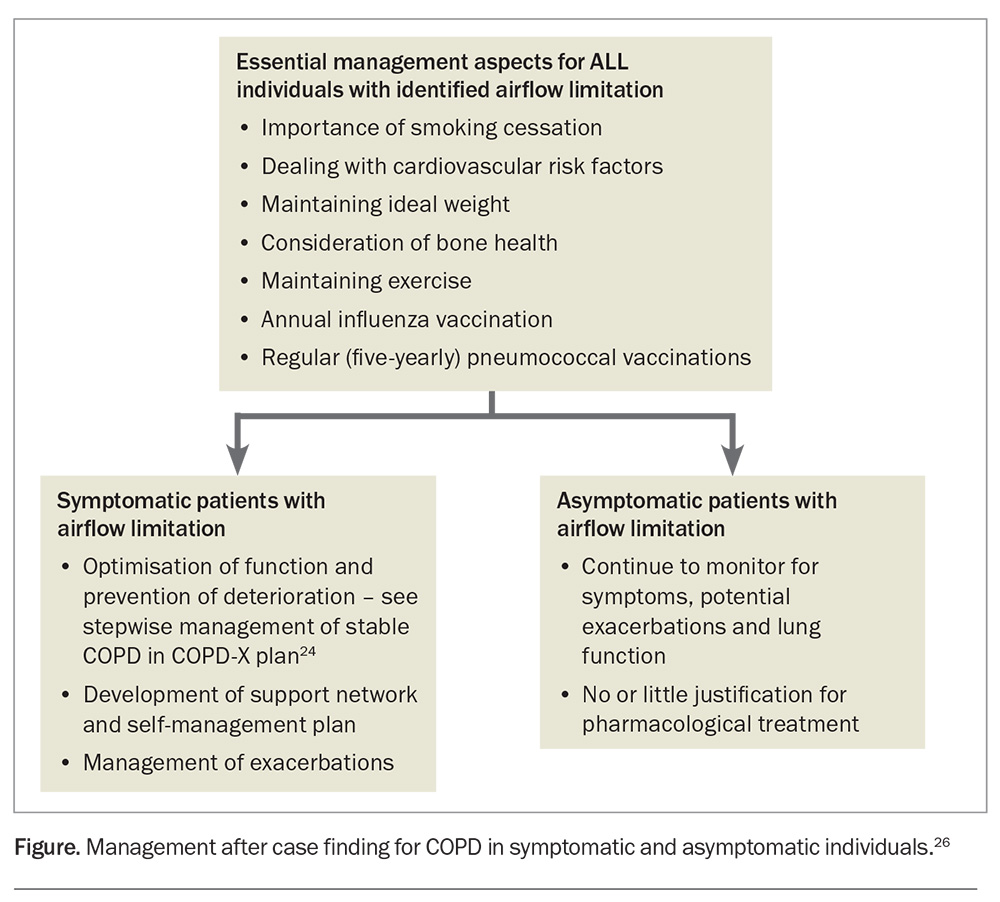
The management of patients with COPD, symptomatic and asymptomatic, is summarised in the Figure.26 There have been few early intervention studies involving earlier initiation of inhaled COPD treatment in treatment-naïve participants.27,28 The results of two recent studies were negative in terms of altering FEV1 loss. It is thus clear that a better paradigm of pathophysiology is required to produce effective disease-modifying treatment. This means developing tools beyond our current CT imaging, blood and sputum analyses, and spirometry to find an effective early intervention for COPD, which recent technological advancements can bring.
Can we alter progression of the disease?
Smoking cessation is the only intervention that can alter the progression of COPD. Although the rate of decline in FEV1 was shown to be reduced in patients with moderate COPD by treatment with a high-dose, combination inhaled corticosteroid/long-acting β2-agonist or a long-acting antimuscarinic agent, the effects were small and of uncertain clinical significance.19,29 Treatment with short-acting antimuscarinic drugs has no effect on the rate of decline in FEV1.30 Nevertheless, there is great heterogeneity in the rate of decline in FEV1, presence of symptoms, systemic disease and exacerbation rates between patients, with some progressing quickly in terms of COPD severity while others remain stable for many years.
Currently, there are no clinically useful markers to identify patients with COPD who will decline rapidly or to predict those in whom drug treatment reduces the rate of decline. It is known that airway hyperresponsiveness, acute bronchodilator reversibility, respiratory symptoms, reduced FEV1/FVC ratio, low baseline FEV1, emphysema, mucus hypersecretion and episodes of lower respiratory tract illness are associated with increased rate of loss of FEV1. This may influence optimal management (e.g. earlier and more intensive interventions). However, their predictive ability in an individual is likely to be poor and they are not routinely used for this purpose.31-38
It is recognised that for a given impairment in FEV1, there is a wide range of symptom severity and exacerbation risk in COPD. This heterogeneity is reflected in the international GOLD strategy document, in which symptoms are included in the severity assessment.1 The presence of symptoms as defined by the GOLD severity classification is associated with an increased risk of exacerbation as well as of mortality for the same degree of airflow limitation defined by spirometry.39
Drug treatment with either single or combination inhaled corticosteroids, long-acting β-agonists and/or long-acting antimuscarinic agents improves symptoms and exacerbation risk in patients with moderate COPD (FEV1, 50 to 80% of predicted), as well as in patients with more severe COPD.40,41 Inhaled corticosteroid treatment in COPD is generally reserved for patients with coexistent asthma or those experiencing frequent exacerbations (≥two moderate acute exacerbations of COPD or ≥one severe acute exacerbation of COPD requiring hospitalisation) with an eosinophil count >0.3 × 109/L despite dual inhaled bronchodilator treatment.1 Although individuals in the general population with milder disease are less likely to report any symptoms than those with lower FEV1, there is great variability. Overall, such patients benefit from pharmacological treatment in terms of improved quality of life and reduced exacerbation risk, with the decision ideally based on a risk–benefit assessment in each individual. It is worthwhile noting that patients entering clinical studies are more likely to be symptomatic because their symptoms identified them as having COPD prior to enrolment. The absence of symptoms or previous exacerbations after thorough history-taking in a patient with moderate airflow limitation (moderate COPD) is associated with a very low risk of exacerbations in the following year – around 2%.40 Mortality risk is also low at 0.6%.40 Therefore, asymptomatic individuals who have COPD do not necessarily warrant drug treatment, particularly if they have only mild to moderate FEV1 impairment.
Thus, the most important treatment in a patient newly diagnosed with airflow limitation whose FEV1 is greater than 50% of predicted and who is asymptomatic is smoking cessation. Other considerations in such a patient are dealing with cardiovascular risk factors, maintaining ideal weight, considering bone health and maintaining exercise. However, there is no or little justification for pharmacological treatment for COPD because there is little known of the benefits of such treatment in patients with asymptomatic, mild to moderate COPD. This is an area that requires further research.
Future developments in COPD
There is widespread agreement about the need for more research into COPD phenotyping (i.e. clinical, biochemical and inflammatory characterisation) because of the potential for clinical benefit.42-44 COPD represents a spectrum of disorders that share airflow limitation as their common underlying pathophysiological process but behave differently in many aspects between individuals. Understanding the heterogeneity of COPD better might allow earlier detection as well as development of treatment methods that are targeted specifically at certain phenotypic subgroups.
Current methods in practice to phenotype COPD include CT imaging to establish, for example, the presence of underlying emphysema. Although there is firm evidence to support a correlation between the extent of emphysema determined by CT and by histological examination,using CT imaging for this purpose has the disadvantages of cost and radiation exposure.45-50 New lung function methods that are more sensitive to small airway dysfunction and might potentially allow improved phenotypic classification include oscillometry and the multiple breath nitrogen washout technique; however, the latter remains predominantly a research tool.51-53
Disease phenotyping (outward clinical appearance) and endotyping (underlying pathophysiology) is a current area of research because of the potential to help improve COPD outcomes by allowing targeted or personalised treatment. Certain COPD phenotypes and endotypes might respond differently to different treatments (e.g. differing bronchodilator responsiveness). The current tools have not produced clinically useful markers of COPD susceptibility (because only about 20% of smokers develop COPD) or progression. This unmet need in COPD is arguably the most urgent priority in COPD research.
Ultimately, being able to identify the clinical links between phenotypes and the complex relation with genetic, molecular, cellular and environmental components may translate into the ability to practise individualised medicine rather than a generalised ‘one-fits-all’ approach to COPD. This could lead to better patient outcomes in terms of morbidity and mortality by delaying progression of disease and improving overall survival.43,44 Such an approach would be of particular relevance for patients with mild COPD.
Conclusion
It is worthwhile identifying patients with mild COPD and early disease, but this is possible only if case finding occurs in primary care. Detection of airflow limitation when the patient is asymptomatic and confirmation by spirometry of a diagnosis of COPD identifies individuals at risk of future symptoms and complications of the condition. Better stratification of future risk by actively diagnosing COPD may alter the management in individuals who have additional risk factors for COPD progression and a cardiovascular risk. Performing spirometry in current smokers may increase their chances of smoking cessation.
It is important to recognise that when a previously asymptomatic patient with newly diagnosed airflow limitation develops respiratory symptoms, this represents an exacerbation of COPD. This exacerbation needs to be managed accordingly, and not be misdiagnosed as a simple lower respiratory tract infection. Confirmation or exclusion of COPD allows appropriate drug prescription and helps avoid diagnostic confusion.
Management of COPD, including its pharmacotherapy, continues to evolve as understanding of the condition increases, particularly the appreciation of the heterogeneity of the disease. Case finding of COPD raises complex arguments about cost effectiveness, clinical benefit and appropriate treatment. There are a great number of clinical questions that still need answering by well-designed clinical studies to provide a stronger evidence base to guide management in early COPD. MT
COMPETING INTERESTS: Dr King has received travel sponsorships from Boehringer Ingelheim, Novartis, Pfizer, AstraZeneca and GlaxoSmithKline and provides consultancy services related to asthma and COPD. His research group at the Woolcock Institute of Medical Research receives a proportion of the unrestricted grants the Institute receives from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline and Pharmaxis, and also receives grants from local research foundations and the NHMRC, Cooperative Research Centre for Asthma and Airways and Lung Foundation Australia. Dr Farah has received honoraria from AstraZeneca, Chiesi and Sanofi for speaking at educational meetings and attending advisory board meetings. Dr Zimmermann: None.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of Chronic Obstructive Lung Disease (updated 2025). GOLD; 2025. Available online at: http://www.goldcopd.org (accessed February 2025).
2. McKenzie DK, Abramson M, Crockett AJ, et al. on behalf of The Australian Lung Foundation. The COPD-X plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease V2.76, 2024. Available online at: http://www.copdx.org.au/ (accessed February 2025).
3. World Health Organization (WHO). Chronic obstructive pulmonary disease (COPD) key facts. WHO; 2024. Available online at: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed March 2025).
4. Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997; 349: 1498-1504.
5. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977; 1: 1645-1648.
6. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. Lancet 2007; 370: 741-750.
7. Toelle BG. Respiratory symptoms and illness in older Australians: the Burden of Obstructive Lung Disease (BOLD) study. Med J Aust 2013; 198: 144-148.
8. Castillo D, Guayta R, Giner J, et al. COPD case finding by spirometry in high-risk customers of urban community pharmacies: a pilot study. Respir Med 2009; 103: 839-845.
9. Zielinski J, Bednarek M, the Know the Age of Your Lung Study Group. Early detection of COPD in a high-risk population using spirometric screening. Chest 2001; 119: 731-736.
10. Jordan RE, Lam KH, Cheng KK, et al. Case finding for chronic obstructive pulmonary disease: a model for optimising a targeted approach. Thorax 2010; 65: 492-498.
11. Seemungal TA, Hurst JR, Wedzicha JA. Exacerbation rate, health status and mortality in COPD – a review of potential interventions. Int J Chron Obstruct Pulmon Dis 2009; 4: 203-223.
12. Hogg JC. A pathologist’s view of airway obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186(5): v-vii.
13. Hogg JC. State of the art. Bronchiolitis in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3: 489-493.
14. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 2006; 11: 54-61.
15. James AL, Palmer LJ, Kicic E, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med 2005; 171: 109-114.
16. Walters JAE, Crockett AJ, McDonald VM. COPD: practical aspects of case finding, diagnosing and monitoring. Med Today 2013; 14(2): 32-40.
17. Koegler H, Metzdorf N, Glaab T, Welte T. Preselection of patients at risk for COPD by two simple screening questions. Respir Med 2010; 104: 1012-1019.
18. Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ 2008; 336: 598-600.
19. Aaron SD, Vandemheen KL, Whitmore A, et al. Early diagnosis and treatment of COPD and asthma — a randomized, controlled trial. N Engl J Med 2024; 390: 2061-2073.
20. Lin JS, Webber EM, Thomas RG. Screening for chronic obstructive pulmonary disease: a targeted evidence update for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2022. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK580641/ (accessed March 2025).
21. Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775-789.
22. Scanlon PD, Connett JE, Waller LA, et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 381-390.
23. Bednarek M, Gorecka D, Wielgomas J, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax 2006; 61: 869-873.
24. McDonald VM, Higgins I, Wood LG, Gibson PG. Multidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense? Thorax 2013; 68: 691-694.
25. McDonald VM, Simpson JL, Higgins I, Gibson PG. Multidimensional assessment of older people with asthma and COPD: clinical management and health status. Age Ageing 2011; 40: 42-49.
26. Lung Foundation Australia. Stepwise management of stable COPD. Brisbane: Lung Foundation Australia; 2012. Available online at: http://www.lungfoundation.com.au/wp-content/uploads/2012/01/ALF-Stepwise-Management-of-COPD-A4-April-2013.pdf (accessed January 2014).
27. Thamrin C, Martin A, Badal T, et al. Dual bronchodilator treatment for prevention of COPD in at-risk smokers. Respirology 2022; 27: 983-986.
28. Han MK, Ye W, Wang D, et al. Bronchodilators in tobacco-exposed persons with symptoms and preserved lung function. N Engl J Med 2022; 387: 1173-1184.
29. Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009; 374: 1171-1178.
30. Anthonisen N, Connett J, Kiley J, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994; 272: 1497-1505.
31. Tashkin DP, Altose MD, Connett JE, Kanner RE, Lee WW, Wise RA. Methacholine reactivity predicts changes in lung function over time in smokers with early chronic obstructive pulmonary disease. The Lung Health Study Research Group. Am J Respir Crit Care Med 1996; 153: 1802-1811.
32. Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011; 365: 1184-1192.
33. Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited. Am J Respir Crit Care Med 2009; 180: 3-10.
34. Drummond MB, Hansel NN, Connett JE, Scanlon PD, Tashkin DP, Wise RA. Spirometric predictors of lung function decline and mortality in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185: 1301-1306.
35. Yuan R, Hogg JC, Paré PD, et al. Prediction of the rate of decline in FEV1 in smokers using quantitative computed tomography. Thorax 2009; 64: 944-949.
36. Mohamed Hoesein FAA, de Hoop B, Zanen P, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax 2011; 66: 782-787.
37. Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996; 153: 1530-1535.
38. Kanner RE, Anthonisen NR, Connett JE, The Lung Health Study Research Group. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the Lung Health Study. Am J Respir Crit Care Med 2001; 164: 358-364.
39. Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med 2012; 186: 975-981.
40. Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009; 10: 59.
41. Decramer M, Celli B, Kesten S, et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet 2009; 374: 1171-1178.
42. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010; 182: 598-604.
43. Park TS, Lee JS, Seo JB, et al. Phenotyping of chronic obstructive pulmonary disease: heterogeneity and its clinical relevance. Curr Respir Care Rep 2012; 1: 189-198.
44. Parr DG. Patient phenotyping and early disease detection in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2011; 8: 338-349.
45. Coxson HO, Rogers RM, Whittall KP, et al. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med 1999; 159: 851-856.
46. Gelb AF, Hogg JC, Müller NL, et al. Contribution of emphysema and small airways in COPD. Chest 1996; 109: 353-359.
47. Kinsella M, Müller NL, Abboud RT, Morrison NJ, DyBuncio A. Quantitation of emphysema by computed tomography using a ‘density mask’ program and correlation with pulmonary function tests. Chest 1990; 97: 315-321.
48. Lee YK, Oh YM, Lee JH, Kim EK, Lee JH, Kim N. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung 2008; 186: 157-165.
49. Müller NL, Staples CA, Miller RR, Abboud RT. ‘Density mask’. An objective method to quantitate emphysema using computed tomography. Chest 1988; 94: 782-787.
50. Verbanck S, Schuermans Dl, Meysman M, Paiva M, Vincken W. Noninvasive assessment of airway alterations in smokers: the small airways revisited. Am J Respir Crit Care Med 2004; 170: 414-419.
51. Timmins SC, Diba C, Farrow CE, et al. The relationship between airflow obstruction, emphysema extent, and small airways function in COPD. Chest 2012; 142: 312-319.
52. Verbanck S, Schuermans D, Van Muylem A, et al. Conductive and acinar lung-zone contributions to ventilation inhomogeneity in COPD. Am J Respir Crit Care Med 1998; 157: 1573-1577.
53. Verbanck S, Schuermans Dl, Meysman M, Paiva M, Vincken W. Noninvasive assessment of airway alterations in smokers: the small airways revisited. Am J Respir Crit Care Med 2004; 170: 414-419.