New developments in COPD
COPD is a heterogeneous disease that presents many management challenges. This review presents an update on promising advances from research.
- Patients with chronic obstructive pulmonary disease (COPD) should be encouraged to maintain regular physical exercise, and referral to pulmonary rehabilitation is recommended.
- Inhaled corticosteroids should be reserved for patients with moderate to severe COPD to reduce exacerbations; and patients with a blood eosinophil count of 0.3 x 109/L or greater are most likely to benefit.
- Inhaled corticosteroids may be withdrawn safely in patients with COPD who do not have frequent exacerbations and have a blood eosinophil count of less than 0.3 x 109/L (excluding patients with an asthma overlap syndrome).
- Highly selected patients may be suitable for endobronchial valve placement or lung volume reduction surgery. Referral to a respiratory specialist and an expert centre is recommended.
Chronic obstructive pulmonary disease (COPD) is characterised by fixed airway obstruction on spirometry, persistent respiratory symptoms including dyspnoea, chronic cough, wheeze or sputum production and a history of exposure to noxious stimuli, generally cigarette smoke.1 COPD was the fifth leading cause of death in 2021 and affects almost 8% of Australians aged over 45 years.2 Presentations due to COPD have a substantial impact on health service use, contributing to 1% of general practice encounters, and are the second leading cause of avoidable hospital admissions.3,4
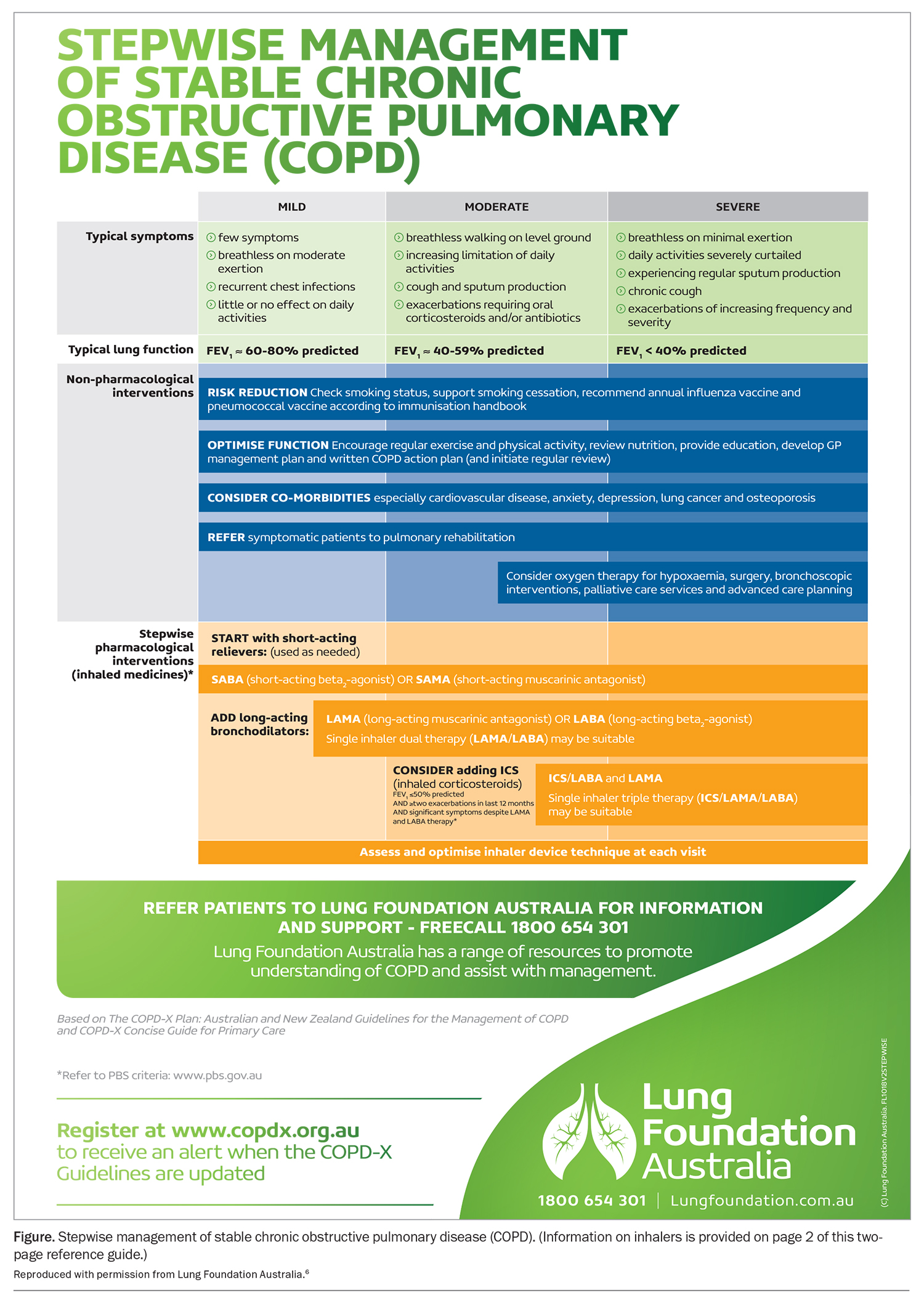
The COPD-X guidelines are a useful resource for investigation, diagnosis and management of COPD.5 COPD should be considered in people aged over 35 years with a history of smoking or exposure to dust, gases or fumes. The diagnosis must be confirmed on spirometry with a forced expiratory volume in 1 second (FEV1) to forced vital capacity ratio of below 0.70.1 There is no evidence that COPD treatments have any benefit in patients without COPD diagnosed on spirometry. Smoking cessation is paramount to prevent further deterioration in lung function and symptoms. Other nonpharmacological interventions including annual influenza vaccination, COVID-19 vaccination and referral to pulmonary rehabilitation are strongly recommended. For COPD patients with persistent symptoms or frequent exacerbations, a stepwise approach to inhaler therapy can be initiated (Figure).6
It has been identified that eosinophilic inflammation may play a role in COPD, and therefore may present a potential target for treatment options. Selected patients with severe COPD and persistent breathlessness despite optimal medical treatment may be suitable for advanced therapies including endobronchial valve placement or lung-volume reduction surgery. Frailty has been identified as a clinical syndrome and has significant impacts in patients with COPD. This article examines these three topics in further detail.
The role of eosinophils in COPD
Eosinophils are innate immune cells that are believed to play a role in host defences in allergic disease. Historically, eosinophilic inflammation in airways disease has been considered to be suggestive of asthma, with neutrophilic inflammation predominantly seen in COPD. However, over recent years there has been increasing interest in the role of eosinophilic inflammation in COPD including its impact on morbidity and the potential role for treatment targeting this form of inflammation.
A normal blood eosinophil count is less than 0.5 × 109/L. A blood eosinophil count of 0.3 × 109/L or greater, which may still be within the normal range, is associated with an increased risk of acute exacerbations of COPD.7,8 In 37 to 68% of people with COPD, blood eosinophil count was shown to be consistently 0.3 × 109/L or greater and to correlate with elevated levels of eosinophils in sputum.9,10 In a small retrospective cohort study undertaken in Canada involving 167 patients hospitalised with an acute exacerbation of COPD, an elevated blood eosinophil count at initial admission was associated with an increase in readmissions for exacerbations (odds ratio [OR], 3.59; 95% confidence interval [CI], 1.65-7.82; p = 0.01) and an increase in all-cause readmissions over a 12-month period (OR, 2.32; 95% CI, 1.10-4.92; p = 0.03). Patients with blood eosinophilia also had a shorter time to first exacerbation.11
Eosinophils have been proposed to be a useful biomarker in COPD for prognostic purposes and to guide treatment with inhaled and oral corticosteroids. It has been suggested that patients with COPD and elevated blood eosinophil counts (defined as ≥0.3 × 109/L) may benefit from inhaled corticosteroid therapy to reduce the risk of exacerbations; however, this has not been assessed prospectively in a randomised controlled trial.12,13
Withdrawal of inhaled corticosteroids (ICS) is safe in some patients with COPD, as shown by a randomised, double-blind, multicentre controlled trial involving 1053 patients with COPD who were receiving long-term triple therapy. Withdrawal of ICS to de-escalate to dual bronchodilator therapy with a long-acting muscarinic antagonist (LAMA) and long-acting beta agonist (LABA) in patients without evidence of eosinophilic inflammation was safe, with no difference in exacerbation rate over a six-month period compared with participants who remained on triple therapy.12 However, in patients with an elevated blood eosinophil count (≥0.3 × 109/L), ICS withdrawal was associated with an increased risk of exacerbations and a small decrease in lung function.
Data suggest that treatment with oral corticosteroids for acute exacerbations of COPD may also be directed by baseline blood eosinophil count. Retrospective analysis of three randomised controlled trials comparing prednisolone to placebo showed a higher rate of treatment failure in patients with an elevated blood eosinophil count of 0.3 × 109/L or greater who did not receive prednisolone, whereas in patients with a blood eosinophil count of less than 0.3 × 109/L there was no difference in treatment failure in the prednisolone group compared with the placebo group.14 However, in at least one of these trials, patients with a history of asthma were not specifically excluded. Asthma-COPD overlap is an increasingly recognised condition and corticosteroid treatment for acute exacerbations remains a key management strategy in patients with this condition. A 2024 UK trial randomised 308 patients in primary care presenting with an exacerbation of COPD to eosinophil-guided prednisolone or standard care. Patients in the intervention arm only received prednisolone if their blood eosinophil count was above 2%. This trial found that eosinophil guided prednisolone use was noninferior to standard care.15
The current evidence on the role of eosinophils in COPD is intriguing; however, the data are retrospective and mostly based on post-hoc analyses. There are no current clear cut-offs for blood eosinophil count to guide management, and further prospective trials are required to determine the association and guide treatment recommendations.
Biologic therapy in COPD
The introduction of targeted biologic therapy in eosinophilic asthma has had a significant impact among patients with symptomatic severe asthma. Benralizumab and mepolizumab are humanised monoclonal antibodies that are administered subcutaneously and block interleukin-5, thereby reducing peripheral circulating eosinophils in blood and tissue.16,17 Mepolizumab significantly reduces exacerbation rates and symptoms and improves quality of life in patients with severe eosinophilic asthma.18
The data with regard to COPD are still emerging. A systematic review of randomised controlled trials comparing anti-IL5 therapy with placebo (three trials with mepolizumab and three trials with benrolizumab) suggested that anti-IL5 therapy was likely to reduce exacerbations in people with COPD and higher eosinophil levels, but did not improve health-related quality life.19
Dupilumab, a monoclonal antibody targeting IL-4 and IL-13, is used to prevent exacerbations in moderate to severe asthma. A 2023 randomised controlled trial showed that dupilimab reduced exacerbations in patients with COPD, chronic cough and sputum, and an eosinophil count above 0.3 × 109/L.20Although monoclonal antibody therapy may be beneficial in a specific subgroup of patients with COPD and eosinophilia, these therapies are not currently approved for use in COPD in Australia.
Endobronchial valves
Insertion of endobronchial valves may be an option for carefully selected patients with severe COPD with an FEV1 of less than 50% predicted, hyperinflation with a total lung capacity of more than 100% predicted and residual volume of more than 175%.21 Valves are inserted via bronchoscopy and occlude emphysematous lobes to block inspiratory airflow while allowing expiratory airflow, with the aim of reducing gas trapping by creating areas of atelectasis and shunting airflow elsewhere.22
Retrospective analysis of the large early endobronchial valve trials showed that patients with intact pleural fissures were most likely to benefit.23 Several subsequent randomised controlled studies only recruited COPD patients with intact pleural fissures and showed a significant improvement in FEV1, six-minute walk distance (6MWD) and quality of life in carefully selected patients.24-28 However, it is important to recognise that there are potential major complications with endobronchial valve placement including pneumothorax (in 1.4 to 26% of patients) and COPD exacerbation (in 4 to 20% of patients).29
Endobronchial valves are not yet recommended as routine care. However, in highly selected patients with significant breathlessness despite optimised medical care and pulmonary rehabilitation, endobronchial valves may be a potential treatment option and patients should be assessed in a centre of expertise.30
Lung volume reduction surgery
Lung volume reduction surgery (LVRS) involves resection of emphysematous lung to decrease hyperinflation with the proposed benefits of improving diaphragmatic function, reducing respiratory muscle fatigue and intrathoracic pressure and improving cardiac ventricular filling.31-34
Patients with severe COPD who may benefit from LVRS include those aged under 75 years with persistent dyspnoea despite optimal medical treatment and pulmonary rehabilitation, who have heterogeneous emphysema (with varying emphysema tissue destruction between pulmonary lobes) and a 6MWD of greater than 140 metres.35
The National Emphysema Treatment Trial (NETT) was a large multicentre study involving 1218 patients with severe emphysema who were randomly allocated to either lung volume reduction surgery or standard medical care following completion of a pulmonary rehabilitation program.35 Patients in the surgical group had a significant improvement in exercise capacity, FEVand quality of life scores. There was an increased 90-day mortality in the surgical group (7.9% compared with 1.3%); however, there was no overall difference in mortality at the end of follow up (mean follow up, 29 months). A recent systematic review and meta-analysis – heavily influenced by NETT data – concluded that LVRS reduces gas trapping and significantly improves FEV1 and quality of life with an early increase in mortality but no difference in overall mortality.29 Postoperative complications included prolonged air leak.
LVRS may be a suitable treatment option for highly selected patients with severe COPD. Patients should undergo pulmonary rehabilitation before considering surgery and should be assessed in an expert centre with a multidisciplinary panel including a respiratory physician, thoracic surgeon, radiologist and interventional pulmonologist.30 A meta-analysis pooling all modalities of lung volume reduction (surgical and endobronchial) showed benefits in lung function, health related quality of life and 6MWD but reported that the odds ration for a severe adverse event including death was six times higher in the intervention group.29
Frailty and COPD
Frailty is a clinical syndrome in which there is a decline in physiological and functional reserve associated with increasing age, resulting in a reduced ability to cope with daily and acute stressors.36,37 Frailty affects 7 to 13% of the older population aged above 65 years with the prevalence increasing with advancing age.38-40 The syndrome is characterised by the presence of three or more of the following characteristics: loss of weight, slow walking speed, low physical activity, reduced grip strength and reduced endurance.38 The phenotype is useful for identifying people at risk of poor health outcomes and is an independent predictor of increased risk of falls, hospitalisation and mortality among the elderly population.38
People with COPD are twice as likely to be frail as people without the condition, with the prevalence of frailty ranging between 19 and 57%.37,40-43 Frailty in people with COPD has significant impacts, including increased risk of acute exacerbations and of readmission due to a new exacerbation episode during the 90 days after hospitalisation for an acute exacerbation of COPD.40,42 Furthermore, frailty in COPD is associated with worsening impairment of lung function, poorer exercise tolerance (including a reduced 6MWD), increased levels of depression and anxiety and low socioeconomic status.40,41,43 People with COPD and frailty are more likely to have multiple comorbidities, in particular cardiovascular disease.40
Exercise has been shown to improve frailty, and the addition of nutritional intervention is associated with further benefits.44,45 Although exercise appears to be an essential component in addressing frailty, the optimal exercise program has not yet been determined. Despite the benefits of pulmonary rehabilitation in patients with COPD being widely known, to date there have been no randomised controlled trials assessing the impact of pulmonary rehabilitation on frailty markers in COPD. A single centre prospective cohort study undertaken in the UK involving 816 patients with COPD and frailty showed that pulmonary rehabilitation improved exercise capacity, dyspnoea, hand grip strength, anxiety and depression. However, patients with frailty were also significantly less likely to complete pulmonary rehabilitation owing to either progression of their frailty or hospitalisation. Among the frail patients who persevered, 60% improved their frailty status to either prefrail or robust after completion of pulmonary rehabilitation.43
Increasing recognition of frailty among older adults and the population with COPD has highlighted the complex interplay between physiological, psychological and social factors. Although further data on effective exercise and nutritional interventions are needed, individuals at high risk of frailty may benefit from early identification and prompt referral to pulmonary rehabilitation.
Conclusion
Despite falling smoking rates in Australia, the burden of disease due to COPD remains substantial and has significant impacts on health care resources and utility. Although preventive health measures such as smoking cessation are vital, early identification and diagnosis of COPD is important to maintain lung function and prevent progression of symptoms. Both nonpharmacological and pharmacological interventions are recommended to reduce symptoms, prevent exacerbations and maintain lung function. Smoking cessation (beyond the scope of this article) is vital to prevent disease progression. Maintenance of physical activity and prevention of frailty is imperative in older adults with COPD and may improve their health outcomes. Further prospective studies assessing the impact of eosinophilic inflammation and targeted antieosinophilic treatments may be of benefit. In carefully selected patients with severe and persistent breathlessness in advanced COPD, advanced therapies including endobronchial valves or lung volume reduction surgery may be accessible. We recommend the COPD-X Guidelines as a useful resource to assist in identification, diagnosis and management of patients with COPD. MT
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2019 report. Available online at: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf (accessed January 2020).
2. Australian Institute of Health and Welfare (AIHW). Deaths in Australia. Updated 11 July 2023. Available onlibe at: https://www.aihw.gov.au/reports/life-expectancy-deaths/deaths-in-australia/contents/leading-causes-of-death (accessed February 2024).
3. Britt H, Miller GC, Henderson J, et al. General practice activity in Australia 2015-16. General Practice Series No. 40. Sydney: Sydney University Press; 2016.
4. Page A, Ambrose S, Glover J, Hetzel D. Atlas of avoidable hospitalisations in Australia: ambulatory care-sensitive conditions. Adelaide: Public Health Information Development Unit, University of Adelaide; 2007.
5. Yang IA, Brown JL, George J, et al. The COPD-X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease. Version 2.59, August 2019. Available online at https://copdx.org.au (accessed February 2020).
6. Stepwise management of stable chronic obstructive pulmonary disease. Milton, Qld: Lung Foundation Australia; 2019. Available online at: https://lungfoundation.com.au/wp-content/uploads/2018/09/Information-Paper-Stepwise-management-of-stable-COPD-Feb2019.pdf (accessed February 2020).
7. Zeiger RS, Tran TN, Butler RK, et al. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract 2018; 6: 944-954.e5. Epub 2017 Nov 15.
8. Yun JH, Lamb A, Chase R, et al ; COPDGene and ECLIPSE Investigators. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2018; 141: 2037-2047.
9. Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 2014; 44: 1697-1700.
10. Kim VL, Coombs NA, Staples KJ, et al ; AERIS Study Group. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J 2017; 50(4): pii: 1700853.
11. Couillard S, Larivee P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest 2017; 151: 366-373.
12. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med 2018; 198: 329-339.
13. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435-442.
14. Bafadhel M, Davies L, Calverley PMA, Aaron SD, Brightling CE, Pavord ID. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J 2014; 44: 789-791.
15. Ramakrishnan R, Jeffers H, Langford-Wiley B, et al. Blood eosinophil-guided oral prednisolone for COPD exacerbations in primary care in the UK (STARR2): a non-inferiority, multicentre, double-blind, placebo-controlled, randomised controlled trial. Lancet 2024; 1: 67-77.
16. Hart TK, Cook RM, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clin Immunol 2001; 108: 250-257.
17. Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica G. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol 2016; 16): 186-200.
18. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651-659.
19. Donovan T, Milan SJ, Wang R, Banchoff E, Bradley P, Crossingham I. Anti-IL-5 therapies for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2020; 12(12): CD013432.
20. Bhatt SP, Rabe KF, Hanania NA, et al; BOREAS Investigators. Dupilumab for COPD with yype 2 inflammation indicated by eosinophil counts. N Engl J Med 2023; 389: 205-214.
21. Slebos DJ, Shah PL, Herth FJ, Valipour A. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration 2017; 93: 138-150.
22. Toma TP, Hopkinson NS, Hillier J, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003; 361: 931-933.
23. Sciurba FC, Ernst A, Herth FJF, et al; VENT Study Research Group. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010; 363: 1233-1244.
24. Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015; 386: 1066-1073.
25. Klooster K, ten Hacken NHT, Hartman JE, Kerstjens HAM, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015; 373: 2325-2335.
26. Valipour A, Slebos DJ, Herth F, et al; IMPACT Study Team. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016; 194: 1073-1082.
27. Criner GJ, Sue R, Wright S, et al; LIBERATE Study Group. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med 2018; 198: 1151-1164.
28. Kemp SV, Slebos DJ, Kirk A, et al; TRANSFORM Study Team. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med 2017; 196: 1535-1543.
29. van Geffen WH, Slebos D-J, Herth FJ, Kemp SV, Weder W, Shah PL. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systematic review and meta-analysis. Lancet Respir Med 2019; 7: 313-324.
30. Herth FJF, Slebos DJ, Rabe KF, Shah PL. Endoscopic lung volume reduction: an expert panel recommendation - update 2017. Respiration 2017; 94: 380-388.
31. Lando Y, Boiselle PM, Shade D, et al. Effect of lung volume reduction surgery on diaphragm length in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 796.
32. Gorman RB, McKenzie DK, Butler JE, Tolman JF, Gandevia SC. Diaphragm length and neural drive after lung volume reduction surgery. Am J Respir Crit Care Med 2005; 172: 1259-1266.
33. Bloch KE, Li Y, Zhang J, et al. Effect of surgical lung volume reduction on breathing patterns in severe pulmonary emphysema. Am J Respir Crit Care Med 1997; 156 (2 Pt 1): 553-560.
34. Jorgensen K, Houltz E, Westfelt U, Nilsson F, Schersten H, Ricksten S. Effects of lung volume reduction surgery on left ventricular diastolic filling and dimensions in patients with severe emphysema. Chest 2003; 124: 1863-1870.
35. Fishman A, Martinez F, Naunheim K; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348: 2059-2073.
36. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011; 27: 1-15.
37. Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003-2006). Heart Lung 2013; 42: 163-170.
38. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol 2001; 56A: 146-156.
39. Widagdo I, Pratt N, Russell M, Roughead E. How common is frailty in older Australians? Australas J Ageing 2015; 34: 247-251.
40. Gale NS, Albarrati AM, Munnery MM, et al. Frailty: a global measure of the multisystem impact of COPD. Chron Respir Dis 2018; 15: 347-355.
41. Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest 2018; 154: 21-40.
42. Bernabeu-Mora R, Garcia-Guillamon G, Valera-Novella E, Gimenez-Gimenez LM, Escolar-Reina P, Medina-Mirapeix F. Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: a longitudinal study. Ther Adv Respir Dis 2017; 11: 383-392.
43. Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016; 71: 988-995.
44. de Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millan-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr 2015; 15: 154.
45. Dedeyne L, Deschodt M, Verschueren S, Tournoy J, Gielen E. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: a systematic review. Clin Interv Aging 2017; 12: 873-896.
COMPETING INTERESTS: Dr Ng: None. Associate Professor Dabscheck is Co-Chair of the COPD Guidelines Committee.