Postmenopausal osteoporosis: is there a role for menopausal hormone therapy?

Menopausal hormone therapy (MHT) increases bone density and prevents fracture. However, the publication and accompanying media coverage of the Women’s Health Initiative study made many women fearful of MHT and many doctors reluctant to prescribe it. There is increasing recognition that MHT does have a place in health management of postmenopausal women, including for fracture prevention.
- Oestrogen-alone and combined oestrogen–progestogen therapies increase bone mineral density and reduce vertebral and nonvertebral fractures in postmenopausal women.
- Use of menopausal hormone therapy (MHT) in general, and for osteoporosis specifically, has been controversial since the early closure of the Women’s Health Initiative (WHI) study.
- In the final analysis of the WHI study, the benefits of MHT were found to be considerable and, particularly for younger, recently postmenopausal women, may outweigh the risk of harm.
- Commencing MHT is a valid option for postmenopausal women under the age of 60 years and within 10 years of menopause, with no specific contraindications, not only for vasomotor symptoms but also for bone protection.
To paraphrase Professor Bronwyn Stuckey, menopause is a consequence of improved public health: most women now not only survive childbirth but also their childbearing years and live long enough to experience loss of ovarian function (at an average age of 51 years). Menopause is generally rare among mammals, occurring only in humans, short-finned pilot whales and killer whales.1
This article focuses on the use of menopausal hormone therapy (MHT) with respect to bone health, and discusses the evidence for combined oestrogen–progestogen therapy, oestrogen-alone therapy in women who have had a hysterectomy, selective oestrogen receptor modulators (SERMs), tissue-selective oestrogen complexes (TSECs) and tibolone. Use of MHT in glucocorticoid-induced osteoporosis, premature or early menopause (cessation of menses before the age of 40 or 45 years, respectively) and functional hypothalamic amenorrhoea (e.g. anorexia nervosa) is not covered.
MHT and bone
Oestrogen
It is hard to overestimate the importance of oestrogen to the skeleton. The rapid rise in bone mineral density (BMD) from increasing levels of gonadal hormones at puberty is predominantly due to oestrogen, in both sexes. Oestrogen secretion causes growth plate closure and thus determines a person’s final height. Oestrogen is important for osteoblasts, osteoclasts and osteocytes and maintains both cortical and trabecular bone.2 It also mediates the skeleton’s response to mechanical loading via sclerostin suppression.3
Oestrogen deficiency causes rapid bone loss at menopause – with greater than 10% of bone mass at the lumbar spine and greater than 9% at the femoral neck being lost in the decade after menopause, mostly in the year before and two years after the final menstrual period – and contributes to ongoing bone loss thereafter.4
The four major endogenous oestrogens are oestrone, oestradiol, oestriol and oestetrol, with oestradiol being the most biologically active form. There are multiple pharmacological oestrogen formulations available in Australia, including 17-beta oestradiol, combined oestrogens and the synthetic oestrogens ethinylestradiol and mestranol. Conjugated equine oestrogens (CEEs), originally derived from pregnant mares’ urine, contain various oestrogens, with the predominant form being oestrone sulfate, which is metabolised to oestrone and then oestradiol.
Oestrogen binds to nuclear oestrogen receptors (ERs). There are two subtypes (ER-alpha and ER-beta), expressed differently in different tissues, with ER-alpha the predominant ER in cortical bone, breast and endometrium. Upon ligand binding, ERs undergo conformational change and dimerisation, ultimately affecting DNA transcription.2
In the 1970s, the use of oestrogen-alone therapy in women with an intact uterus caused endometrial hyperplasia and increased the risk of endometrial cancer. These complications were almost entirely obviated by the addition of progesterone, particularly if given continuously.5
Use of intravaginal oestrogen has a theoretical risk of endometrial hyperplasia; however, compared with placebo or baseline incidence rates, no increase in endometrial hyperplasia or carcinoma has been observed at 12 months, using endometrial biopsy or ultrasound, respectively.6,7 Data regarding long-term risks are lacking, but progesterone coadministration is generally thought unnecessary.8
Selective oestrogen receptor modulators and tissue-selective oestrogen complexes
SERMs have oestrogenic effects in some tissues (e.g. bone) but oestrogen-antagonistic effects in others (e.g. breast and uterus). Similar to oestrogen, SERMs interact with nuclear ERs. The tissue-specific agonist versus antagonist effects of SERMs depend not only on ER subtype distribution and binding, but also on differing compositions of the ER–ligand complex, its dimerisation and conformational change, and the coactivators and/or corepressors available in any particular cell type.9
Two SERMS are available in Australia: raloxifene and bazedoxifene. Lasofoxifene, another SERM, is currently unavailable. At the endometrium, raloxifene has a neutral effect (as does lasofoxifene), whereas bazedoxifene is antioestrogenic.10 Thus, bazedoxifene can be coadministered with oestrogen without additional endometrial protection.11
TSEC therapy refers to the combination of a SERM plus oestrogen and is available in Australia as bazedoxifene plus CEE.
Tibolone
Tibolone is a biologically inactive synthetic steroid, but its three active metabolites are weak agonists of the ER (e.g. bone and vaginal tissue), progesterone receptor (e.g. endometrium) and androgen receptor.
Progestogens
Progesterone is the major progestogen in humans and is secreted predominantly by the ovaries, affecting the uterus, vagina, cervix, breasts and brain. Progesterone binds to the progesterone receptor (both nuclear and membrane forms) and to other nonaromatised steroid receptors; namely, androgen, glucocorticoid and mineralocorticoid receptors. For example, progesterone antagonises aldosterone at the mineralocorticoid receptor, altering fluid balance during the menstrual cycle.
There are many progestogens available in Australia. Progesterone itself is available orally as micronised progesterone. Synthetic progestogens (collectively termed progestins) include medroxyprogesterone acetate, etonogestrel, levonorgestrel, dydrogesterone, drospirenone and norethisterone. Progestogens are also available in combination with oestrogen, in both oral and transdermal formulations. In addition to binding to the progesterone receptor, different progestogens have varying profiles at the other steroid receptors. Thus, side effects differ between agents.
Androgens
Androgens affect the skeleton indirectly (via aromatisation to oestradiol and the ER) and possibly directly (through the androgen receptor).12 In hypogonadal men, the main mechanism by which testosterone prevents bone loss is through conversion of testosterone to oestradiol.13 A meta-analysis on the safety and efficacy of testosterone in women did not show any effect of testosterone on BMD.14 In women, an independent effect of testosterone on bone remains poorly defined.15
Dehydroepiandrosterone (DHEA) and DHEA-sulfate
Dehydroepiandrosterone (DHEA) is derived predominantly from the adrenal cortex (90%), with only 10% produced by the gonads. DHEA-sulfate (DHEA-S) is produced almost exclusively by the adrenal gland. Both DHEA and DHEA-S can be metabolised to oestrogens and androgens. Their contribution to overall postmenopausal gonadal hormone levels (which are, in any case, low) is not well defined. Whether either has an independent skeletal effect is unknown.
Does MHT improve bone health?
Oestrogen-alone and combined oestrogen–progestogen therapies
Both oestrogen-alone and combined oestrogen–progestogen therapies improve BMD and prevent vertebral and nonvertebral fractures (including hip fractures) in postmenopausal women.
Two randomised controlled trials (RCTs) showed oestrogen improved BMD,16,17 consistent with observational data that it decreased fracture risk. The Postmenopausal Estrogen/Progestin Interventions Trial (PEPI) demonstrated efficacy of MHT in improving BMD. The trial enrolled 875 healthy women aged 45 to 64 years (mean age 56) within 10 years of menopause, who were randomly assigned to receive either placebo or one of four active regimens, CEE 0.625 mg/day alone, or combined with medroxyprogesterone acetate (either cyclically or continuously), or micronised progesterone. Participants assigned to the placebo group lost 1.8% BMD at the lumbar spine and 1.7% at the hip at 36 months, whereas those in the active regimen groups gained BMD at the hip and spine. CEE with continuous medroxyprogesterone acetate use was associated with a significantly greater increase in spinal BMD (5%) compared with the other three active regimens (3.8%).16
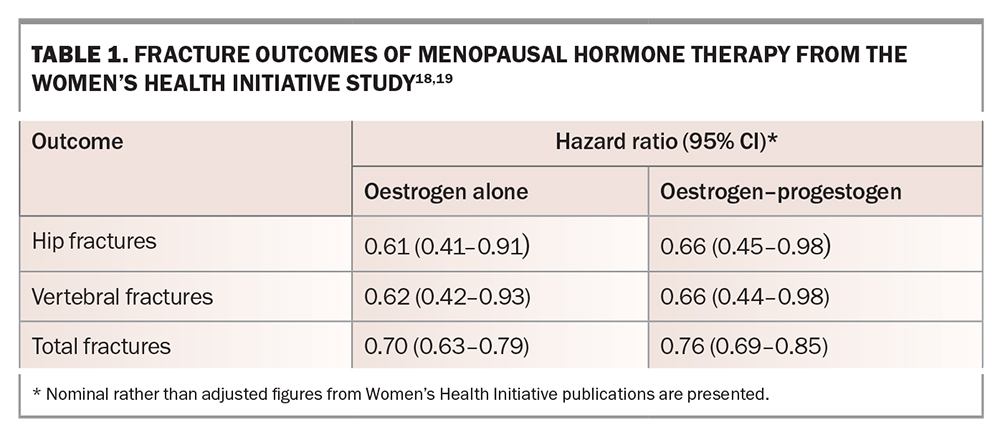
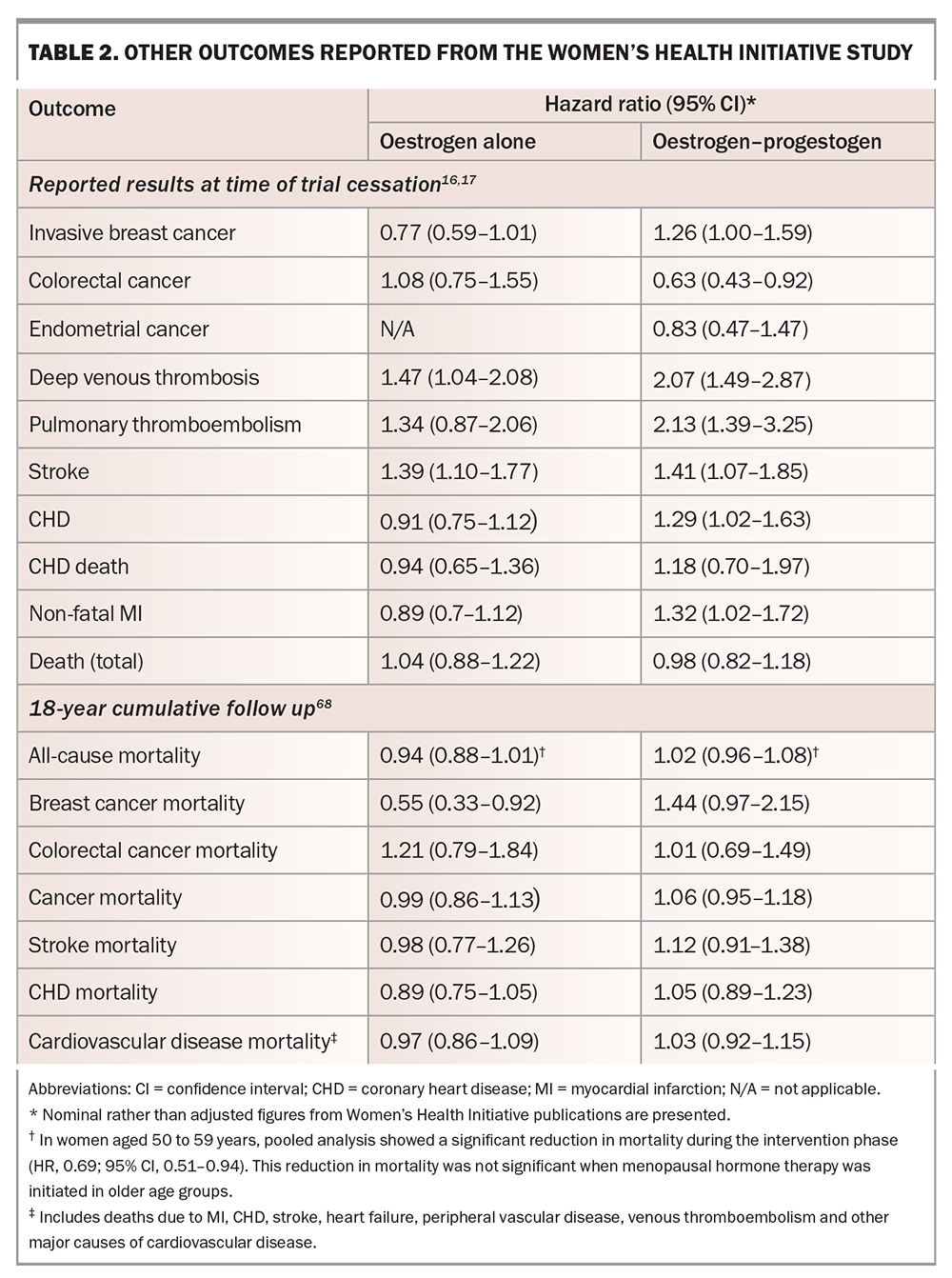
One of the indisputable findings of the Women’s Health Initiative (WHI) study, involving 27,347 women, was that MHT reduced fracture risk. The trial included participants aged 50 to 79 years who were not stratified by baseline BMD and received either CEE 0.625mg/day plus medroxyprogesterone acetate 2.5 mg/day (n=8506) or placebo (n=8102). Compared with placebo, oestrogen alone reduced hip, clinical vertebral and total fractures by 30 to 39%, and oestrogen–progestogen therapy reduced fractures by 24 to 34% (Table 1).18,19
Later analyses of the WHI data found that the positive effect of MHT on bone health persisted after treatment discontinuation for up to five years, although unsurprisingly, the degree of protection decreased over time.20 However, the placebo-controlled Women’s International Study of Long-Duration Oestrogen after Menopause (WISDOM) RCT, evaluating the long-term risks and benefits of MHT in postmenopausal women aged 50 to 69 years, prematurely closed during recruitment after early findings from the WHI study suggested an increased cardiovascular risk associated with stopping MHT. With less than a year’s follow up and only 26% of required recruitment (22,300 planned to ensure adequate power), little can be concluded from this study.21
A 2016 systematic review and meta-analysis of 28 studies involving 33,426 women found fracture risk reduced with MHT use, with a relative risk (RR) of 0.74 for total fractures (95% confidence interval [CI], 0.69–0.80), 0.72 for hip fractures (95% CI, 0.53–0.98) and 0.63 for vertebral fractures (95% CI, 0.44–0.91).22
Does the dose and/or formulation of MHT matter?
Different doses and preparations of MHT may have differing effect sizes on bone health. Data regarding the efficacy of low-dose oestrogens and transdermal oestrogens are scarce. A small 2019 Chinese study of 123 women in early menopause comparing half dose of CEE (0.3 mg) with the standard dose (0.625 mg) combined with progesterone found that, although half dose CEE increased overall BMD and decreased bone turnover markers, standard MHT dose was more efficacious in increasing BMD at the lumbar spine.22 The Million Women Study showed that oral oestrogen was associated with a greater decreased fracture risk than transdermal oestrogen (hazard ration [HR], 0.60; 95% CI, 0.53–0.68 vs HR, 0.76; 95% CI, 0.65–0.86; p=0.04).23 The meta-analysis suggested that oestradiol use was associated with a greater decrease in total fracture risk than CEE (p=0.01).22 Although small trials have shown that intravaginal oestrogen improves BMD, fracture data are lacking.24,25 Intravaginal oestrogen is not recommended for bone health.
MHT may have synergistic effects with calcium and vitamin D on bone health. In the calcium plus vitamin D arm of the WHI study (16,089 participants), women receiving MHT (either oestrogen alone or oestrogen–progestogen) and calcium plus vitamin D had a lower risk of hip fracture compared with women taking calcium plus vitamin D but not MHT (HR, 0.58; 95% CI, 0.37–0.93 vs HR, 1.15; 95% CI, 0.81–1.61; p for interaction, 0.07).26-28 However, within the group of women taking MHT, there is no direct available comparison between those who did and did not receive calcium plus vitamin D.16
Do oestrogen-alone and oestrogen–progestogen therapies benefit all postmenopausal women or only those at highest risk of fracture?
The WHI study population was unusually healthy from a bone perspective; hip fracture rates in the placebo group were about 50% lower than expected for an age-matched cohort.21 Nonetheless, a reduction in all fracture types was observed in both study arms. BMD was only measured in 5.7% of participants, and this subgroup was not thought to be representative of the cohort overall. Thus, the capacity of the WHI to assess who best to target for fracture prevention (women with low BMD and osteoporosis or osteopenia, women with other clinical risk factors for fracture, or all postmenopausal women) is limited.
In the oestrogen-alone arm, ‘the effect of CEE on hip and total fractures was remarkably consistent, almost irrespective of individual characteristics’.29 A summary fracture risk score (including age, current smoking status, body mass index and previous fracture, but not BMD) was calculated. There was significant interaction between this score and total fracture risk reduction, such that those with the highest fracture risk score had the greatest reduction in total fracture risk (high risk: HR, 0.66; moderate risk: HR, 0.68; lowest risk: HR, 0.86; p for interaction, 0.04). A similar but nonsignificant trend was observed for risk of hip fracture specifically. Total hip BMD was measured at baseline in 938 women: 5.7% had a T-score less than –2.5 and 38.7% had a T-score between –1 and –2.5. Within this small group, total fracture risk reduction with CEE was insignificant (49 fractures with CEE vs 64 with placebo; HR, 0.77), and no significant interaction of fracture risk reduction with BMD was observed (osteoporotic group: HR, 0.83; osteopenic group: HR, 0.83; women with normal BMD: HR, 0.99; p for interaction, 0.17).
In the combined oestrogen–progesterone arm, similar but not identical analyses were performed. Reduction in risk of hip fracture was observed regardless of baseline characteristics, with the single exception that reduction in hip fracture risk was only observed in women with a daily calcium intake greater than 1200 mg.30 No interaction was seen between the summary fracture risk score and either hip fracture or total fracture risk reduction. Total hip BMD was measured in 1024 women, and women with a T-score less than –3.0 were excluded from participation. The risk reduction for all fractures in women with a T-score less than –2.5 was 0.53 (with a CI crossing 1; no formal p value was presented), compared with 0.87 in the group with BMD greater than –2.5 (p for interaction of fracture reduction with BMD, 0.15). It is unclear how many women overall had a T-score less than –2.5, and the fracture numbers were small (e.g. 11 fractures with combined oestrogen–progesterone vs 22 with placebo in the low BMD group).30
In 2022, a post hoc combined analysis of 25,389 postmenopausal women aged 50 to 79 years enrolled in the two WHI trials was performed to determine if the antifracture efficacy of MHT differed by baseline falls and fracture risk. Compared with placebo, MHT was associated with a significantly reduced risk of any clinical fracture (HR, 0.72; 95% CI, 0.65–0.78), major osteoporotic fracture (clinical spine, hip, forearm or humerus) (HR, 0.60; 95% CI, 0.53–0.69) and hip fracture (HR, 0.66; 95% CI, 0.45–0.96), regardless of baseline fracture risk and falls risk.31 In extracting meaning from these results, even if the relative risk reduction is the same across the population, the absolute reduction in fractures will be greatest in the group with the highest fracture rate.
SERMs and TSECs
SERMs improve BMD and prevent vertebral fracture. Among 6828 women in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene improved BMD at the femoral neck and spine and reduced vertebral fractures (RR, 0.7; 95% CI, 0.5–0.8, p=0.05).32 However, it had no effect on nonvertebral fractures (RR, 0.9; 95% CI, 0.8–1.1; p=0.24) or hip fractures (RR, 1.1; 95% CI, 0.6–1.9; p=0.71).32 Bazedoxifene has similar efficacy to raloxifene in terms of increased BMD at the hip and spine and vertebral fracture prevention.33 In contrast, lasofoxifene reduces both vertebral and nonvertebral fractures.34 In a 2019 network meta-analysis, both raloxifene (RR, 0.59; 95% CI, 0.46–0.76) and bazedoxifene reduced vertebral fracture (RR, 0.61; 95% CI, 0.41–0.90).35
TSECs may be more effective for osteoporosis prevention than SERMs alone. The Selective Estrogen Menopause and Response to Therapy (SMART-1) trial found greater BMD gains at the lumbar spine and hip with a TSEC (bazedoxifene plus CEE) compared with either raloxifene alone or placebo, although this study was not adequately powered for fracture comparison.36
Tibolone
Tibolone prevents bone loss, increases BMD and prevents fracture.27,37,38 The Long-Term Intervention on Fractures with tibolone (LIFT) study, involving 4538 women, showed that tibolone significantly reduced vertebral fractures at the low dose of 1.25 mg daily (standard dose 2.5 mg daily)(HR, 0.55; 95% CI, 0.41–0.74; p <0.001) and nonvertebral fractures (HR, 0.74; 95% CI, 0.58–0.93; p=0.01) compared with placebo.37 A recent meta-analysis of 107 studies that included tibolone confirmed that tibolone reduced both vertebral (RR, 0.56; 95% CI, 0.36–0.87) and nonvertebral fractures (RR, 0.73; 95% CI, 0.58–0.94) compared with placebo.35
Isolated progestogen
Whether progesterone has a direct skeletal effect is unresolved.39 The isolated use of progestogens (including transdermal preparations from compounding pharmacies) to prevent or treat osteoporosis is of unproven benefit. Supraphysiological doses, as are used in progestin-only contraceptives, are associated with bone loss (through hypogonadotrophic hypogonadism and secondary oestrogen deficiency), although this appears to be reversible, and increased fracture risk has not been reported.40,41 High-dose progestogens may also stimulate glucocorticoid receptors.39 At lower doses, progestogens may act synergistically with oestrogen: combined oestrogen–progestogen increases BMD about 1% more than oestrogen alone.42 This difference is statistically significant, but whether it is clinically meaningful is debatable.
Androgens
Although some multivariate analyses suggest an independent effect of testosterone on BMD and fracture risk in women, parsing unique and independent effects of individual sex steroids is difficult, even in an experimental setting.12 Moreover, testosterone does not consistently improve BMD, even allowing for its aromatisation to oestradiol.43,44 Data regarding fracture outcomes and cardiovascular safety are lacking. Testosterone is not recommended for treating postmenopausal osteoporosis.
DHEA and DHEA-S
In ovariectomised mice, DHEA-S showed some oestrogen-independent effects preventing bone loss.45 The clinical relevance of this finding for humans is unclear. A recent multivariate analysis suggested that DHEA-S levels correlated with BMD in both men and women, but this study did not adjust for other sex hormones, including oestrogen.46 Robust evidence that DHEA or DHEA-S independently improves BMD or reduces fracture risk is lacking.43
How does MHT compare with other options for treating osteoporosis?
Pharmacological management of postmenopausal osteoporosis was presented in a US Endocrine Society clinical practice guideline.47 A meta-analysis of pharmacological therapies for postmenopausal women (107 trials involving 193,987 postmenopausal women) found that MHT (oestrogen alone vs oestrogen–progestogen), bazedoxifene, raloxifene, lasofoxifene, tibolone, bisphosphonates, teriparatide and denosumab are all effective treatments for osteoporosis.
All forms of MHT are effective in preventing vertebral fractures. Oestrogen alone and oestrogen–progestogen are effective for preventing hip fractures, whereas neither SERMs nor tibolone are effective for this outcome.35 Most pivotal trials of bisphosphonates, denosumab and SERMs are in older postmenopausal women; trials in younger postmenopausal women have usually assessed BMD rather than fracture as the primary outcome.48,49
There are few head-to-head studies comparing MHT with other treatments for osteoporosis, whether assessing BMD or fracture, and entry criteria differ considerably between trials. A study in young postmenopausal women aged 45 to 59 years found similar increases in BMD with alendronate versus combined oestrogen–medroxyprogesterone acetate.49 In contrast, a study of older postmenopausal women (aged 65 to 90 years) found greater increases in BMD with alendronate compared with oestrogen (with or without medroxyprogesterone).50 Neither study assessed fracture risk. In considering MHT versus bisphosphonates, bone loss will restart promptly after cessation of MHT, whereas the effect of bisphosphonates may persist after cessation (the duration of persistence varies with different compounds).51
Combination treatment
Trials combining MHT with bisphosphonate therapy have produced contradictory results.50,52,53 A small study of 331 postmenopausal women with osteoporosis compared raloxifene, alendronate and both therapies combined. Combination therapy resulted in greater BMD gains than either medication alone.54 There is no clear role for these combinations at present.
Limited studies combining MHT (including raloxifene) with teriparatide show greater BMD gains.55 No trials have combined MHT with denosumab.
Given its efficacy, why is MHT so controversial?
Two decades ago, oestrogen-alone and combined oestrogen–progestogen therapies were widely used by postmenopausal women. In addition to reducing vasomotor symptoms, observational studies (e.g. Nurses’ Health Study) suggested that MHT was beneficial for cardiovascular health.56 Other studies (e.g. Framingham Study) did not support these findings.57 The WHI study was established specifically to assess the effect of MHT on cardiovascular health, with recruitment deliberately skewed towards older women (almost 70% of participants were aged 60 years or older).
The WHI compared placebo with oestrogen-alone (CEE) in 10,739 women who had undergone hysterectomies and compared placebo with combined oestrogen–progestogen treatment (CEE and medroxyprogesterone acetate) in 16,608 women who had not had hysterectomies.58 Secondary outcomes included breast cancer, colon cancer, stroke, thromboembolism and fracture risk. Both WHI arms were terminated early – the combined therapy arm in 2002, after 5.2 years, and the oestrogen-alone arm in 2004, after 6.6 years. The termination of the oestrogen–progestogen arm was announced in a publication alongside a contemporaneous press release – without prior knowledge of the principal investigators, and before final analyses had been done.59 Subsequent (and final) analyses of the WHI study have not necessarily agreed with the initial publications, particularly their tone.
Around the same time, results of the Million Women Study, an observational study of 1,084,110 women with longitudinal follow up, were also published. This study concluded that invasive breast cancer was increased in women currently using MHT, particularly those using combined MHT, compared with those who had never used it.60
Consequently, MHT prescribing declined drastically worldwide, including in Australia.61 Some studies have since suggested a decrease in breast cancer incidence in women over the age of 50 years, attributed to the decreased use of MHT. For example, a 6.7% decrease in the incidence of breast cancer in Australia was reported in the year after publication of the WHI trial, although this would be inconsistent with the usual timeframe between cancer initiation and clinical presentation.62 Other changes during this time included increased intensity of breast cancer screening regimens in multiple countries (including the United Kingdom and Australia), increased rates of obesity and reduced rates of smoking. Notably, in 2007, there was a 34% increase in breast cancer compared with expected rates projected from 1987, despite widespread abandonment of MHT.63
More recently, there has been increasing recognition that the risks and benefits of MHT are more nuanced than were initially presented and that, for many women, MHT may be both beneficial and low risk.59
WHI and cancer risk
Breast cancer
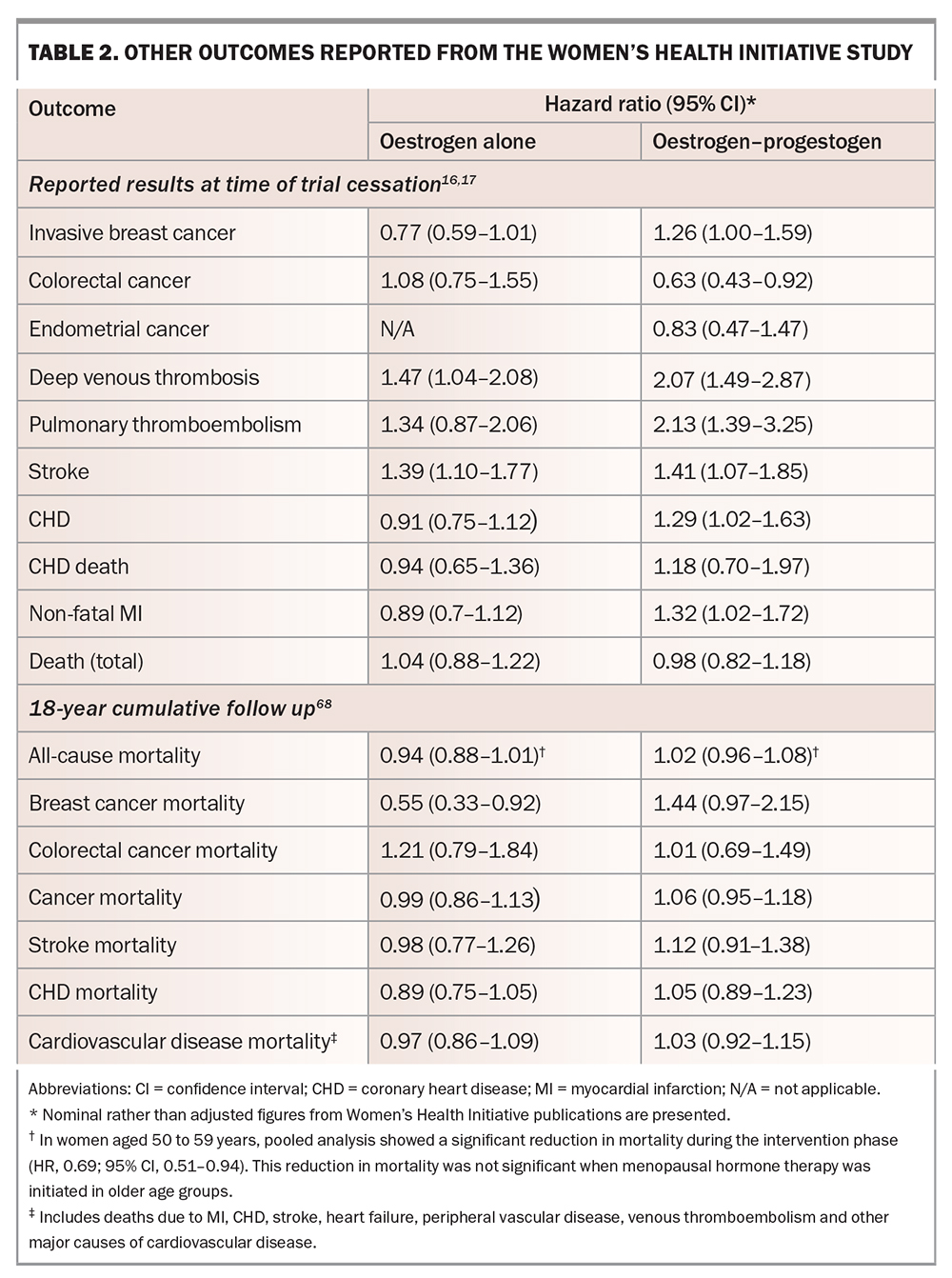
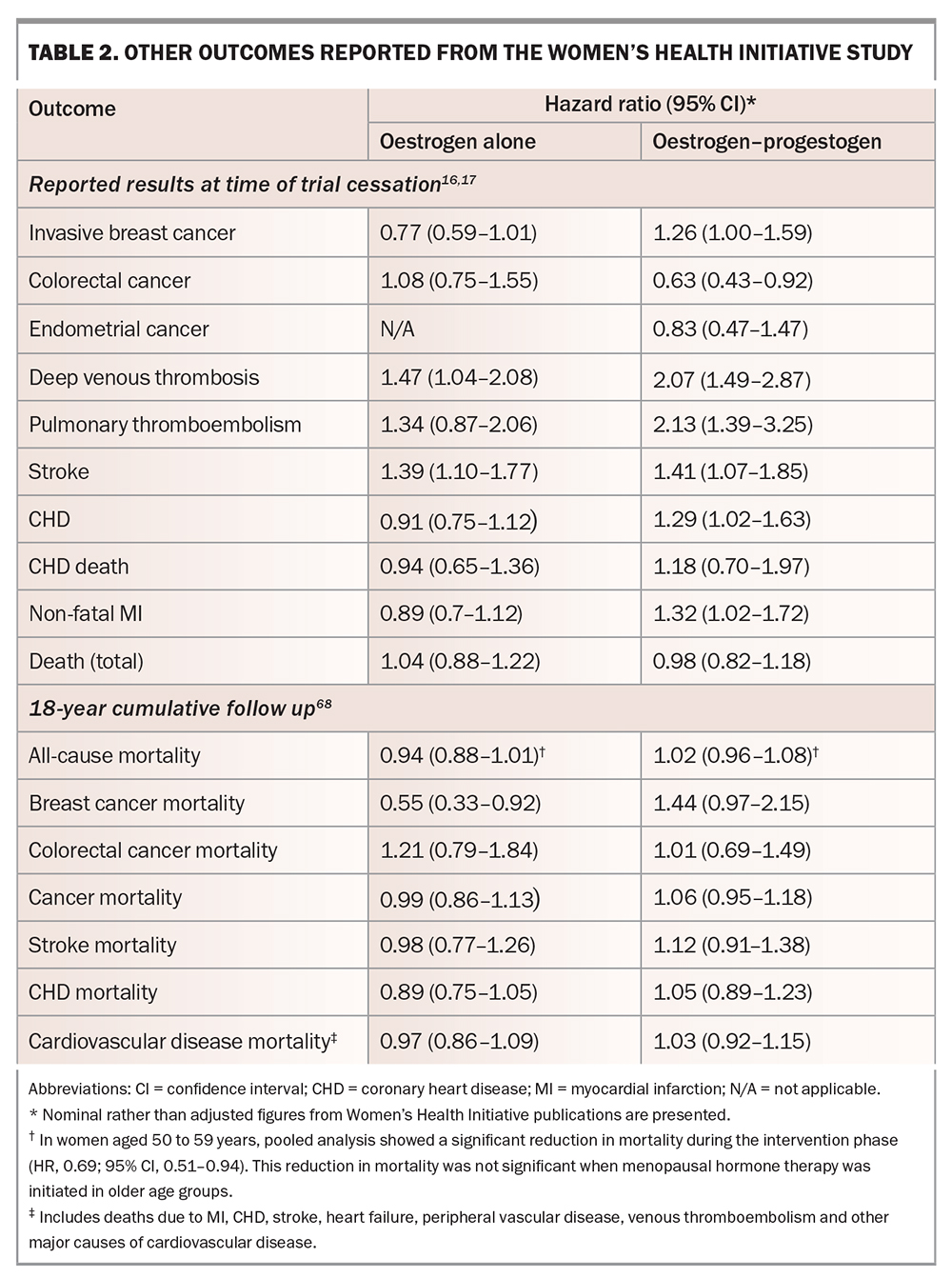
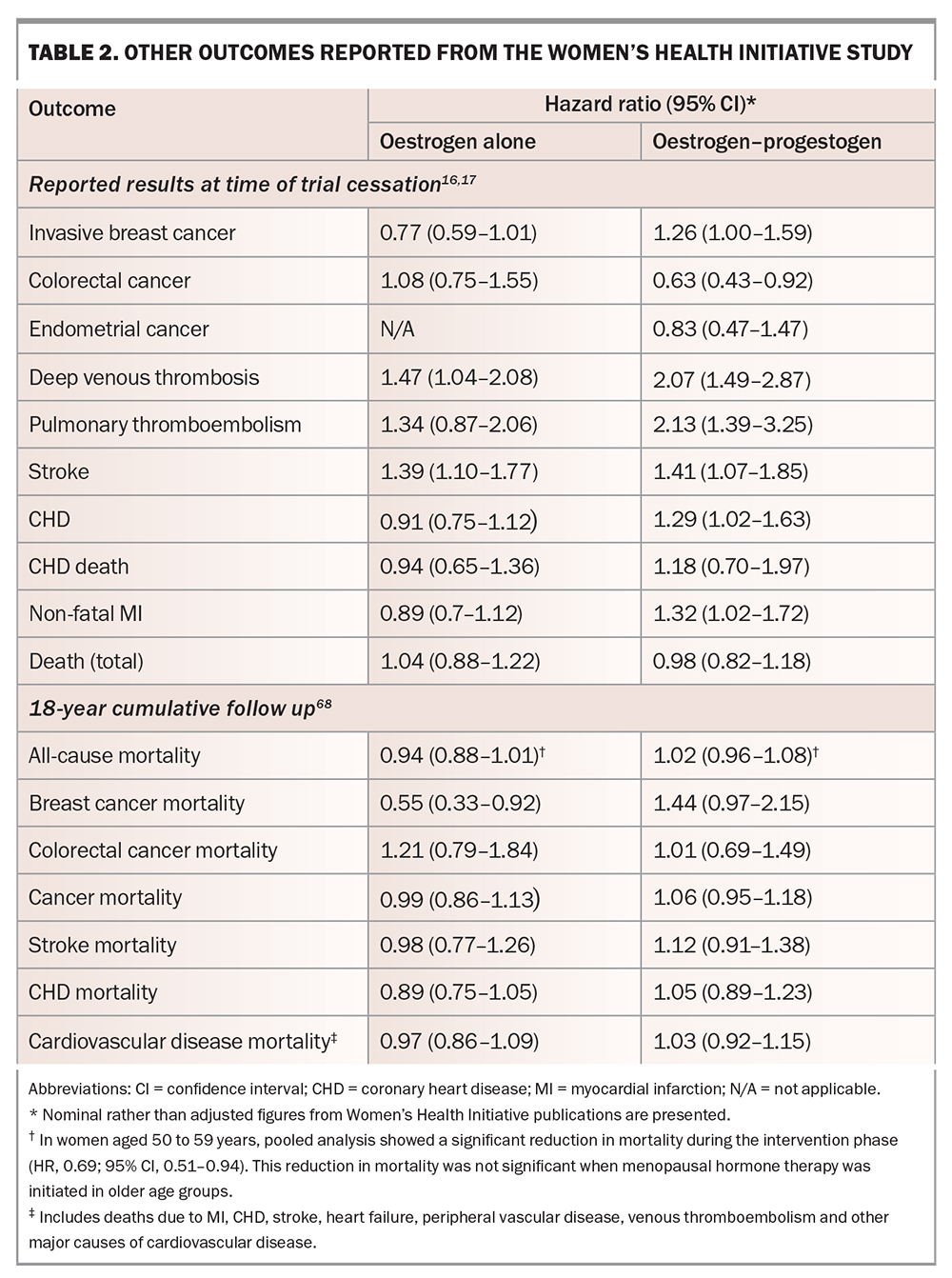
Initial publications and press releases from the WHI study strongly implied that MHT increased the risk of breast cancer. However, the data showed that oestrogen alone did not increase breast cancer, with an absolute risk of seven fewer cases of invasive breast cancer per 10,000 person-years of treatment in the oestrogen-alone arm compared with placebo.18 Combined oestrogen–progestogen slightly increased the risk of invasive breast cancer and all breast cancer, with an absolute risk of nine breast cancer cases per 10,000 person-years of treatment (i.e. less than 0.1% per person-year; Table 2).16,17,19,56 This risk is similar to that seen with postmenopausal obesity and decreased physical activity.64,65 Importantly, extended follow up (18 years, including the intervention phase) found no difference in mortality from breast cancer in the pooled intervention arms compared with placebo.66
Two recent nested case-control studies analysed data from 98,611 women with breast cancer aged 50 to 79 years and 457,498 matched controls, to investigate the association between MHT and breast cancer risk. In these studies, short-term MHT use was defined as use for less than five years; long-term use was defined as use for more than five years. Recent MHT use was defined as a prescription more than one year and less than five years before the index date, whereas past exposure was defined as those who had used MHT more than five years prior to the index date. These studies found that both short-term and long-term use of oestrogenonly and combined oestrogen – progestogen therapies were associated with an increased risk of breast cancer compared with never having used MHT. The odds ratio for oestrogen-only therapy was 1.15 (95% CI, 1.09–1.21) and for combined oestrogen– progestogen therapy was 1.79 (95% CI, 1.73–1.85). The highest risk for combined progestogens was for norethisterone (RR, 1.88; 95% CI 1.79–1.99) and the lowest risk was for dydrogesterone (RR, 1.24; 95% CI, 1.03–1.48). These studies found that both short-term (including current) use of oestrogen-only therapy (OR, 1.15; 95% CI, 1.09–1.21) and combined oestrogen– progestogen therapy (OR, 1.79; 95% CI, 1.73–1.85) were associated with an increased risk of breast cancer compared with never having used MHT. Additionally, past long-term use of combined oestrogen–progestogen therapy was associated with an increased risk (RR, 1.16; 95% CI, 1.11–1.21), wheras past long-term use of oestrogen-only therapy did not show an increased risk. In terms of absolute risk, oestrogen-only users had between three and eight extra cases of breast cancer per 10,000 women-years; combined MHT users had between nine and 36 extra cases per 10,000 woman-years. No excess breast cancer risk was associated with oestrogen creams or vaginal preparations.67
Different results were seen in long-term follow up of two placebo-controlled trials involving 27,347 postmenopausal women aged 50 to 79 years with no history of breast cancer, randomised to receive either CEE plus MPA, CEE alone or placebo. After a median follow up of 20 years, CEE alone in women with a prior hysterectomy (n=10,739) was associated with a lower risk of breast cancer (HR, 0.79; 95% CI, 0.65–0.93; p=0.005) and lower breast cancer mortality (HR, 0.60; 95% CI, 0.37–0.97, p=0.04). In contrast, CEE plus MPA was associated with a higher incidence of breast cancer (HR, 1.28; 95% CI, 1.13–1.45; p<0.001), but without a significant difference in breast cancer mortality.68 These findings are consistent with those of the WHI.56
Endometrial cancer
Women in the oestrogen-alone arm of the WHI study had all undergone hysterectomy.18 There was no difference in endometrial cancer with combined oestrogen–progestogen compared with placebo (Table 2).19
Colorectal cancer
Combined oestrogen–progestogen reduced colorectal cancer compared with placebo.19 No difference was observed with oestrogen alone compared with placebo (Table 2).18
WHI and cardiovascular disease
Coronary heart disease
This was the primary outcome measure of WHI. Early reports of the WHI study described an increased incidence of coronary heart disease (CHD) with combined oestrogen–progestogen compared with placebo, but not with oestrogen alone (Table 2).19 Post hoc analyses suggested a critical 10-year window after menopause: in women aged 50 to 59 years, there was no increase in CHD in either arm and indeed a nonsignificant trend toward cardioprotection, particularly with oestrogen alone, translating to 11 fewer cases of CHD per 10,000 patient-years.56 In contrast, an analysis combining the results of both treatment arms of the WHI study showed that MHT initiated more than 10 years after menopause conveyed no cardiac benefit, with a trend to increased risk.69
These conclusions are supported by the Early versus Late Intervention Trial with Estradiol (ELITE), which found that, compared with placebo, oestrogen (plus vaginal progesterone in women with a uterus) reduced the rate of increase of carotid artery intima–media thickness in women in whom MHT was initiated within six years of menopause, but not in those in whom it was initiated more than 10 years after menopause.70 A Cochrane review also found decreased CHD and lower mortality in women who started MHT within 10 years of menopause.71
A systematic review and meta-analysis of 26 RCTs and 47 observational studies conducted between 2000 and 2019 was conducted to assess the association between MHT and cardiovascular disease. Populations in the RCTs were older than in observational studies (median age, 63.6 years vs 60.1 years, respectively), and with higher comorbidities at baseline. In most of the RCTs, oral MHT was used, whereas some observational studies used transdermal and vaginal oestrogen. Overall, MHT was not associated with all-cause death (summary estimate [SE], 1.00; 95% CI, 0.96–1.04 in RCTs; SE, 0.90; 95% CI; 0.79–1.02 in observational studies) or cardiovascular death (SE, 0.96; 95% CI, 0.83–1.12 in RCTs; SE, 0.81; 95% CI, 0.61–1.07 in observational studies).72 In the pooled results, MHT was not associated with myocardial infarction in RCTs, and was associated with a reduced risk of myocardial infarction in observational studies (SE, 0.79; 95% CI, 0.75–0.84), regardless of regimen, timing of initiation or underlying disease at baseline. In a subgroup analysis of observational studies, a decreased risk of all-cause death was observed in oestrogen-only users (SE, 0.85; 95% CI, 0.77–0.95) and early users after menopause (defined as age under 60 years or initiation within 10 years since menopause) (SE, 0.68; 95% CI, 0.51–0.92).72
Concerningly, stopping MHT may have adverse cardiovascular consequences. Several studies, including the Women’s International Study of Long-Duration Oestrogen after Menopause (WISDOM), were stopped prematurely because of the WHI publications. A Finnish study found that there was an increased risk of cardiac death in women aged under 60 years in the year after MHT discontinuation (standardised mortality ratio [SMR], 1.52; 95% CI, 1.13–2.00 after less than or equal to five years’ exposure; SMR, 2.08; 95% CI, 1.44–2.90 after more than five years’ exposure).73
Pulmonary thromboembolism and deep venous thrombosis
Pulmonary thromboembolism (PTE) and deep venous thrombosis (DVT) are increased with some forms of MHT. In the WHI study, DVT and PTE were increased with combined oestrogen–progestogen, with an absolute risk of eight additional PTEs per 10,000 patient-years and with the greatest risk in the first two years of use.19 With oestrogen alone, only the increase in DVT reached statistical significance; the increase in PTE risk was not significant (Table 2).18 In the systematic review and meta-analysis discussed above, oral oestradiol was associated with increased risk of VTE in both RCTs (SE, 1.70; 95% CI, 1.33–2.16) and observational studies (SE, 1.32; 95% CI, 1.13–1.54), especially in combined oestrogen–progestogen users, late users (more than 10 years after menopause) and women with underlying comorbidities at baseline. PTE risk was also increased with MHT use in both RCTs (SE, 1.26; 95% CI, 1.06–1.50) and observational studies (SE, 1.44; 95% CI, 1.17–1.76).72
Stroke
Stroke was increased in both treatment arms of the WHI study, compared with placebo, amounting to an absolute risk of 12 additional strokes per 10,000 person-years in the oestrogen-alone arm and eight in the combined treatment arm (Table 2). Stroke risk was not affected by time since menopause at time of MHT initiation. Extended follow up has not shown any increase in stroke mortality.66 In a pooled analysis of predominantly oral MHT, MHT was associated with increased risk of stroke in RCTs (SE, 1.14; 95% CI, 1.04–1.25) but not in observational studies (SE, 0.98; 95% CI, 0.85–1.13). In subgroup analyses of RCTs, increased risk of stroke was associated with combined oestrogen-progestogen use, MHT duration greater than five years, late users after menopause, and in those with underlying cerebrovascular disease at baseline. In subgroup analyses of observational studies, decreased stroke risk was observed in women administered nonoral MHT (SE, 0.86; 95% CI, 1.04–1.18).72
Does the type of oestrogen or oestrogen–progestogen matter for CHD risk?
Oral oestrogens increase clotting factors and inflammatory markers, an effect not seen with transdermal oestrogen, which bypasses first-pass hepatic metabolism.74,75 Observational studies have suggested reduced risks of cardiovascular disease (CVD), stroke and PTE with transdermal compared with oral oestrogen preparations.76-79 However, this has not been assessed in head-to-head studies.
The promiscuity of progestogen binding to multiple receptor types, with differing affinity and action (agonist vs antagonist effects), can result in quite different risk profiles for different progestogens, which may include CVD risk. However, this is conjectural, and the contribution of progesterone to thrombogenesis is unknown.
It is important to note that the WHI study used oral CEE and medroxyprogesterone acetate, and most women were more than 10 years post menopause, with an average age of 63 years. This does not reflect current prescribing practices which includes lower-dose oestrogens, transdermal oestrogens and newer progestogens including localised delivery (e.g. placement of a hormonal intrauterine device), with prescribing predominately in younger women.80
Additional side effects of oestrogen–progesterone
A recent analysis of pooled data from 20 trials (n=39,145) and three cohort studies (n=1,155,410) assessed the effects of oestrogen-only and combined oestrogen–progestogen therapies. Oestrogen-only therapy was associated with lower rates of diabetes (134 per 10,000 persons) and fractures (388 fewer per 10,000 persons) compared with placebo, but higher rates of gallbladder disease (377 per 10,000 persons), stroke (79 per 10,000 persons), venous thromboembolism (VTE; 77 per 10,000 persons) and urinary incontinence (885 per 10,000 persons).81 Combined oestrogen–progestogen was associated with lower risk of colorectal cancer (34 per 10,000 persons), diabetes (78 per 10,000 persons) and fractures (230 per 10,000 persons), but increased risks for invasive breast cancer (51 per 10,000 persons), gallbladder disease (260 per 10,000 persons), stroke (52 per 10,000 persons) and VTE (120 per 10,000 persons). Additionally, combined oestrogen–progestogen therapy was associated with a probable increased risk of dementia (88 per 10,000 persons) and urinary incontinence.82 However, there is conflicting evidence regarding the impact of MHT use on dementia risk. Specifically, among women with the APOE gene (associated with Alzheimer’s disease risk) in the European Prevention of Alzheimer’s Disease (EPAD) cohort, those using MHT showed improvement in delayed memory stores and increased hippocampal volumes compared with non-users.82
Side effects of SERMs, TSECs and tibolone
Both raloxifene and lasofoxifene reduce the risk of breast cancer.32,34 Preclinical evidence suggests bazedoxifene may also reduce risk of breast cancer.83,84
SERMs increase risk of thromboembolism (both DVT and PTE).32,34,85 An increased risk of thromboembolic disease has not been reported with tibolone.37
Raloxifene increases the risk of fatal but not overall stroke (HR, 1.49; absolute risk of seven more cases per 10,000 women).86 In contrast, lasofoxifene reduces the risk of stroke.34 Tibolone is associated with increased stroke risk in older women.37
Overall, raloxifene does not reduce coronary artery disease, although the Raloxifene Use for The Heart (RUTH) study (in women with established CHD or risk factors for CHD) suggested a protective effect in women aged under 60 years (p for interaction of age with coronary artery disease: 0.01).32,87 Lasofoxifene reduces CHD at higher (0.5 mg) but not lower (0.25 mg) daily doses.34
SERMs may worsen vasomotor symptoms, whereas TSECs may reduce them. Tibolone is less effective than oestrogen or oestrogen–progestogen for vasomotor symptoms.88
Clinical indications for MHT use for bone health
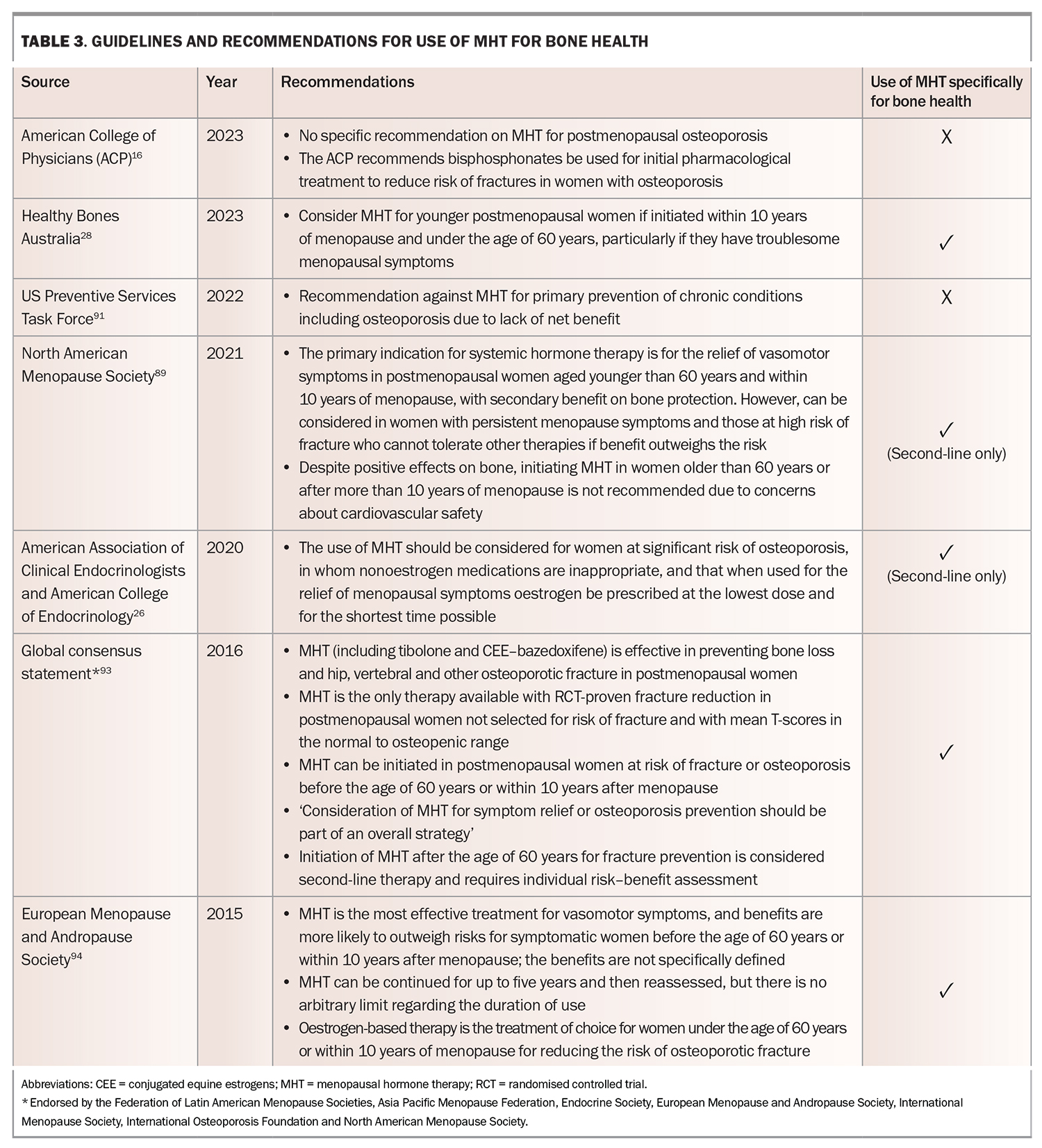
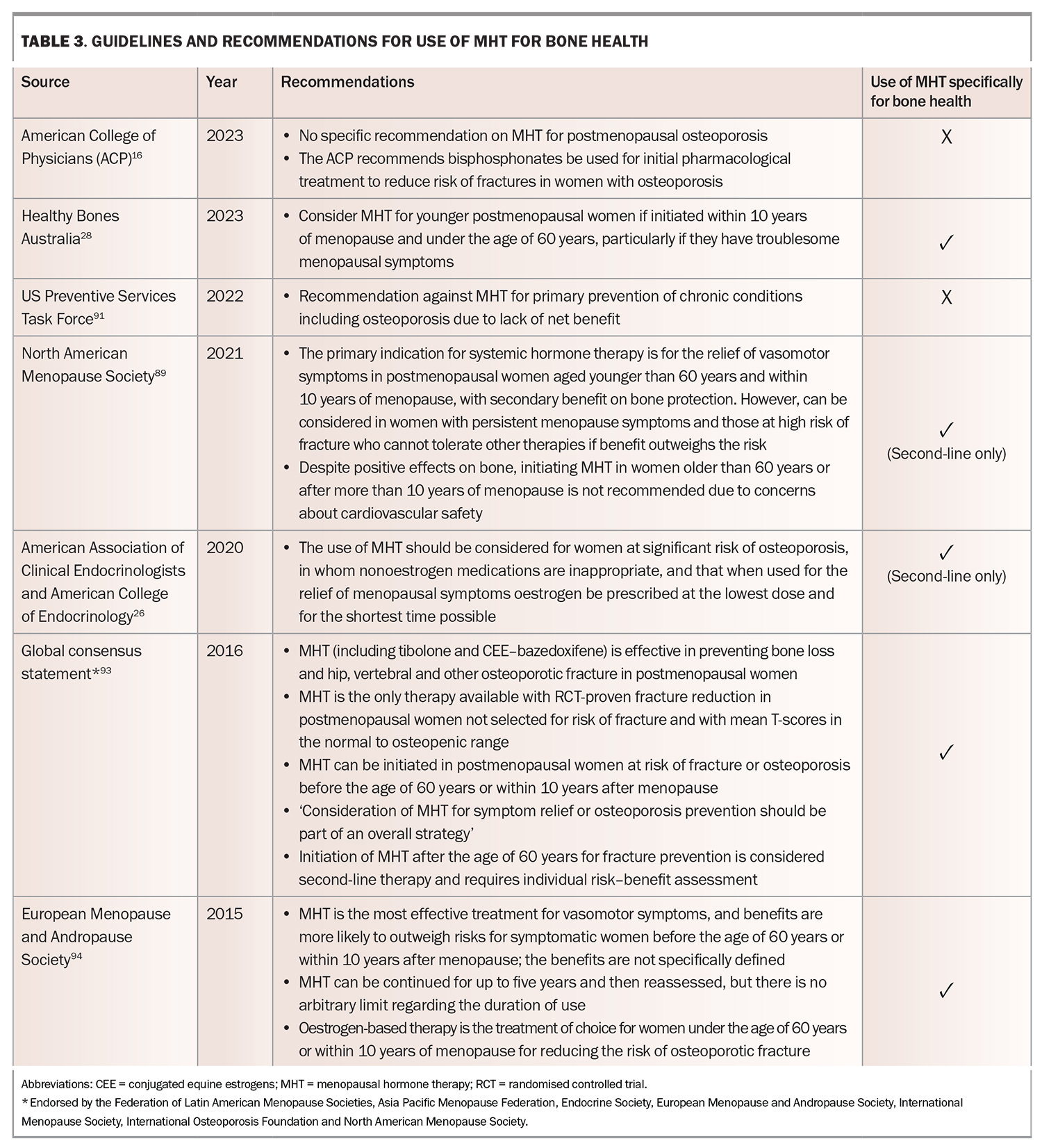
There are many guidelines regarding use of MHT.89-91 Generally, there is concordance that MHT is appropriate and effective for vasomotor symptoms, assuming no contraindications.92 There is much less consensus regarding the use of MHT primarily for prevention or treatment of osteoporosis, particularly as a first-line agent, and questions regarding age of initiation, duration and monitoring of MHT are largely unanswered. Updated recommendations and guidelines for MHT use for bone health are summarised in Table 3.26,28,89-91,93,94
Recommendations for MHT use: update since 2019
Recommendations on MHT use for women with postmenopausal osteoporosis continue to vary among organisations. The 2022 US Preventative Services Task Force (USPSTF) guidelines still currently recommend against combined oestrogen–progestogen, or oestrogen alone (in postmenopausal women, for the prevention of chronic conditions, including osteoporosis), concluding a lack of net benefit.91 The American Association of Clinical Endocrinologists/American College of Endocrinology Practice Guideline 2020 update also still recommends that MHT only be considered for women who are at significant risk of osteoporosis and in whom nonoestrogen medications are inappropriate, and that if used for relief of menopausal symptoms, oestrogen be prescribed at the lowest dose and for the shortest time possible.26
However, the 2022 Hormone Therapy Position Statement of the North American Menopause Society recommends risk stratification by age and time since menopause, and consider that the benefits of MHT outweigh the risks in most healthy women with vasomotor or genitourinary symptoms younger than 60 years and within 10 years of the onset of menopause for the prevention of bone loss.89 Similarly, a revised Global Consensus Statement on Menopausal Hormone Therapy, endorsed by several international organisations, including the International Menopause Society, The North American Menopause Society, the Endocrine Society and The International Osteoporosis Foundation, recommends MHT for the prevention of bone loss in most healthy symptomatic women younger than 60 years and within 10 years of menopause onset.93 Healthy Bones Australia 2023 guidelines suggest MHT as an option for young postmenopausal women, if initiated within 10 years of menopause or under 60 years of age, particularly for women with troublesome menopausal symptoms.28
Monitoring during MHT use for osteoporosis
There are no specific monitoring requirements for MHT used for bone health (e.g. BMD follow up), as opposed to general comments regarding MHT use for treatment of menopausal symptoms. Local guidelines should be consulted.
MHT contraindications and adverse side effects
The North American Menopause Society 2022 position statement recommends the following as contraindications to oral and transdermal MHT: unexplained vaginal bleeding, liver disease, prior oestrogen-sensitive (including breast) cancer, CHD, stroke, myocardial infarction, VTE and personal history or inherited risk of VTE. Observational studies have not demonstrated an increased risk of thromboembolic risk with transdermal oestrogen (see below) and some practitioners consider transdermal therapy can be used with caution in women with a past history of stroke or transient ischaemic attack, myocardial infarction, gall bladder disease and hypertriglyceridaemia.95 Adverse effects that should be discussed with women considering MHT are nausea, bloating, weight gain, fluid retention, mood swings, breakthrough bleeding, worsening migraines, leiomyoma growth and exacerbation of endometriosis.89 Furthermore, modern guidelines do not recommend placing an upper limit on the duration of MHT use, particularly where there is ongoing benefit.89,94,96
Cessation of MHT
After cessation of MHT, cardiovascular risk will increase; this is often forgotten. Rapid bone loss will also ensue after cessation, as is observed after natural menopause. Alternative osteoporosis-specific treatment should be offered to women at high risk of fracture.
Conclusion
Use of MHT is justified in its own right for treating distressing vasomotor symptoms (discussed in multiple societal guidelines listed in Table 3).
MHT improves BMD and reduces fracture risk in women across the BMD spectrum, as seen in the WHI trial. However, the absolute benefit of treatment will be greater in women at higher risk of fracture (e.g. BMD T-score less than–2.5, or previous fracture). The use of MHT for the sole indication of primary prevention of osteoporosis in all postmenopausal women, irrespective of BMD, seems excessive. However, shutting the stable door before the proverbial horse has bolted is a reasonable concern for many women.
MHT is a valid option for younger postmenopausal women within 10 years of cessation of menses who have osteopenia or osteoporosis by BMD criteria, or previous minimal trauma fracture, and without specific contraindications. However, it should be noted that women with previous fracture are eligible for other agents to treat osteoporosis (albeit with their own long-term side effects) and that absolute fracture risk at this young age, even in women with low BMD, is low.
Notwithstanding the above, the influence of the WHI study is such that many women are fearful of MHT due to continuing overemphasis of the risks, and many doctors are reluctant to prescribe it. Ongoing education of the wider medical community regarding the limitations of the WHI study will enable practitioners to prescribe MHT with more confidence. As with any medication, prescribing MHT requires an informed discussion about risks versus benefits. These vary from woman to woman and include age, time since menopause, other risk factors for fracture (e.g. previous fracture) and other risk factors for CVD (e.g. smoking). The choice of MHT preparation should be tailored to the individual patient’s clinical profile and preference. Transdermal therapies confer a lower risk of CVD than oral preparations.
Optimal duration of MHT is unclear and modern guidelines do not recommend an upper limit on the duration of use. Whether the adverse consequences of treatment observed in women initiating MHT at an older age apply to women who start MHT in the immediate postmenopausal period and continue use to this same age is unknown. As a rule of thumb, many endocrinologists would consider initiating, and continuing, MHT until the age of 60 years. The decision to cease MHT is an individual one, and many women may choose to continue treatment beyond this age for various reasons, including improved quality of life. After cessation of MHT, management of cardiovascular risk and fracture risk will need re-evaluation. MT
COMPETING INTERESTS: None
References
1. Yong E. Why killer whales go through menopause but elephants don’t. National Geographic 2015; 5 Mar. Available online at: www.nationalgeographic.com/science/phenomena/2015/03/05/why-killer-whales-go-through-menopause-but-elephants-dont/ (accessed May 2023).
2. Almeida M, Laurent MR, Dubois V, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev 2017; 97: 135-187.
3. Galea GL, Meakin LB, Sugiyama T, et al. Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor beta. J Biol Chem 2013; 288: 9035-9048.
4. Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res 2012; 27: 111-118.
5. Judd MD, Mebane-Sims I, Legault C, et al; Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1996; 275: 370-375.
6. Simon J, Nachtigall L, Ulrich LG, Eugster-Hausmann M, Gut R. Endometrial safety of ultra-low-dose estradiol vaginal tablets. Obstet Gynecol 2010; 116: 876-883.
7. Naessen T, Rodriguez-Macias K. Endometrial thickness and uterine diameter not affected by ultralow doses of 17beta-estradiol in elderly women. Am J Obstet Gynecol 2002; 186: 944-947.
8. NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017; 24: 728-753.
9. Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 2013; 8: 135-155.
10. Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol 2005; 105: 1397-1404.
11. Mirkin S, Pinkerton JV, Kagan R, et al. Gynecologic safety of conjugated estrogens plus bazedoxifene: pooled analysis of five phase 3 trials. J Womens Health (Larchmt) 2016; 25: 431-442..
12. Cauley JA, Danielson ME, Jammy GR, et al. Sex steroid hormones and fracture in a multiethnic cohort of women: The Women’s Health Initiative Study (WHI). J Clin Endocrinol Metab 2017; 102: 1538-1547.
13. Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 2000; 106: 1553-1560.
14. Islam RM, Bell RJ, Green S, Page MJ, Davis SR. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol 2019; 7: 7547-66.
15. Clarke BL, Khosla S. Androgens and bone. Steroids 2009; 74: 296-305.
16. Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. The Writing Group for the PEPI. JAMA 1996; 276: 1389-1396.
17. Genant HK, Lucas J, Weiss S, et al. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med 1997; 157: 2609-2615.
18. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004; 291: 1701-1712.
19. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288: 321-333.
20. Watts NB, Cauley JA, Jackson RD, et al. No increase in fractures after stopping hormone therapy: results from the Women’s Health Initiative. J Clin Endocrinol Metab 2017; 102: 302-308.
21. Vickers MR, MacLennan AH, Lawton B, et al. Main morbidities recorded in the women’s international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ 2007; 335: 239.
22. Zhu L, Jiang X, Sun Y, Shu W. Effect of hormone therapy on the risk of bone fractures: a systematic review and meta-analysis of randomized controlled trials. Menopause 2016; 23: 461-470.
23. Banks E, Beral V, Reeves G, Balkwill A, Barnes I, Million Women Study C. Fracture incidence in relation to the pattern of use of hormone therapy in postmenopausal women. JAMA 2004; 291: 2212-2220.
24. Salminen HS, Saaf ME, Johansson SE, Ringertz H, Strender LE. The effect of transvaginal estradiol on bone in aged women: a randomised controlled trial. Maturitas 2007; 57: 370-381.
25. Naessen T, Berglund L, Ulmsten U. Bone loss in elderly women prevented by ultralow doses of parenteral 17-estradiol. Am J Obstet Gynecol 1997; 177: 115-119.
26. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis- 2020 Update Executive Summary. Endocr Pract 2020; 26: 564-570.
27. Pavlov PW, Ginsburg J, Kicovic PM, van der Schaaf DB, Prelevic G, Bennink HJ. Double-blind, placebo-controlled study of the effects of tibolone on bone mineral density in postmenopausal osteoporotic women with and without previous fractures. Gynecol Endocrinol 1999; 13: 230-237.
28. Ebeling PR, Seeman E, Center J, et al. Position Statement on the Management of Osteoporosis. Healthy Bones Australia. 2022. Available online at: https://healthybonesaustralia.org.au/wp-content/uploads/2023/01/position-statement-on-osteoporosis-dec-2022.pdf (accessed May 2023).
29. Zhu SY, Deng Y, Wang YF, Xue W, Ma X, Sun A. Bone protection for early menopausal women in China: standard or half-dose estrogen with progestin? A one-year prospective randomized trail. Gynecol Endocrinol 2019; 35: 165-169.
30. Jackson RD, Wactawski-Wende J, LaCroix AZ, et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the Women’s Health Initiative randomized trial. J Bone Miner Res 2006; 21: 817-828.
31. Lorentzon M, Johansson H, Harvey NC, et al. Menopausal hormone therapy reduces the risk of fracture regardless of falls risk or baseline FRAX probability-results from the Women’s Health Initiative hormone therapy trials. Osteoporos Int 2022; 33: 2297-2305.
32. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282: 637-645.
33. Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 2008; 23: 525-235.
34. Cummings SR, Ensrud K, Delmas PD, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med 2010; 362: 686-696.
35. Barrionuevo P, Kapoor E, Asi N, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab 2019; 104: 1623-1630.
36. Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 2009; 92: 1045-1052.
37. Cummings SR, Ettinger B, Delmas PD, et al. The effects of tibolone in older postmenopausal women. N Engl J Med 2008; 359: 697-708.
38. Ederveen AG, Kloosterboer HJ. Tibolone exerts its protective effect on trabecular bone loss through the estrogen receptor. J Bone Miner Res 2001; 16: 1651-1657.
39. Prior JC. Progesterone for the prevention and treatment of osteoporosis in women. Climacteric 2018; 21: 366-374.
40. Guilbert ER, Brown JP, Kaunitz AM, et al. The use of depot-medroxyprogesterone acetate in contraception and its potential impact on skeletal health. Contraception 2009; 79: 167-177.
41. Green W. The FDA, contraceptive marketing approval and products liability litigation: Depo-Provera and the risk of osteoporosis. Food Drug Law J 2013; 68: 115-135, i.
42. Prior JC, Seifert-Klauss VR, Giustini D, Adachi JD, Kalyan S, Goshtasebi A. Estrogen-progestin therapy causes a greater increase in spinal bone mineral density than estrogen therapy - a systematic review and meta-analysis of controlled trials with direct randomization. J Musculoskelet Neuronal Interact 2017; 17: 146-154.
43. Elraiyah T, Sonbol MB, Wang Z, et al. Clinical review: The benefits and harms of systemic testosterone therapy in postmenopausal women with normal adrenal function: a systematic review and meta-analysis. J Clin Endocrinol Metab 2014; 99: 3543-3550.
44. Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev 2005; (4): CD004509.
45. Zhang N, Gui Y, Qiu X, et al. DHEA prevents bone loss by suppressing the expansion of CD4(+) T cells and TNFa production in the OVX-mouse model for postmenopausal osteoporosis. Biosci Trends 2016; 10: 277-287.
46. Park SG, Hwang S, Kim JS, Park KC, Kwon Y, Kim KC. The association between dehydroepiandrosterone sulfate (DHEA-S) and bone mineral density in Korean men and women. J Bone Metab 2017; 24: 31-36.
47. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2019; 104: 1595-1622.
48. Hosking D, Chilvers CE, Christiansen C, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med 1998; 338: 485-492.
49. Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med 1999; 131: 935-942.
50. Greenspan SL, Beck TJ, Resnick NM, Bhattacharya R, Parker RA. Effect of hormone replacement, alendronate, or combination therapy on hip structural geometry: a 3-year, double-blind, placebo-controlled clinical trial. J Bone Miner Res 2005; 20: 1525-1532.
51. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2002; 137: 875-883.
52. Yoon BK, Lee DY, Park MC, Cho SH, Park HM, Choi YM. Effects of combination therapy of alendronate and hormonal therapy on bone mineral density in postmenopausal Korean women: multicenter, randomized controlled clinical trial. J Korean Med Sci 2017; 32: 992-998.
53. Evio S, Tiitinen A, Laitinen K, Ylikorkala O, Valimaki MJ. Effects of alendronate and hormone replacement therapy, alone and in combination, on bone mass and markers of bone turnover in elderly women with osteoporosis. J Clin Endocrinol Metab 2004; 89: 626-631.
54. Johnell O, Scheele WH, Lu Y, Reginster JY, Need AG, Seeman E. Additive effects of raloxifene and alendronate on bone density and biochemical markers of bone remodeling in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2002; 87: 985-992.
55. Song J, Jin Z, Chang F, Li L, Su Y. Single and combined use of human parathyroid hormone (PTH) (1-34) on areal bone mineral density (aBMD) in postmenopausal women with osteoporosis: evidence based on 9 RCTs. Med Sci Monit 2014; 20: 2624-2632.
56. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013; 310: 1353-1368.
57. Wilson PW, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. The Framingham Study. N Engl J Med 1985; 313: 1038-1043.
58. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol 2003; 13 (9 Suppl): S5-S17.
50. Langer RD. The evidence base for HRT: what can we believe? Climacteric 2017; 20: 91-96.
60. Beral V, Million Women Study C. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003; 362: 419-427.
61. Main P, Robinson M. Changes in utilisation of hormone replacement therapy in Australia following publication of the findings of the Women’s Health Initiative. Pharmacoepidemiol Drug Saf 2008; 17: 861-868.
62. Canfell K, Banks E, Moa AM, Beral V. Decrease in breast cancer incidence following a rapid fall in use of hormone replacement therapy in Australia. Med J Aust 2008; 188: 641-644.
63. Sitas F, Gibberd A, Kahn C, et al. Cancer incidence and mortality in people aged less than 75 years: changes in Australia over the period 1987-2007. Cancer Epidemiol 2013; 37: 780-787.
64. Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007; 335: 1134.
65. Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med 2008; 42: 636-647.
66. Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA 2017; 318: 927-938.
67. Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ 2020; 371: m3873.
68. Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA 2020; 324: 369-380.
69. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297: 1465-1477.
70. Hodis HN, Mack WJ, Henderson VW, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 2016; 374: 1221-1231.
71. Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev 2015; 2015(3): CD002229.
72. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep 2020; 10: 20631.
73. Venetkoski M, Savolainen-Peltonen H, Rahkola-Soisalo P, et al. Increased cardiac and stroke death risk in the first year after discontinuation of postmenopausal hormone therapy. Menopause 2018; 25: 375-379.
74. Vehkavaara S, Silveira A, Hakala-Ala-Pietila T, et al. Effects of oral and transdermal estrogen replacement therapy on markers of coagulation, fibrinolysis, inflammation and serum lipids and lipoproteins in postmenopausal women. Thromb Haemost 2001; 85: 619-625.
75. Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol 1997; 17: 3071-3078.
76. Simon JA, Laliberte F, Duh MS, et al. Venous thromboembolism and cardiovascular disease complications in menopausal women using transdermal versus oral estrogen therapy. Menopause 2016; 23: 600-610.
77. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ 2010; 340: c2519.
78. Laliberte F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause 2018; 25: 1297-1305.
79. Bezwada P, Shaikh A, Misra D. The effect of transdermal estrogen patch use on cardiovascular outcomes: a systematic review. J Womens Health 2017; 26: 1319-1325.
80. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant metaanalysis of the worldwide epidemiological evidence. Lancet 2019; 394: 1159-1168.
81. Gartlehner G, Patel SV, Reddy S, Rains C, Schwimmer M, Kahwati L. Hormone therapy for the primary prevention of chronic conditions in postmenopausal persons: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2022; 328: 1747-1765.
82. Saleh RNM HM, Ritchie CW, Minihane AM. Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimers Res Ther 2023; 15: 10.
83. Pickar JH, Boucher M, Morgenstern D. Tissue selective estrogen complex (TSEC): a review. Menopause 2018; 25: 1033-1045.
84. Tian J, Chen X, Fu S, et al. Bazedoxifene is a novel IL-6/GP130 inhibitor for treating triple-negative breast cancer. Breast Cancer Res Treat 2019; 175: 553-566.
85. Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 2008; 23: 1923-1934.
86. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355: 125-137.
87. Collins P, Mosca L, Geiger MJ, et al. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the Raloxifene Use for The Heart trial: results of subgroup analyses by age and other factors. Circulation 2009; 119: 922-930.
88. Formoso G, Perrone E, Maltoni S, et al. Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database Syst Rev 2016; 10(10): CD008536.
89. Management of Osteoporosis in Postmenopausal Women: The Position Statement of The North American Menopause Society’’ Editorial Panel. Management of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. Menopause 2021; 28: 973-997.
90. Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med 2023; 176: 224-238.
91. Mangione CM, Barry MJ, Nicholson WKet al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal persons: US Preventive Services Task Force Recommendation Statement. JAMA 2022; 328: 1740-1746.
92. Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev 2017; 1(1): CD004143.
93. de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Maturitas 2016; 91: 153-155.
94. Neves ECM, Birkhauser M, Samsioe G, et al. EMAS position statement: the ten point guide to the integral management of menopausal health. Maturitas 2015; 81: 88-92.
95. Davis SR, Baber RJ. Treating menopause - MHT and beyond. Nat Rev Endocrinol 2022; 18: 490-502.
96. Hamoda H, Davis SR, Cano A, et al. BMS, IMS, EMAS, RCOG and AMS joint statement on menopausal hormone therapy and breast cancer risk in response to EMA Pharmacovigilance Risk Assessment Committee recommendations in May 2020. Post Reprod Health 2021; 27: 49-55.