Atopic dermatitis – an update on management in general practice
Atopic dermatitis is a common, chronic and often debilitating inflammatory skin disorder with a large burden of its diagnosis and management falling within primary care. New advancements and updates to management are significantly improving the outcomes for patients with this condition.
Correction
A correction for this article was published in the May 2022 issue of Medicine Today. The online version and the full text PDF of this article (see link above) have been corrected.
- Atopic dermatitis is a common condition and is largely managed in the primary care setting.
- Understanding and quantifying the impact of the condition on quality of life is important when tailoring therapy for patients.
- First-line therapy involves robust education around general measures and commencement of appropriate topical corticosteroid therapy.
- Should patients fail to respond to optimised general measures and topical corticosteroids after one month, they should be referred to a dermatologist.
- Dupilumab and upadacitinib are new advanced therapies that have profound benefits for patients with severe atopic dermatitis and have recently been added to the Pharmaceutical Benefits Scheme.
Atopic dermatitis is a common, chronic and often debilitating inflammatory skin disorder characterised by pruritis, erythema and excoriations. It affects between 15 and 20% of the Australian population, with the burden steadily increasing.1 The highest incidence is associated with infancy and early childhood, with 20% of this age population being affected at some point. However, there is also a substantial prevalence of around 6% in adulthood.1
Atopic dermatitis is closely associated with other atopic diseases, including asthma, food allergies and rhinosinusitis. Its pathogenesis is complex and multifactorial, involving epidermal barrier abnormalities and immune dysregulation in genetically susceptible individuals, as well as environmental triggers, such as heat, infection and potential allergies.2
Atopic dermatitis has a profound impact on quality of life, with associated mood and sleep disturbance.3-5 Traditional treatment regimens are centred on symptomatic control through general measures, topical agents, light therapy and other immunosuppressants. Inadequate response to treatment and potential toxicity has previously limited treatment benefit.6 The recent addition of advanced therapies, monoclonal antibodies and Janus kinase (JAK) inhibitors to the Australian Pharmaceutical Benefits Scheme (PBS) is significantly shaping the management of atopic dermatitis and improving the long-term outcomes for patients with severe disease.
Atopic dermatitis is consistently identified as the most frequent new dermatological consultation in general practice, and most patients with mild disease are managed in primary care.7 This article provides a framework for the management of this highly prevalent condition, including new advances in therapy.
Pathogenesis
The pathogenesis of atopic dermatitis is complex, led by dysregulation of innate and adaptive immunity, as well as genetic risk factors and environmental triggers. It is driven by type 2 helper (Th2) cell inflammation, the key cytokines involved in this being interleukin (IL) 4 and IL-13.2 Poor skin barrier function is associated with mutations in the epidermal protein filaggrin.2 This predisposes patients to infection with micro-organisms, in particular bacteria and viruses, as there is greater penetration of these through the skin.8 Environmental triggers include climate (low humidity), behaviours that increase skin dryness (e.g. soap use, hot showers, chlorinated swimming pools) and exposure to irritants and allergens.
Clinical appearance
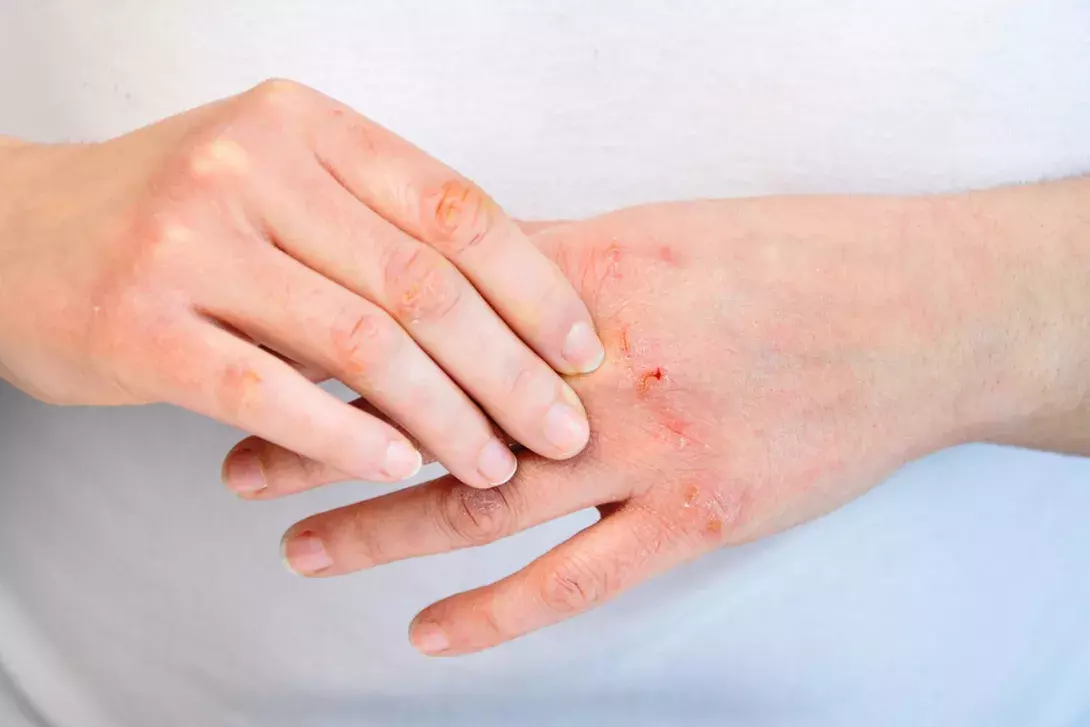
Atopic dermatitis manifests as pruritic, poorly demarcated erythematous scaly lesions (Figure 1 and Figure 2).9 The distribution can vary, but is often flexural, affecting the antecubital fossae, popliteal fossae, face and neck. Over time, the skin becomes thickened due to chronic rubbing and scratching. Dyspigmentation can be seen, depending on skin phototype. The condition may be relapsing with acute flares and periods of remission, but in more severe cases there is continuous involvement.
Complications and psychological impact
Atopic dermatitis carries a large psychological burden and can have a significant detrimental impact on quality of life. There is greater prevalence of mood-related, sleep and behavioural disorders in patients with severe disease, generally driven by pruritus.3-5 Additionally, there is a large cost burden relating to hospital admissions, treatments and potential time off school or work.10
Skin infections are common in patients with atopic dermatitis because of the deficient barrier function and antimicrobial activity.8 Staphylococcus aureus colonisation is frequent, with infections exhibiting the characteristic features of honey- coloured crust and pustules. S. aureus can also be found in higher numbers in non-lesional skin, so antibiotics are not required on the basis of this finding on a swab result alone. Bleach baths are often recommended both as prevention (once or twice a week) and active treatment (daily) of infected eczema. The dilution is 12 mL bleach (e.g. plain, fragrance-free household bleach) per 10 L water, and the immersion time two to five minutes. No rinsing is required. This can minimise the need for oral antibiotics. In the case of severe bacterial infection, oral cephalexin (12.5 to 25 mg/kg, three times daily for seven to 10 days) may be necessary.
Viruses, including herpes simplex virus, herpes zoster virus and poxvirus (molluscum contagiosum), are also common causes of cutaneous infection and flares of atopic dermatitis. Herpetic infection presents with crops of small, painful, punched-out ulcers or vesicles on an erythematous base. For suspected herpes infection, empirical oral aciclovir, famciclovir or valaciclovir is given for five days. If there are lesions around the eyes, urgent referral for ophthalmological assessment is required.
Diagnosis and scoring
Several scoring tools are available for the diagnosis of severity and to monitor patient progress against objective measures. The Dermatology Life Quality Index (DLQI) is a commonly used simple patient questionnaire for the clinical evaluation of the impact of eczema.11 Table 1 shows the interpretation of the scoring involved in the DLQI; the full questionnaire can be found at https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnaires/dermatology-life-quality-index. The Eczema Area and Severity Index (EASI) is a validated scoring system that grades the physical signs of atopic dermatitis in each region of the body and gives a score equating to the percentage of total affected surface area.12 Further information about the EASI can be found at https://dermnetnz.org/topics/easi-score.
These tools can form useful adjuncts in the management of atopic dermatitis. In the primary care setting however, where time may be limited, the emphasis should be on eliciting the severity of itch, impact on sleep and frequency of flares.13
Management of atopic dermatitis
General measures
Education and patient involvement are imperative in the management of atopic dermatitis, regardless of severity. It is important to listen to the patient’s perspectives to identify any suspected triggers and discuss their treatment goals and potential limitations to adherence. Disease quiescence cannot be achieved without maintaining a constant background of general measures to restore barrier function and prevent infections.13 All patients should be instructed to keep cool, moisturise twice daily (ideally with a ceramide-containing moisturiser) and avoid soaps and fragrances (Box 1).
Topical therapies
Mild atopic dermatitis is typically managed in the primary care setting. The basis of treatment is formed through the above general measures and the addition of topical corticosteroids (TCS) and/or topical calcineurin inhibitors.
Education, counselling and guidance for application of topical therapies are paramount. These therapies require patient motivation and can be time consuming and burdensome for both patients and their families.10 Patients often underuse their topical treatments because of phobias and misunderstanding.14 Lack of adherence leads to failure. It is important to encourage liberal use without strict time limits (which unfortunately only enhances their fears regarding these medications). Strategies to improve adherence include ensuring adequate quantities of topical medications are prescribed, arranging regular follow up and developing written action plans, including protocols for flares.
When used appropriately, TCS are safe and well tolerated.15 Skin thinning (atrophy) is an overexaggerated side effect and is rarely seen. The most common side effect of the use of a potent TCS on the face is periorificial dermatitis, a form of steroid-induced rosacea that is treated with a tetracycline antibiotic. Striae are the most concerning side effect of potent TCS use in the groin or axillae as they are irreversible, unlike atrophy, which is minimally visible and reversible. Cataracts and glaucoma are the most concerning adverse effect of potent TCS use on the eyelids. For these reasons, a potent TCS should not be used on the face, and hydrocortisone 1% and topical calcineurin inhibitors are the treatments of choice for the face and genitalia. Pimecrolimus cream is safe and generally well tolerated, and is affordable as it is listed on the PBS. However, its use can be limited by stinging and lack of efficacy. Topical tacrolimus 0.1% is compounded as either ointment or cream in Australia and is significantly more effective then pimecrolimus, although more expensive. Topical calcineurin inhibitors should be avoided in the setting of herpetic infection and can also potentially trigger periorificial dermatitis.
The ideal duration of therapy for topical therapies is between two and four weeks, although ongoing low potency TCS use and intermittent long-term medium potency TCS use is considered safe.13 Principles and strategies guiding choice of potency and vehicle of corticosteroid, based on severity, location and patient factors, are summarised in Box 2.
Crisaborole is a topical phosphodiesterase-4 inhibitor that is a key regulator in the inflammatory cascade in atopic dermatitis. It has been shown to be a well-tolerated and efficacious, albeit expensive, alternative as it is not yet listed on the PBS. It can be used as a nonsteroidal option for patients in whom TCS are ineffective or contraindicated, or in patients who require a break from TCS therapy.16
Escalation of therapy
Treatment failure is guided by the patient’s opinion of worsening of lesions after one to two weeks, or the physician’s opinion of an unchanged clinical score four weeks after therapy (according to the DLQI or Physician Global Assessment [PGA] scores – a 5- or 6-point scoring system used to assess disease severity).13 It is important to assess the factors relating to treatment response, including severity of disease, infection, compliance, and social and psychological factors. Dermatology referral should be made promptly for treatment failure, or in the setting of frequent number of flares, significant interference with quality-of-life, including sleep or ability to function at school or work, and recurrent bacterial or viral infections.
Phototherapy
Narrowband ultraviolet B (nbUVB) can be used as an adjunct in patients who fail to respond to topical therapies. In one recent large cohort study, it was found that 70% of patients received significantly less TCS in the 12 months following a course of nbUVB, and nearly 50% of patients were scored as clear or almost clear at the end of a two-month course of treatment.17 Considerations before starting phototherapy include skin type (very fair skin and a history of skin cancer are relative contraindications), availability and logistics. Sessions ideally take place three times a week for around six to eight weeks, although this can vary depending on the patient and their response.13 Phototherapy has a favourable safety profile and is well tolerated among most users. Risk of skin cancer is considered low, provided the number of treatments remains below 200 to 400.13 Other possible adverse effects include burning, tanning and hyperpigmentation, depending on the patient’s skin type.
Oral corticosteroid treatment
Systemic corticosteroid therapy should be reserved for patients with severe atopic dermatitis as rescue therapy for significant flares. A standard course for an acute flare of atopic dermatitis would be prednisolone 0.5 mg/kg (25 to 37.5 mg) for four days, 0.25 mg/kg (12.5 to 15 mg) for four days, 0.125 mg/kg (5 to 7.5 mg) for four days, then cease. Well described short- and long-term complications are involved with its use, and any patient requiring multiple courses per year needs to be assessed by a dermatologist.
Other immunosuppressant treatment
Other systemic therapies include immunosuppressants such as ciclosporin, azathioprine, methotrexate and mycophenolate mofetil. These have been important steroid-sparing agents for severe atopic dermatitis patients. However, there can be significant systemic toxic adverse effects and these should only be prescribed by a specialist; close clinical and laboratory monitoring is required.
Ciclosporin is the only oral immunosuppressant with an approved indication for use in atopic dermatitis. It is used in doses of 3 to 5 mg/kg. It is not recommended for continuous use of longer than two years because of the high risk of hypertension and renal impairment. Rebound flares are common on cessation. Close monitoring of blood pressure, renal function and for systemic infection is required.
Methotrexate in doses of 5 to 20 mg weekly has long been used as a steroid-sparing agent in atopic dermatitis as it is relatively low-risk and cost effective. It can be given either orally or as a subcutaneous injection (if there are compliance or absorption concerns). Nausea, liver toxicity and lack of efficacy are drawbacks to its use.
Azathioprine can be an effective treatment in a relatively small proportion of patients with atopic dermatitis. Nausea, infections and severe drug reactions can be seen, however, and regular monitoring of haematological parameters and liver function tests are required.
Mycophenolate mofetil has become more popular in the last few years as a treatment option for atopic dermatitis now it is more easily available and cheaper. The effective dose range is from 500 mg to 4 g per day. However, as with the other agents, many patients fail to achieve substantive improvement. Nausea, gastrointestinal upset and infections are the major side effects.
Biologic therapy
Dupilumab is a monoclonal antibody that specifically reduces the action of IL-4 and IL-13 cytokines by blocking their binding to their shared receptor. It is considered a targeted immunomodulator that addresses the type 2 inflammation without causing immunosuppression. It was listed on the PBS in April 2021 for use in patients aged 12 years and over with severe atopic dermatitis that has not responded to four weeks of moderate-potency TCS use. Large scale clinical trials have shown its efficacy and long-term safety.18,19 Meaningful improvements in sleep, pruritus and overall quality of life can be experienced in as little as a few weeks and are sustained with long-term use.12 ‘Before and after’ images of severe atopic dermatitis-affected skin treated with dupilumab for six weeks are shown in Figure 3.
Dupilumab is administered subcutaneously with an initial loading dose of 600 mg and then a 300 mg dose every two weeks. No blood test monitoring is required. The most common side effects of therapy are ocular symptoms, including dry eyes and conjunctivitis; these can usually be treated with topical therapies and rarely require cessation of therapy, although referral to an ophthalmologist may be required in some cases.
Other advanced therapies
The first of the JAK inhibitors, upadacitinib, was approved by the PBS in March 2022 for severe atopic dermatitis in patients aged 12 years and older. JAK inhibitors are small molecules that inhibit intracellular signalling. The initial dose is 15 mg daily, orally. Upadacitinib appears to be highly effective for atopic dermatitis, but not effective for atopic comorbidities, such as asthma. This class of medication requires monitoring of blood tests for anaemia, lymphopenia and hyperlipidaemia. Side effects include acne and an increase in infections, particularly herpes zoster, but no ocular issues as are seen with dupilumab. Patients are encouraged to be vaccinated against herpes zoster before starting upadacitinib.
Other novel therapies are being investigated and awaiting approval for use in Australia for the treatment of atopic dermatitis.20,21
Conclusion
Atopic dermatitis is a common condition that can range from mild to severe, and can have significant impacts on patients’ quality of life. First-line therapy involves education around general measures and commencement of appropriate topical therapy with regular follow up. Dermatologist referral should be arranged for patients who have failed to respond to first-line measures, as there are now additional advanced treatments available for patients with severe disease. MT
