What’s the diagnosis?
A woman with a recurring blister on her elbow
Case presentation
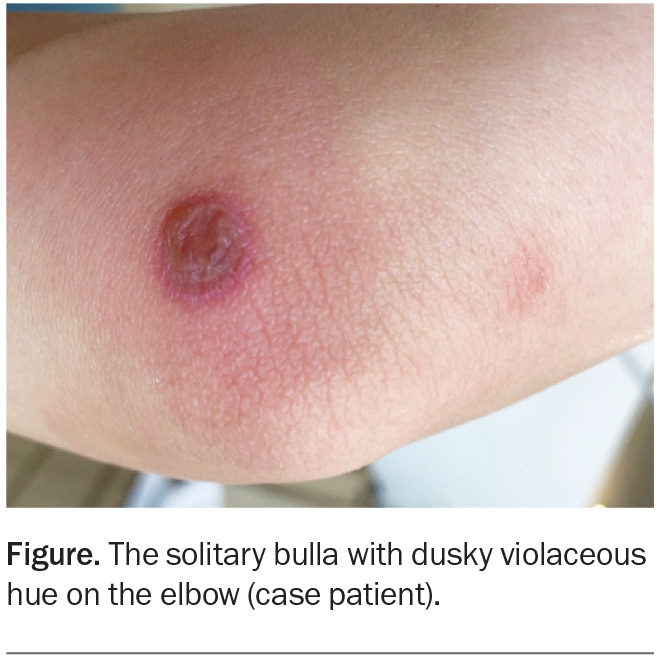
A 29-year-old woman presents with an itchy and painful lesion of sudden onset on her elbow, which has occurred in exactly the same location multiple times (Figure). She denies recent trauma to the area and has had no direct contact with chemicals or other agents, such as plants or corrosive substances. She reports no systemic symptoms.
The patient has no significant medical conditions. She takes the combined oral contraceptive pill. She has a new partner and underwent recent screening for sexually transmitted infections, which returned negative.
On examination, a solitary, sharply-demarcated, discoid bulla, about 25 mm in diameter, is observed on the right medial elbow. The lesion has a dusky violaceous hue and surrounding erythema. There is no other similar lesion or rash elsewhere on her body.
Differential diagnoses
Conditions to consider among the differential diagnoses for a patient with a solitary bulla on the arm include the following.
Bullous insect bite reaction
There are various clinical manifestations of insect bite reactions. These include vesiculobullous lesions, which are most commonly caused by fleas or bedbugs.1 The lesions are filled with serous fluid and typically intensely pruritic. Vesicles and bullae may be present on a background of normal skin or located within erythematous papules. When caused by bedbugs the lesions will usually appear in a linear arrangement, which is known as the ‘breakfast, lunch and dinner’ sign.
The pathogenesis of insect bite reactions involves both IgE- and cell-mediated immunity.1 A biopsy is not usually required but is sometimes performed to confirm the diagnosis. Histopathological examination will commonly reveal epidermal spongiosis, which is occasionally accompanied by vesicles that group together over time and form subepidermal bullae.1,2 A superficial and deep lymphocytic infiltrate, as well as perivascular and periadnexal eosinophil infiltrates, are also frequently seen.2
This is not the correct diagnosis for the case patient. The lesion is larger than a typical insect bite reaction and its dusky violaceous hue is not usual (insect bites are generally more erythematous). Also, patients with bullous insect bite reactions usually present with multiple bullae.
Bullous impetigo
Impetigo is a common, contagious superficial skin infection. It can affect people of all ages but is mostly seen in young children.
There are two main types of impetigo: bullous (about 30% of cases) and nonbullous.3 Impetigo is caused by Staphylococcus aureus or Streptococcus species. S. aureus can cause bullous impetigo when exfoliative toxins A and B cleave desmoglein 1 and lead to acantholysis and subsequent bullae formation.1,4
Impetigo most commonly occurs on the face, trunk, axillae, extremities, buttocks and perineum. The disease manifests initially as small vesicles, which turn into flaccid bullae. These typically rupture spontaneously, releasing serous yellow fluid and leaving a collarette of scale. Surrounding erythema is not usually present.5
This is not the correct diagnosis for the case patient, whose lesion is solitary (bullous impetigo lesions are almost never solitary) with prominent surrounding erythema.
Herpes simplex virus infection
Cutaneous herpes simplex virus (HSV) infections are commonly caused by HSV-1 and HSV-2. Worldwide, in 2016 an estimated 66.6% of people aged under 50 years had HSV-1 infection at any site and 13.2% of people aged 15 to 49 years were living with HSV-2 infection.6 HSV-1 is most frequently acquired nonsexually through the orolabial mucosa whereas HSV-2 is sexually transmitted.7-9
Transmission of HSV results in a lifelong infection. During the primary infection, the virus replicates in epithelial cells of the affected mucocutaneous site, producing infectious viral particles that travel by retrograde axonal flow to the dorsal root ganglia, where it establishes latency. Upon reactivation, new viral particles are produced and travel in an anterograde fashion to the skin or mucosa, resulting in typical HSV lesions, but they occasionally travel to the central nervous system, resulting in herpes meningoencephalitis.10 Reactivation can happen spontaneously or in response to a range of triggers, such as local trauma, immunosuppression, emotional stress, exposure to UV light, fever and menstruation.11-16
HSV infection has a wide range of dermatological presentations but the classic mucocutaneous presentations are closely grouped vesicles (often umbilicated), erosions, ulcers with scalloped borders, and pustules on an erythematous base.
For the case patient, the solitary appearance, shape and location of her lesion do not support a diagnosis of HSV infection.
Erythema multiforme
Erythema multiforme (EM) is an acute, immune-mediated and self-limiting mucocutaneous disease characterised by targetoid lesions. Patients typically present with multiple lesions, which have a predilection for the extremities and face.1,17 Early lesions (‘iris lesions’) may have only two zones: a central dusky, violaceous area of epidermal damage, manifesting as bulla or crust, and an erythematous outer zone.1 Over time, the lesions become targetoid in appearance, with an additional middle oedematous ring that is light in colour.17 Patients commonly feel systemically unwell. The incidence is highest in men under the age of 50 years.18
In the past, EM was considered to be part of the spectrum of disease that includes Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN); in addition, the name of the more severe form, EM major, was used interchangeably with SJS.19 However, EM is now recognised as a separate disease entity, distinct from SJS, SJS/TEN overlap or TEN.17,20 EM is most commonly caused by infectious agents, such as HSV and Mycoplasma pneumoniae, whereas SJS and TEN are primarily induced by drugs.21
Studies of HSV-associated EM have led to a proposed disease mechanism in which HSV DNA fragments are released into the blood and transported by CD34+ cells to be deposited on epidermal keratinocytes. This leads to the recruitment of HSV-specific CD4+ type 1 T-helper cells, which produce interferon-gamma and triggers an inflammatory cascade and promotes the recruitment of autoreactive T-cells, resulting in epidermal damage.22-24 It is unclear whether a similar pathogenesis applies to EM precipitated by other causes.
This is not the correct diagnosis for the case patient. Although her lesion fits the description of an iris lesion, EM typically manifests with multiple lesions. In addition, patients with EM often experience systemic symptoms, which were absent in this case.
Trauma/burn
Injuries – such as trauma and partial-thickness (second-degree) thermal or chemical burns – can result in vesicles and bullae. The formation of blisters in the immediate setting after an injury is well recognised, but delayed blister formation has been observed in the weeks to months following partial-thickness thermal burns, including in graft donor and recipient sites, and has also been observed over laser-resurfaced skin.25-29
This is not the correct diagnosis for the case patient, who has no history of trauma, thermal injury or contact with chemical substances affecting the site of the lesion.
Fixed drug eruption
This is the correct diagnosis. A fixed drug eruption (FDE) is a cutaneous allergic reaction where a lesion occurs at the same site after initial exposure and subsequent re-exposures to a causative agent. The most common causes include NSAIDs, paracetamol and antibiotics.30 Others, such as fluconazole, influenza and COVID-19 vaccines, PDE-5 inhibitors, H1 antihistamines and proton pump inhibitors, have also been reported.30
A FDE is a type IV hypersensitivity reaction. Following the initial exposure, memory CD8+ T-cells at the dermoepidermal junction are activated and release interferon-gamma, causing epidermal damage.31 Typically, activated T-cells are removed by apoptosis at the end of the inflammatory cascade. However, the lesions in FDEs are unusual – a high concentration of CD8+ T-cells remains in the inflamed site long after the clinical resolution.31-33 After the offending agent is removed and the epidermis starts to heal, regenerating basal keratinocytes have been shown to release IL-15, which is likely crucial to the long-term survival of the resident memory CD8+ T-cells in the location where the FDE occurs.34 These T-cells subsequently remain quiescent but are rapidly activated again after re-exposure to the offending agent.
A FDE most commonly manifests as a solitary, well-demarcated, erythematous or violaceous patch or plaque (round or oral in shape), which may blister or ulcerate. It is generally asymptomatic but can be painful or pruritic. The lesion can occur anywhere on the body, but the extremities, lips and anogenital areas are the most commonly affected sites.35 The average time to onset is usually within two days after the initial exposure and within 24 hours following subsequent exposures.36,37
For the case patient, the appearance and site of the lesion are typical of a FDE. Further questioning revealed that the lesion occurred recurrently and was associated with menstruation, when she would take oral naproxen for menstrual cramps.
Management
A FDE is a benign and self-limiting reaction. The most important aspect of management is discontinuing and avoiding future exposure to the offending agent. Topical corticosteroids can be prescribed if there is pruritus or pain associated with the lesion.
Outcome
The case patient was diagnosed with a FDE caused by an NSAID. The lesion faded over the next few days, leaving postinflammatory hyperpigmentation, which is expected for a FDE. This typically continues to fade over time but may take up to two years to resolve. MT
COMPETING INTERESTS: None.
References
1. Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018.
2. Miteva M, Elsner P, Ziemer M. A histopathologic study of arthropod bite reactions in 20 patients highlights relevant adnexal involvement. J Cutan Pathol 2009; 36: 26-33.
3. Hartman-Adams H, Banvard C, Juckett G. Impetigo: diagnosis and treatment. Am Fam Physician 2014; 90: 229-235.
4. Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med 2006; 355: 1800-1810.
5. Nardi NM, Schaefer TJ. Impetigo [internet]. Treasure Island, FL: StatPearls Publishing; 2023.
6. James C, Harfouche M, Welton NJ, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ 2020; 98: 315-329.
7. Nahmias A, Dowdle W, Naib Z, Josey WE, McLone D, Domescik G. Genital infection with type 2 herpes virus hominis. A commonly occurring venereal disease. Br J Vener Dis 1969; 45: 294-298.
8. Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis 2002; 186 Suppl 1: S3-S28.
9. AlMukdad S, Farooqui US, Harfouche M, Aldos L, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 2 in Canada, Australia, and New Zealand: systematic review, meta-analyses, and meta-regressions. Sex Transm Dis 2022; 49: 403-413.
10. Zhu S, Viejo-Borbolla A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021; 12: 2670-2702.
11. Huang W, Xie P, Xu M, Li P, Zao G. The influence of stress factors on the reactivation of latent herpes simplex virus type 1 in infected mice. Cell Biochem Biophys 2011; 61: 115-122.
12. Ichihashi M, Nagai H, Matsunaga K. Sunlight is an important causative factor of recurrent herpes simplex. Cutis 2004; 74(5 Suppl): 14-18.
13. Chida Y, Mao X. Does psychosocial stress predict symptomatic herpes simplex virus recurrence? A meta-analytic investigation on prospective studies. Brain Behav Immun 2009; 23: 917-925.
14. Horn EE, Turkheimer E, Strachan E. Psychological distress, emotional stability, and emotion regulation moderate dynamics of herpes simplex virus type 2 recurrence. Ann Behav Med 2014; 49: 187-198.
15. Suzich JB, Cliffe AR. Strength in diversity: Understanding the pathways to herpes simplex virus reactivation. Virology 2018; 522: 81-91.
16. El Hayderi L, Delvenne P, Rompen E, Senterre JM, Nikkels AF. Herpes simplex virus reactivation and dental procedures. Clin Oral Investig 2013; 17: 1961-1964.
17. Auquier-Dunant A, Mockenhaupt M, Naldi L, et al. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol 2002; 138: 1019-1024.
18. Sanchis J, Bagán J, Gavaldá C, Murillo J, Diaz JM. Erythema multiforme: diagnosis, clinical manifestations and treatment in a retrospective study of 22 patients. J Oral Pathol Med 2010; 39: 747-752.
19. Roujeau J-C. What is going on in erythema multiforme? Dermatology 1994; 188: 249-250.
20. Soares A, Sokumbi O. Recent updates in the treatment of erythema multiforme. Medicina (Kaunas) 2021; 57: 921.
21. Grunwald P, Mockenhaupt M, Panzer R, Emmert S. Erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis – diagnosis and treatment. J Dtsch Dermatol Ges 2020; 18: 547-553.
22. Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J 2003; 9: 1.
23. Aurelian L, Burnett J. Current understanding of herpes simplex virus-associated erythema multiforme. Exp Rev Dermatol 2008; 3: 491-499.
24. Ono F, Sharma BK, Smith CC, et al. CD34+ cells in the peripheral blood transport herpes simplex virus DNA fragments to the skin of patients with erythema multiforme (HAEM). J Invest Dermatol 2005; 124: 1215-1224.
25. Compton CC. The delayed postburn blister: a commonplace but commonly overlooked phenomenon. Arch Dermatol 1992; 128: 249-252.
26. Anolik R, Loyd A, Patel R, Magro C, Franks AG Jr. Delayed and recurring blisters in the donor graft site of a burn patient. Dermatol Online J 2010; 16: 13.
27. Dhawan AK, Grover C, Bisherwal K, Garg S. Delayed burn blister. Indian J Dermatol 2015; 60: 323.
28. Chetty BV, Boissy RE, Warden GD, Nordlung JJ. Basement membrane and fibroblast aberration in blisters at the donor, graft, and spontaneously healed sites in patients with burns. Arch Dermatol 1992; 128: 181-186.
29. Alora MB, Dover JS. Spontaneous bullae over laser resurfaced skin. J Am Acad Dermatol 2000; 42(2 Pt 1): 288-290.
30. McClatchy J, Yap T, Nirenberg A, Scardamaglia L. Fixed drug eruptions – the common and novel culprits since 2000. J Dtsch Dermatol Ges 2022; 20: 1289-1302.
31. Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol 2007; 17: 201-208.
32. Shiohara T, Mizukawa Y, Teraki Y. Pathophysiology of fixed drug eruption: the role of skin-resident T cells. Curr Opin Allergy Clin Immunol 2002; 2: 317-323.
33. Mizukawa Y, Yamazaki Y, Teraki Y, et al. Direct evidence for interferon-gamma production by effector-memory-type intraepidermal T cells residing at an effector site of immunopathology in fixed drug eruption. Am J Pathol 2002; 161: 1337-1347.
34. Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol 2008; 158: 1230-1238.
35. Flowers H, Brodell R, Brents M, Wyatt JP. Fixed drug eruptions: presentation, diagnosis, and management. South Med J 2014; 107: 724-727.
36. Ben Fadhel N, Chaabane A, Ammar H, et al. Clinical features, culprit drugs, and allergology workup in 41 cases of fixed drug eruption. Contact Dermatitis 2019; 81: 336-340.
37. Brahimi N, Routier E, Raison-Peyron N, et al. A three-year-analysis of fixed drug eruptions in hospital settings in France. Eur J Dermatol 2010; 20: 461-464.
Skin lesions
Medicines