A solitary tender, erythematous nodule in a healthy farmer
Test your diagnostic skills in our regular dermatology quiz. What is the cause of this tender lesion on a woman’s forearm?
Case presentation
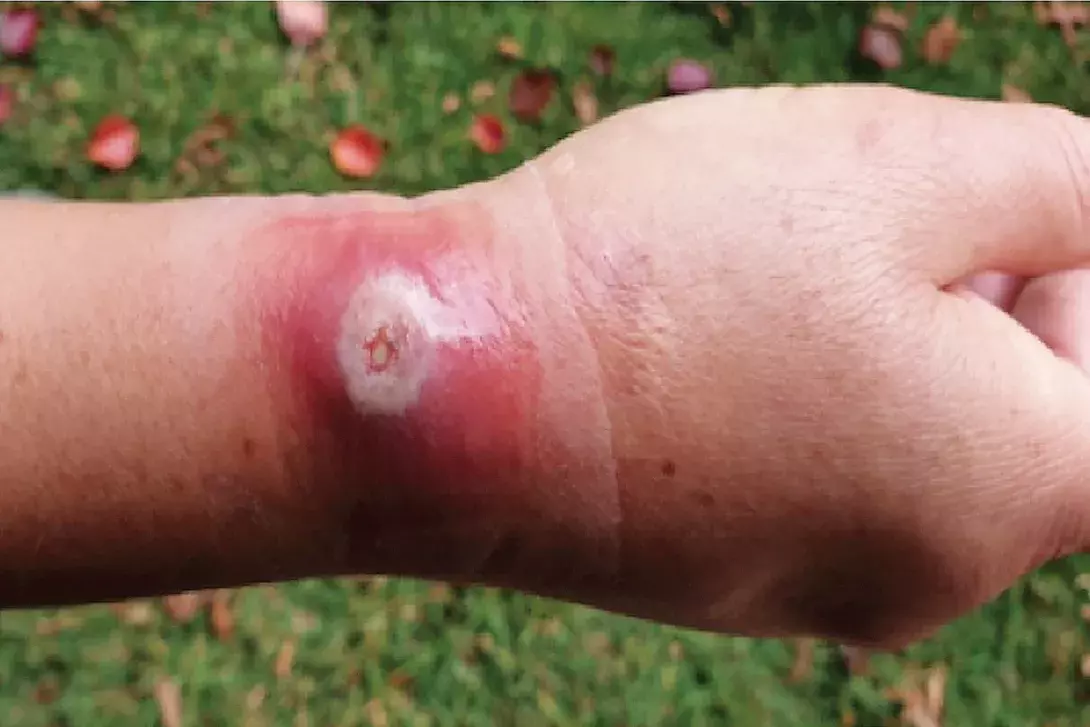
A 55-year-old woman presents with a tender, erythematous nodule on her right forearm (Figure 1). There is no discharge from the lesion. It initially appeared five days earlier as an erythematous macule and has since developed into a nodule that is tender to palpation. She denies any trauma or insect bites to the affected area.
The patient is systemically well, with no fevers or malaise, and she has no other medical issues. There is no family history of note. She lives on a farm, where she has regular exposure to cows, sheep and horses.
Differential diagnoses
Conditions to consider among the differential diagnoses for a healthy farmer who has a nondischarging erythematous nodule include the following.
Sporotrichosis
Sporotrichosis is a fungal infection caused by the dimorphic fungus Sporothrix schenckii, which is found worldwide but particularly in tropical and subtropical regions.1 It has the potential to affect people of any age and gender.
S. schenckii is commonly found in soil, plants and animals. Inoculation often occurs via direct contact to traumatised or eroded skin.1
Sporotrichosis primarily affects the skin, subcutaneous tissue and adjacent lymphatic vessels, with potential to disseminate to other organs.1,2 Risk factors include occupations or recreational activities where exposure to organic matter colonised by S. schenckii is increased (e.g. agriculture, gardening, mining, wood chopping), immunosuppression and animal bites/scratches.1,2
Sporotrichosis is classified based on the location of the lesions, which can be cutaneous, mucosal or extracutaneous. Initially it appears as a nontender, cutaneous lesion with surrounding erythema at the site of inoculation. In more than 75% of cases, a subcutaneous nodule develops, which may ulcerate and discharge. Despite ulceration, the lesion typically remains painless.3 However, it may also morphologically appear verrucous, tuberous, infiltrated with plaque or vegetative.1 Primary lesions are commonly found on extremities that may be most exposed to trauma, particularly the hands and forearms.1 As the infection progresses, secondary lesions develop along regional lymph nodes and may disseminate without extracutaneous features at isolated sites; this phenomenon is known as ‘sporotrichoid spread’. The mucosal forms are lesions involving the conjunctivae and nasal mucosa.
Immunocompromised patients are at an increased risk of developing the extracutaneous form, which includes osteoarticular, pulmonary or meningeal involvement.1 In these patients, associated clinical features could include arthritis, cough, dyspnoea or fevers, which are often reflected by an elevated erythrocyte sedimentation rate (ESR). Definitive diagnosis of sporotrichosis is made by a positive S. schenckii fungal culture from a skin biopsy specimen, although this may be negative due to the difficulty in culturing the fungus.1 Fungal microscopy would demonstrate S. schenckii, characterised by cigar-shaped yeast associated with narrow based buds.3
Sweet’s syndrome
Sweet’s syndrome, also known as febrile neutrophilic dermatosis, is an uncommon cutaneous inflammatory condition characterised by a neutrophilic infiltrate that is often linked to systemic disease (Figure 2).4 There are three types of Sweet’s syndrome: classic, malignancy-associated and drug-induced.4
The definitive pathogenesis of Sweet’s syndrome remains unclear due to its multifactorial associations. However, an affiliation between interleukins operating as cytokine mediators and tumour necrosis factors has been established.5 Epidemiologically is occurs worldwide without racial exclusivity. The classic variant primarily affects women, who are often, but not always, between the ages of 30 and 60 years.5 Risk factors include a recent upper respiratory or gastrointestinal infection, pregnancy or a history of inflammatory bowel disease.5 One-third of these individuals are at risk of developing recurrence.5 Malignancy-associated Sweet’s syndrome affects both genders equally. Up to 21% of patients with Sweet’s syndrome have a background of either haematological malignancy, particularly acute myelogenous leukaemia, or solid malignancies.5 Drug-induced Sweet’s syndrome has been predominantly identified in relation to administration of granulocyte-colony stimulating factor. However, various antibiotics, antiepileptics, antihypertensives (e.g. hydralazine) and antipsychotics (clozapine) have been identified as causative agents.5
Individuals with Sweet’s syndrome commonly present with a variety of symptoms and may be noticeably unwell. These symptoms include solitary or multiple cutaneous eruptions, pyrexia (which may develop days to weeks after skin lesions) and leukocytosis with associated neutrophilia on full blood count.5 The skin lesions are asymmetrically distributed, tender, erythematous papules, nodules or plaques. Additionally, due to oedema developing within the upper dermis, lesions may have a clear vesicular appearance that may eventually ulcerate or appear pustular.6 Overall, the regions frequently affected include the neck, face and upper extremities. However, there is also a range of additional extracutaneous manifestations associated with Sweet’s syndrome.5 Associated symptoms may include malaise, myalgia/arthralgia and headache.5
Diagnosis of Sweet’s syndrome is confirmed with a skin biopsy, often in combination with an elevated ESR and neutrophilia.5 The histopathological features favouring a diagnosis of Sweet’s syndrome include diffuse infiltration of mature neutrophils within the upper dermis, in conjunction with oedematous papillary dermal and endothelial cells.5
Cutaneous mycobacterial infections
Cutaneous mycobacterial infections are classified according to the causative organism.
Cutaneous infections can be caused by nontuberculous mycobacteria, which are either slow or rapidly growing. These present as single or multiple lesions that appear as discoloured nodules, papules, ulcers, abscesses or cellulitis.7
Buruli ulcer (formerly Bairnsdale ulcer) was initially identified in Australia in the 1930s. It is caused by the slow growing Mycobacterium ulcerans (nontuberculous mycobacteria), a species that evolved from Mycobacterium marinum and is found in rural areas with wetlands; in Australia it has been found in the Mornington Peninsula in Victoria. The organism can be transmitted via skin injuries or insect bites and has an incubation period of five to eight weeks.7 Buruli ulcer can present as a painless nodule, plaque or swelling that proceeds to ulcerate; the myolactone secreted by M. ulcerans eventually results in tissue destruction, leading to deep ulcerations causing osteonecrosis and potentially limb loss. The lower limbs are primarily affected (>55% of cases).7 It is commonly a clinical diagnosis but can be confirmed by direct microscopy, histopathological examination of skin biopsy specimens, culture and IS2404 PCR testing. Throughout the acute infection phase, the histopathology reveals large numbers of extracellular bacilli.
Cutaneous tuberculosis (caused by Mycobacterium tuberculosis) and leprosy (caused by Mycobacterium leprae and Mycobacterium lepromatosis) can occur in Australia but are rare.
Orf
This is the correct diagnosis. Orf, also known as ecthyma contagiosum, is a zoonotic viral infection found worldwide that is caused by the parapoxvirus, Orfviridae.8,9 The organism, a double-stranded DNA virus without specific receptor requirements for mammalian cell entry, is transmitted to humans from infected sheep and goats by direct contact, where it is able to infiltrate skin abrasions by attaching to basal keratocytes.8 Risk of direct inoculation is often related to an individual’s occupation and travel history, with farmers, butchers, veterinarians and sheepherders at increased risk because of their frequent involvement with livestock.8
Orf cutaneous lesions arise after an incubation period of three to five days and morphologically evolve through six stages within a few weeks.8 Initially orf presents as an erythematous maculopapular lesion that appears targetoid in shape with a developing necrotic centre.8 It progresses to form a suppurative nodule that eventually becomes a crusted, firm papule. Lastly, it cultivates a papillomatous surface that ultimately regresses and has potential to resolve without long-term scarring.8 In more than 90% of cases, the cutaneous lesions of orf primarily affect an individual’s hands and digits. However, additional regions may also be involved, including the scalp, face and genitals.8
Recurrence of orf is rare.8 However, there are preventive measures used to decrease exposure to the virus, such as wearing gloves and vaccinating animals.
Diagnosis and investigations
Orf can potentially be diagnosed clinically on the basis of a relevant history of exposure to the virus together with suggestive skin lesions.8 However, the gold standard for diagnosis is histopathological examination of a punch/shave skin biopsy specimen. Microscopy of parapoxvirus is characterised by cytoplasmic vacuoles that are pale in appearance in addition to acanthotic squamous epithelium.8,9 The upper dermis exhibits keratinocytes largely enclosed with eosinophils, whereas the lower dermis demonstrates infiltration with polymorphic structures and degeneration of spongiform within the follicular structures.8
Another, highly sensitive technique to aid diagnosis of orf is electron microscopy, in which parapoxvirus particles are identified by its classic oval figure.9 At any stage of infection, the DNA of parapoxvirus is also accurately recognised by PCR testing.9
Management
Orf is largely a self-limiting infection that usually resolves spontaneously within four to 12 weeks and is therefore often associated with a positive prognosis.8 In most cases, management involves reassurance and expectant measures such as concealing and thoroughly cleaning the lesion with antiseptic agents to avoid contamination.9 Superimposed bacterial infections, lymphangitis and systemic features (such as fever, malaise and lymphadenopathy) are common complications.8
Immunocompromised individuals are at greater risk of developing larger lesions (giant orf), a variant that can present morphologically similar to granulomas and tumours because of its large surface area.8 In this situation, treatment may include topical antivirals in conjunction with mechanical measures such as surgical excision and cryotherapy.8
Outcome
In this patient, a skin biopsy was taken which confirmed the diagnosis of orf. Bacterial swabs taken from the centre of the nodule demonstrated concomitant Streptococcus pyogenes infection, which resolved with intravenous antibiotics. The orf lesion self-resolved over the following month without scarring. MT
