Hereditary haemorrhagic telangiectasia: diagnosis, screening and management

Hereditary haemorrhagic telangiectasia is an autosomal dominant genetic disorder of abnormal vasculature, characterised by telangiectasias and arteriovenous malformations affecting specific sites. This disorder is underdiagnosed, and both patients and affected family members require screening and treatment to manage the end-organ complications that may occur.
Hereditary haemorrhagic telangiectasia (HHT), also known as Osler–Weber–Rendu syndrome, is an autosomal dominant genetic disorder characterised by the presence of dilated or broken blood vessels near the surface of the skin or mucous membranes (telangiectasias) and arteriovenous malformations (AVMs).1-3 The estimated prevalence is one in 5000 individuals; however, this is probably an underestimation.2-4 Patients with HHT may have AVMs in various visceral organs and experience serious complications because of bleeding or shunting of blood through abnormal vessels.2,3 The prognosis varies, and mortality rates may be as high as double those in the general population for patients younger than 60 years of age, with worse survival in patients with internal organ manifestations.2,5
HHT is underdiagnosed, and there is often a delay in diagnosis.6 Thus, it is important for primary care physicians to recognise the presentation of HHT, as prompt diagnosis allows for appropriate screening and preventative treatment in both patients and affected family members.1 Early screening by GPs and referrals to specialists may reduce morbidity and mortality associated with pulmonary or central nervous system (CNS) AVMs, as patients with these complications are assessed and pre-emptively treated at HHT centres.7 Management is best delivered in conjunction with a multidisciplinary team. This review outlines the diagnosis of HHT and, screening and treatment of HHT-related complications.
Diagnostic criteria
The diagnosis of HHT is clinical and based on the Curaçao criteria (Table 1).1-3,8 A diagnosis of HHT is ‘definite’ if three or more criteria are met, ‘possible or suspected’ if two are present and ‘unlikely’ if none or one are met.1 In current practice, genetic testing is offered to identify a causative mutation in a patient with family members with clinically confirmed HHT, as predictive testing in asymptomatic relatives or to establish a diagnosis in those who do not meet the criteria.1,9 More than 95% of people with definite HHT carry an identifiable causative mutation.1,10
Genetics
The underlying mechanism of HHT lies in mutations in genes involved in the transforming growth factor-beta pathway. There are currently six genes associated with HHT, with mutations in ACVRL1 and ENG constituting more than 90% of cases.11 Mutations in SMAD4, RASA1, GDF2 and EPHB4 have also been reported.12-15 Most mutations are single-nucleotide variations or short deletions or insertions, with large copy number variations found in up to 10% of cases involving ACVRL1 and ENG mutations.16 The penetrance is highly variable.17 Diagnostic genetic testing utilises massively parallel sequencing to screen for mutations and copy number variations simultaneously in these genes.
Clinical manifestations
HHT is a systemic disorder characterised by AVMs in the skin, CNS, lungs, liver and mucous membranes of the nose and gastrointestinal (GI) tract.1 Patients with HHT tend to develop an increasing number of manifestations over their lifetime.3,9 There is a large spectrum of disease severity, and manifestations of HHT can also vary within a single family carrying the same mutation.
Epistaxis
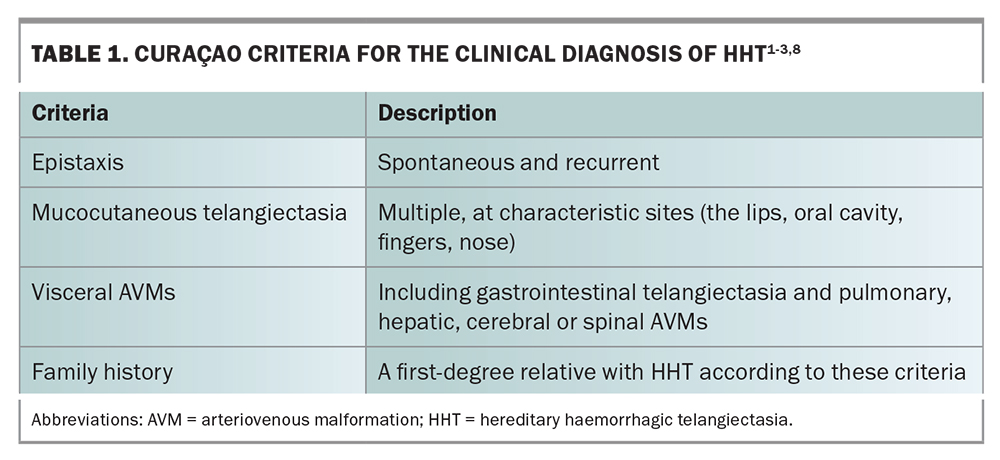
Recurrent epistaxis is the most common symptom of HHT and usually the earliest sign of disease
(Figure 1).9 It occurs in about 50% of patients younger than 10 years, and more than 90% of patients with HHT will develop it in their lifetime.2,3,18 Epistaxis is often spontaneous or follows minimal trauma to the nose.3 The severity of epistaxis is not influenced by the type of mutation, and the symptoms often worsen with age.19 Epistaxis may lead to iron-deficiency anaemia or the need for blood transfusion if severe.20
Skin telangiectasia
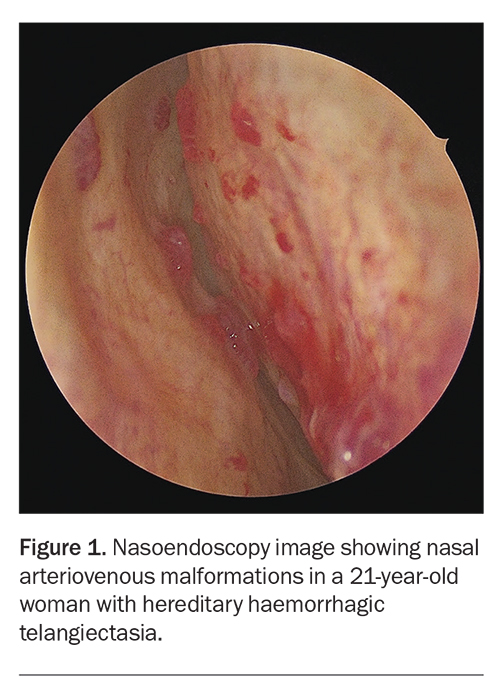
Skin telangiectasias are seen in the oral mucosa, lips, face and fingertips in up to 90% of patients with HHT (Figures 2a and b).2 Telangiectasias are distinguished by their ability to blanch with pressure and then immediately refill.3 The age of onset of visible telangiectasias is generally later than that of epistaxis, and telangiectasias may not be present until adulthood. Mucocutaneous telangiectasias can be subtle and easily missed on clinical examination.
Pulmonary AVMs
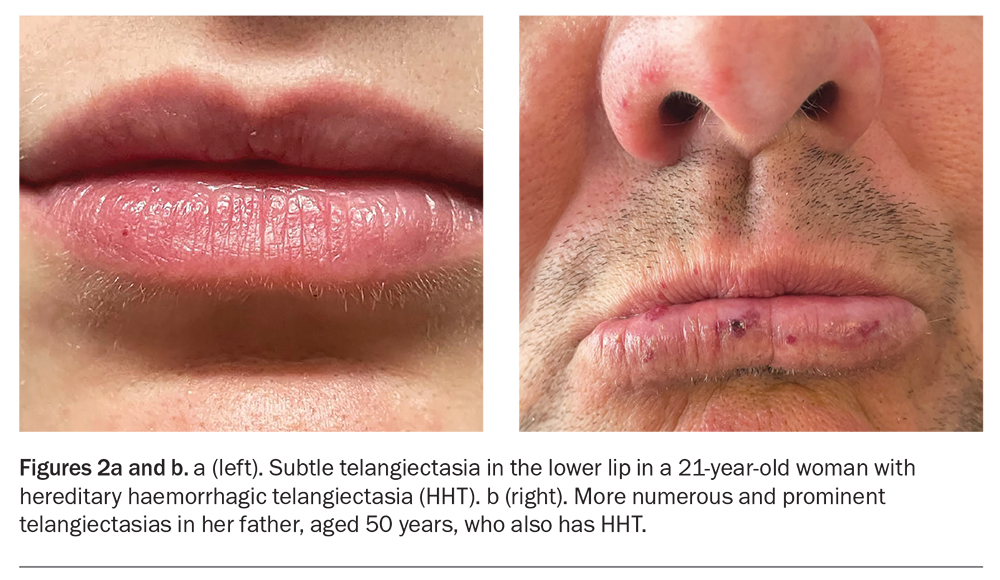
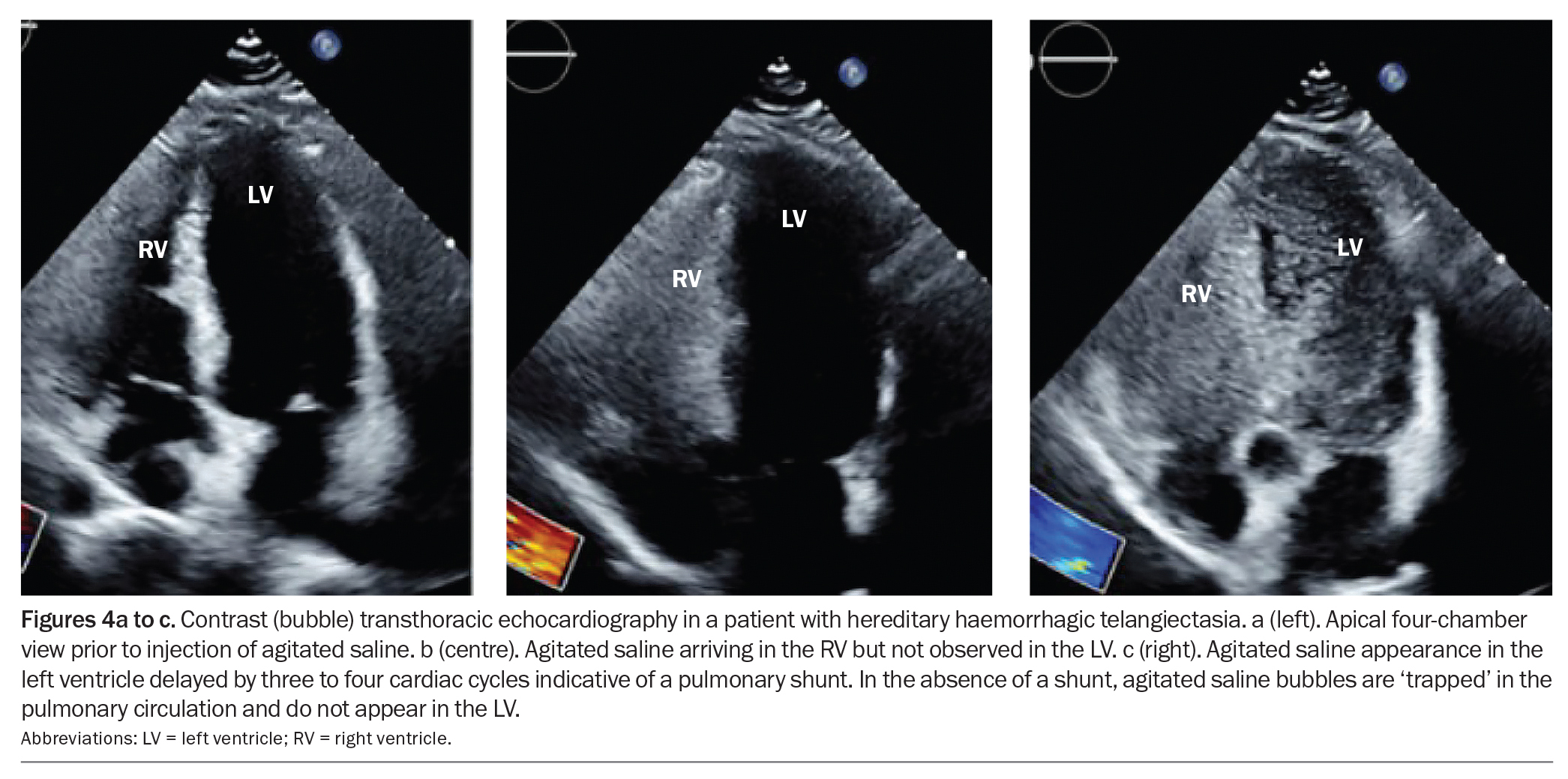
Pulmonary AVMs occur in as many as 50% of patients with HHT (Figures 3a and b).2 When a pulmonary AVM is identified, a diagnosis of HHT must be considered, as more than 70% of pulmonary AVMs are caused by underlying HHT.2 Many patients may be asymptomatic, and the first presentation may be a stroke caused by paradoxical embolism related to the presence of a right-to-left shunt. Pulmonary AVMs can also lead to haemoptysis, dyspnoea, hypoxaemia or digital clubbing.2,9 Migraines are common in patients with pulmonary AVMs.
Pulmonary hypertension is another vascular manifestation, albeit less common than pulmonary AVMs.3 It is usually the result of very high cardiac output related to hepatic shunting. Rarely, pulmonary hypertension is the result of a distal small-vessel vasculopathy indistinguishable from idiopathic pulmonary arterial hypertension.
Hepatic AVMs
Hepatic AVMs are found in up to 70% of patients but are often asymptomatic.2 Major hepatic AVMs can lead to high-output cardiac failure, liver failure or portal hypertension.
Gastrointestinal bleeding
Telangiectasia can be found anywhere in the GI tract but are most commonly present in the stomach and proximal small intestine.3 GI bleeding affects about 20% of all adults.2 Clinical signs of bleeding are more apparent in adulthood, often between 40 and 50 years of age. In patients who have received iron therapy for at least three months, the severity of GI bleeding should be graded according to the following:1
- mild GI bleeding in those who meet their haemoglobin goals with oral iron replacement
- moderate GI bleeding in those who meet their haemoglobin goals with intravenous iron treatment
- severe GI bleeding in those who do not meet their haemoglobin goals despite adequate iron replacement or in those who require blood transfusion.
CNS manifestations
CNS manifestations may affect 10 to 15% of patients with HHT.2,3,9 Cerebral AVMs may be asymptomatic, numerous and present at birth.2,3 These may result in epilepsy, transient ischaemic attacks, stroke or spinal haemorrhage. Spinal AVMs are rarer than brain AVMs. Patients with spinal AVMs usually present with paralysis with or without back pain; most cases are diagnosed and treated in the first decade of life.3
Management
Multidisciplinary care
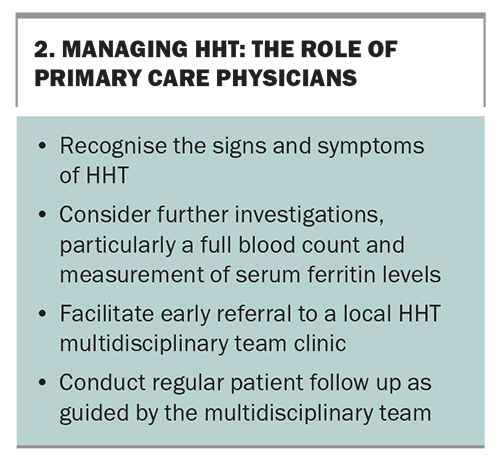
Patients with HHT can have complex multi-organ disease and, ideally, should be assessed at a dedicated HHT multidisciplinary team clinic (Box 1). For many patients with a mild HHT phenotype, a shared-care approach with the primary care physician is appropriate
(Box 2). For complex cases involving significant end-organ disease, the patient’s care should be co-ordinated by an HHT multidisciplinary team clinic with access to subspecialty services, such as ear, nose and throat surgery; respiratory medicine; gastroenterology; haematology; interventional radiology; and genetics.
Genetic counselling
Patients with HHT should be made aware of the autosomal dominant transmission of the disease and offered genetic counselling.21 When a causative mutation has been identified in the proband, predictive testing can be considered in relatives. Preimplantation diagnosis is an option for patients with HHT wish to be parents and to ensure the causative mutation is not passed on to offspring.
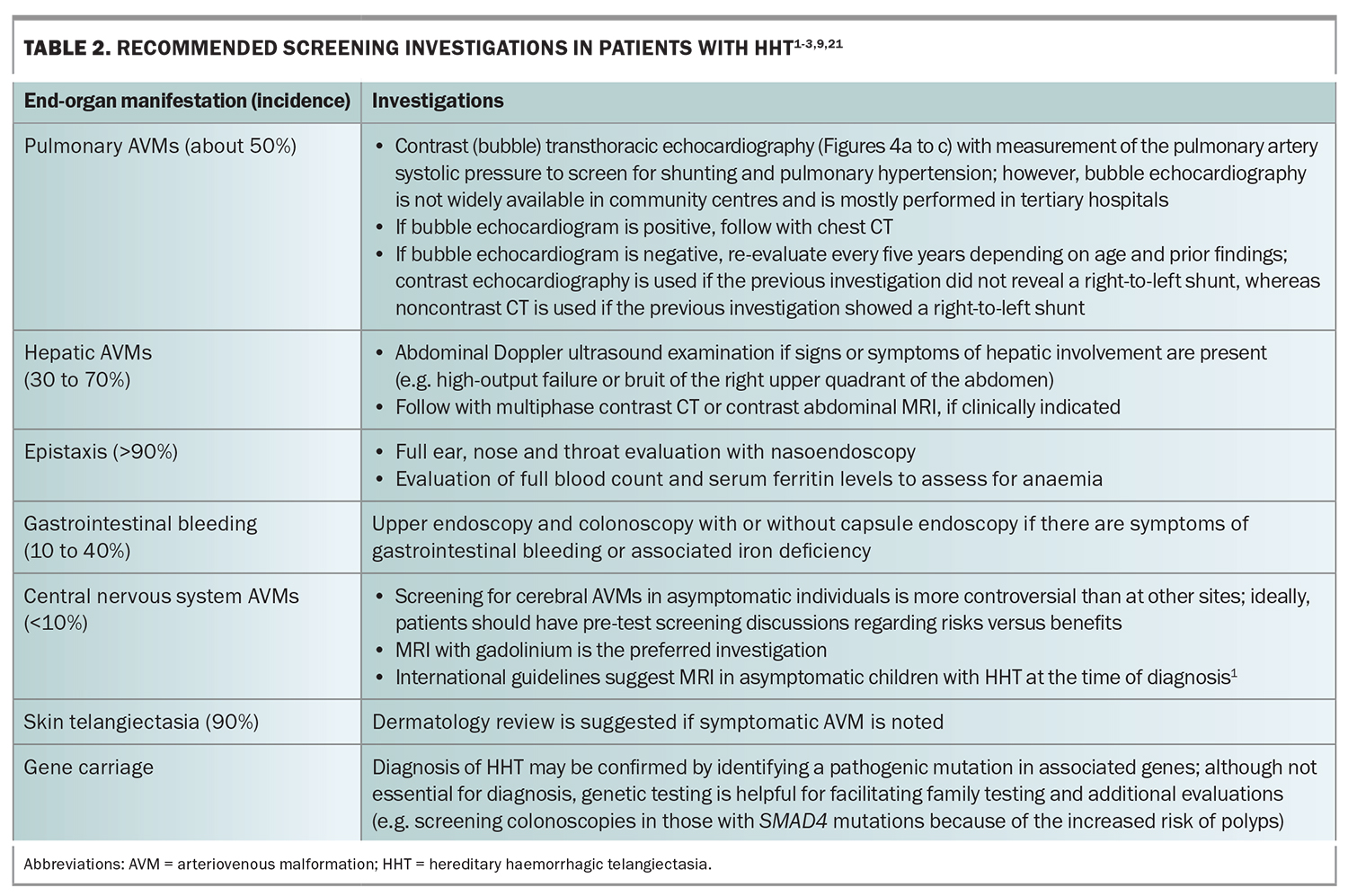
Screening for end-organ manifestations
One of the most important aspects of HHT care is to screen for potential end-organ involvement. The modality and frequency of screening are based on expert opinion, with recommended screening investigations outlined in Table 2 and Figure 4.1-3,9,21 A case example is presented in Box 3.
Specific management of HHT
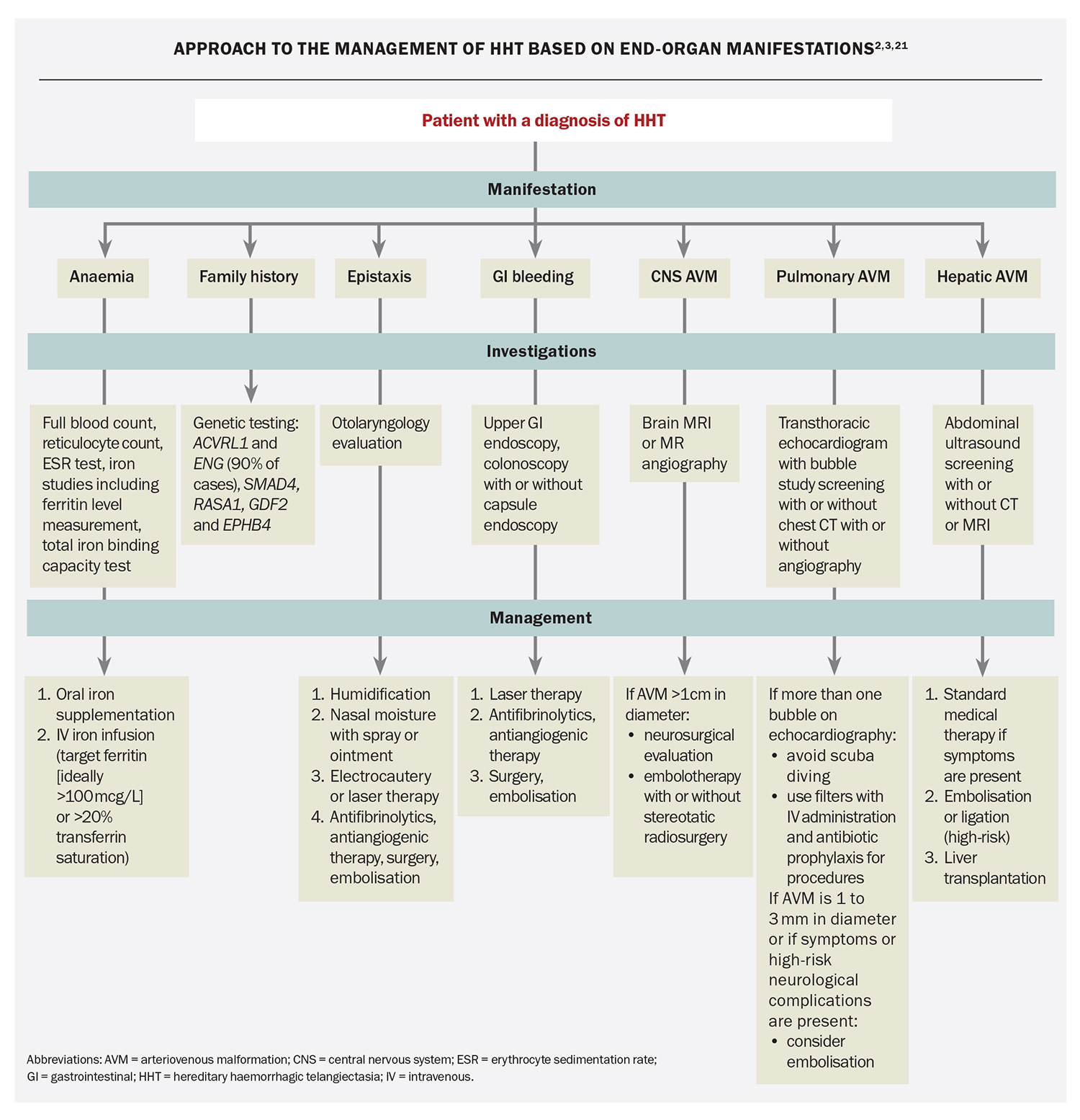
There are no standard medical therapies for HHT given the few randomised trials in this field.2 Management can include supportive care, lesion-specific therapy and systemic treatment
(Flowchart).2,3,21 Supportive measures include iron supplementation (oral and intravenous initially, if not effective) and red blood cell transfusion for bleeding and anaemia.1,3 Directed treatment to ablate bleeding sites and AVMs may require input from ENT, interventional radiology and neurosurgery specialists. Anticoagulants and NSAIDs, such as acetylsalicylic acid and ibuprofen, should be avoided if possible.3
Epistaxis
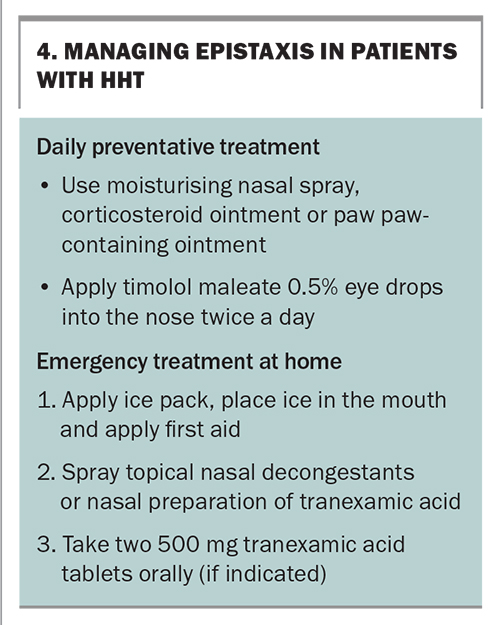
Epistaxis is likely to be the most troublesome day-to-day symptom for patients with HHT and their primary care physicians. Patients with HHT who are reviewed in a multidisciplinary team clinic are provided with education on nasal care, consisting of the use of regular nasal lubricants, such as petroleum jelly, paw paw-containing ointment or moisturising nasal sprays.22 Topical beta blockers can also be introduced as part of the treatment for adult patients (e.g. timolol maleate-based eye drop formulation).23,24 If there are no contraindications, some patients can take regular tranexamic acid (at a dose of 1 g) to manage symptoms.1,25
Assessment and management for epistaxis should ensue as for epistaxis with any other cause (Box 4).26 Patients with HHT with significant epistaxis should be given instructions regarding emergency treatment at home for breakthrough epistaxis. This includes applying an ice pack and basic first aid. Patients are also instructed to keep a supply of nasal decongestants (e.g. oxymetazoline hydrochloride, xylometazoline hydrochloride), as well as compounded nasal tranexamic acid (10% or 2 g/20 mL), which can be used for epistaxis. Additional oral tranexamic acid tablets (dose of 1 g) can also be given.
Patients who continue to be symptomatic despite regular treatment should be considered for ablation of the AVM.1 Larger AVMs can be treated with coblation, and smaller AVMs can be treated using a potassium–titanyl–phosphate laser (wavelength: 532 nm) or blue-light laser (wavelength: 445 nm). In some patients, septodermoplasty (application of a split- thickness skin graft to the nasal septum) may be considered.1,27 Historical procedures, such as the Young’s procedure or free-flap grafting of the nasal cavity, are rarely performed.
Pulmonary AVMs
Management of pulmonary AVMs will depend on their size, symptoms and location and the approaches may include surgery, embolisation or continued surveillance.2 Any pulmonary AVM with a feeding artery 1 to 3 mm in size detected on chest CT should be considered for treatment with transcatheter embolisation by a radiologist with experience in treating pulmonary AVMs.3 The main aim of treating pulmonary AVMs is to prevent paradoxical stroke, but treatment is also indicated for symptoms of dyspnoea and hypoxaemia, as well as for the prevention of haemorrhage. Some patients may require repeated procedures to achieve embolisation of all pulmonary AVMs. With advances in interventional radiology, most pulmonary AVMs are amenable to percutaneous closure, although large, complex pulmonary AVMs may still require surgery. Antibiotic prophylaxis is recommended for dental work and other procedures with a significant risk of bacteraemia in all patients with evidence of a pulmonary shunt.
Gastrointestinal bleeding
Treatment is often unnecessary for GI AVMs, unless aggressive iron therapy has been ineffective in maintaining haemoglobin concentrations.3 However, the presence of symptoms or a sharp decline in haematocrit levels should prompt upper and lower GI endoscopy to assess for GI bleeding.2 If bleeding is not found, capsule endoscopy should be performed.1,3,28,29
Argon plasma coagulation and other local therapies may be administered concurrently with the initial endoscopy for lesions with active bleeding, or lesions 1 to 3 mm in size if nonbleeding, as the mainstay.1,3,5,30 Repeated sessions are avoided to reduce iatrogenic injury to the mucosa.1 If there is recurrent bleeding, multiple AVMs or small-bowel AVMs, additional systemic pharmacotherapy may be needed.2,5,20
Hepatic AVMs
No treatment is recommended for asymptomatic hepatic AVMs.1 Most symptomatic hepatic AVMs can be satisfactorily managed with intensive medical therapy aimed at improving high-output cardiac failure and hepatic dysfunction.3,31 Angiographic treatment of hepatic AVMs may be helpful but is considered a high-risk procedure.2 Referral for liver transplantation could be considered for symptomatic patients in whom medical therapy has failed.1,3
CNS AVMs
Similar to the management of pulmonary AVMs, the management of CNS AVMs will depend on the site, size and symptoms.2,3 The risk of cerebral haemorrhage from these high-flow lesions may warrant treatment, even in young, asymptomatic individuals. Typically, AVMs larger than 1.0 cm in diameter may be treated with neurovascular surgery, embolotherapy or stereotactic radiosurgery.3
Systemic therapy for bleeding
Oral antifibrinolytics can be considered for mild HHT-related bleeding.1 Oral tranexamic acid is preferred and can be safely coadministered with other systemic antiangiogenic therapies. This is contraindicated in patients with recent venous thromboembolism or arterial thrombosis. Antifibrinolytics should also be used with care in patients with known untreated pulmonary AVMs, particularly if there is a history of a paradoxical ischaemic event.
Bevacizumab, a recombinant humanised monoclonal antibody that blocks angiogenesis via vascular endothelial growth factor inhibition, can be considered for refractory epistaxis and GI bleeding.1,2,20,32 This drug is given off label, and administered intravenously as a six-month course initially. Some patients will require either maintenance or repeat dosing if bleeding recurs. In observational and retrospective studies, bevacizumab has been shown to alleviate anaemia and reduce transfusion requirements.7,20,32,33 The side effects include hypertension, delayed wound healing, proteinuria and venous thromboembolism.
Pregnancy
Most pregnancies in women with HHT are uneventful.3,5 Epistaxis and skin telangiectasia may worsen. The most common serious complications include pulmonary haemorrhage and stroke related to untreated pulmonary AVMs, intracranial haemorrhage and high-output cardiac failure. Thus, screening for cerebral and pulmonary AVMs should be performed prior to pregnancy. Pregnant women with untreated pulmonary or CNS AVMs, as well as those who have not been screened, should be considered to have a high risk of haemorrhagic and neurological complications. These patients should be managed by a high-risk obstetrics team with the close support of an HHT multidisciplinary team.1
Conclusion
HHT is an uncommon, genetic bleeding disorder that demands greater attention to reduce its delayed diagnosis, as well as facilitate early screening and treatment for end-organ complications. In general, lesions of the skin and oral and GI mucosa are guided by symptoms; however, AVMs of the lungs and CNS are treated more routinely in asymptomatic patients because of the possibility of life-threatening complications. MT
COMPETING INTERESTS: Dr Karas and Dr Cheong: None. Associate Professor Low is Chair of the Head and Neck Multidisciplinary Team at the Chris O'Brien Lifehouse; and Director of the Sydney Facial Nerve Service.
References
1. Faughnan ME, Mager JJ, Hetts SW, et al. second international guidelines for the diagnosis and management of hereditary hemorrhagic telangiectasia. Ann Intern Med 2020; 173: 989-1001.
2. Kritharis A, Al-Samkari H, Kuter DJ. Hereditary hemorrhagic telangiectasia: diagnosis and management from the hematologist’s perspective. Haematologica 2018; 103: 1433-1443.
3. McDonald J, Baryrak-Toydemir P, Pyeritz RE. Hereditary hemorrhagic telangiectasia: an overview of diagnosis, management, and pathogenesis. Genet Med 2011; 13: 607-616.
4. Dakeishi M, Shioya T, Wada Y, et al. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum Mutat 2002; 19: 140-148.
5. Shovlin CL. Hereditary hemorrhagic telangiectasia (HHT): evaluation and therapy for specific vascular lesions. UpToDate. Wolters Kluwer; 2023. Available online at: https://www.uptodate.com/contents/hereditary-hemorrhagic-telangiectasia-hht-evaluation-and-therapy-for-specific-vascular-lesions (accessed April 2023).
6. Pierucci P, Lenato GM, Suppressa P, et al. A long diagnostic delay in patients with hereditary haemorrhagic telangiectasia: a questionnaire-based retrospective study. Orphanet J Rare Dis 2012; 7: 33.
7. Iyer VN, Apala DR, Pannu BS, et al. Intravenous bevacizumab for refractory hereditary hemorrhagic telangiectasia-related epistaxis and gastrointestinal bleeding. Mayo Clin Proc 2018; 93: 155-166.
8. Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000; 91: 66-67.
9. Shovlin CL. Clinical manifestations and diagnosis of hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome). UpToDate. Wolters Kluwer; 2023. Available online at: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-hereditary-hemorrhagic-telangiectasia-osler-weber-rendu-syndrome (accessed April 2023).
10. McDonald J, Wooderchak-Donahue W, VanSant Webb C, et al. Hereditary hemorrhagic telangiectasia: genetics and molecular diagnostics in a new era. Front Genet 2015; 6: 1.
11. McDonald J, Bayrak-Toydemir P, DeMille D, Wooderchak-Donahue W, Whitehead K. Curaçao diagnostic criteria for hereditary hemorrhagic telangiectasia is highly predictive of a pathogenic variant in ENG or ACVRL1 (HHT1 and HHT2). Genet Med 2020; 22: 1201-1205.
12. Gallione CJ, Repetto GM, Legius E, et al. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 2004; 363: 852-859.
13. El Hajjam M, Mekki A, Palmyre A, et al. RASA1 phenotype overlaps with hereditary haemorrhagic telangiectasia: two case reports. J Med Genet 2021; 58: 645-647.
14. Amyere M, Revencu N, Helaers R, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation 2017; 136: 1037-1048.
15. Wooderchak-Donahue WL, McDonald J, O’Fallon B, et al. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet 2013; 93: 530-537.
16. McDonald J, Damjanovich K, Millson A, et al. Molecular diagnosis in hereditary hemorrhagic telangiectasia: findings in a series tested simultaneously by sequencing and deletion/duplication analysis. Clin Genet 2011; 79: 335-344.
17. McDonald J, Wooderchak-Donahue WL, Henderson K, Paul E, Morris A, Bayrak-Toydemir P. Tissue-specific mosaicism in hereditary hemorrhagic telangiectasia: implications for genetic testing in families. Am J Med Genet A 2018; 176: 1618-1621.
18. Porteous ME, Burn J, Proctor SJ. Hereditary haemorrhagic telangiectasia: a clinical analysis. J Med Genet 1992; 29; 527-530.
19. Anning R, Huang J, Ronan A, Malmanche J, Asher R, Low TH. Improvement in epistaxis management: the experience of a dedicated hereditary haemorrhagic telangiectasia clinic. ANZ J Surg 2022; 92: 499-504.
20. Al-Samkari H, Kritharis A, Rodriguez-Lopez JM, Kuter DJ. Systemic bevacizumab for the treatment of chronic bleeding in hereditary haemorrhagic telangiectasia. J Intern Med 2019; 285: 223-231.
21. Shovlin CL. Hereditary hemorrhagic telangiectasia (HHT): routine care including screening for asymptomatic AVMs. UpToDate: Wolters. Kluwer; 2023. Available online at: https://www.uptodate.com/contents/hereditary-hemorrhagic-telangiectasia-hht-routine-care-including-screening-for-asymptomatic-avms (accessed April 2023).
22. Whitehead KJ, Sautter NB, McWilliams JP, et al. Effect of topical intranasal therapy on epistaxis frequency in patients with hereditary hemorrhagic telangiectasia: a randomized clinical trial. JAMA 2016; 316: 943-951.
23. Ichimura K, Kikuchi H, Imayoshi S, Dias MS. Topical application of timolol decreases the severity and frequency of epistaxis in patients who have previously undergone nasal dermoplasty for hereditary hemorrhagic telangiectasia. Auris Nasus Larynx 2016; 43: 429-432.
24. Mei-Zahav M, Blau H, Bruckheimer E, Zur E, Goldschmidt N. Topical propranolol improves epistaxis in patients with hereditary hemorrhagic telangiectasia - a preliminary report. J Otolaryngol Head Neck Surg 2017; 46: 58.
25. Lee M, Naidoo Y. Managing epistaxis – a guide for GPs. Med Today 2022; 23: (6) 35-43.
26. Gaillard S, Dupuis-Girod S, Boutitie F, et al. ATERO Study Group. Tranexamic acid for epistaxis in hereditary hemorrhagic telangiectasia patients: a European cross-over controlled trial in a rare disease. J Thromb Haemost 2014; 12: 1494-1502.
27. Levine CG, Ross DA, Henderson KJ, et al. Long-term complications of septal dermoplasty in patients with hereditary hemorrhagic telangiectasia. Otolaryngol Head Neck Surg 2008; 138: 721-724.
28. van Tuyl SA, Letteboer TG, Rogge-Wolf C, et al. Assessment of intestinal vascular malformations in patients with hereditary hemorrhagic teleangiectasia and anemia. Eur J Gastroenterol Hepatol 2007; 19: 153-158.
29. Canzonieri C, Centenara L, Ornati F, et al. Endoscopic evaluation of gastrointestinal tract in patients with hereditary hemorrhagic telangiectasia and correlation with their genotypes. Genet Med 2014; 16: 3-10.
30. Kwan V, Bourke MJ, Williams SJ, et al. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol 2006; 101: 58-63.
31. Buscarini E, Leandro G, Conte D, et al. Natural history and outcome of hepatic vascular malformations in a large cohort of patients with hereditary hemorrhagic teleangiectasia. Dig Dis Sci 2011; 56: 2166-2178.
32. Guilhem A, Fargeton AE, Simon AC, et al. Intra-venous bevacizumab in hereditary hemorrhagic telangiectasia (HHT): a retrospective study of 46 patients. PLoS One 2017; 12: e0188943.
33. Al-Samkari H, Kasthuri RS, Parambil JG, et al. An international, multicenter study of intravenous bevacizumab for bleeding in hereditary hemorrhagic telangiectasia: the InHIBIT-Bleed study. Haematologica 2021; 106: 2161-2169.