The ravages of prolonged bed rest: an update on multidisciplinary care for the deconditioned patient

Deconditioning describes functional decline, progressive weakness and physiological changes that result from prolonged bed rest and inactivity. Frailty is a syndrome in which age-related physiological changes are accelerated or exacerbated in multiple systems. Deconditioning is more likely in patients who experience frailty. Due to the complexities of these conditions, optimal management involves a multidisciplinary team comprised of GPs, specialists and allied health professionals.
- Deconditioning is used to describe the physical and physiological changes that result from prolonged inactivity, immobility and bed rest associated with acute or chronic illness, injury and social isolation.
- Multisystem changes can occur within 24 hours of bed rest; these changes worsen with prolonged inactivity, potentially requiring more time to reverse (where possible).
- GPs play an important role in identifying, diagnosing and managing deconditioning.
- Adequately managing the complex changes associated with deconditioning requires a multidisciplinary approach with the involvement of various allied health specialists. Areas on which to focus include exercise, nutrition and psychosocial supports.
Deconditioning is a term used to describe functional decline, progressive physical weakness and associated physiological changes resulting from inactivity and prolonged bed rest after illness and injury. Deconditioning may occur acutely (due to new illness or hospitalisation and associated bed rest), progressively (due to chronic illness and progressive decline in activity), or both acutely and progressively.
Deconditioning can affect people of any age but is more common in those who experience frailty, as they are more vulnerable to the negative effects of bed rest. Additionally, deconditioning is one of the contributors to frailty. Older people and those with multiple chronic comorbidities are at higher risk of developing frailty, which is characterised by a decline in function across multiple physical and physiological systems and an increased vulnerability to psychosocial stressors.1 As such, it is recommended that medical care be complemented by a multidisciplinary team of nurses, psychologists and allied health specialists.
The outcomes of deconditioning and frailty after hospitalisation are common and well recognised. Evidence suggests that addressing nutrition and increasing activity and exercise during hospitalisation may reduce deconditioning and the risk of frailty associated with hospitalisation.2,3 In community settings, the impacts of COVID-19 and associated social isolation during lockdown inadvertently created a secondary pandemic of deconditioning and frailty; this serves to highlight the significant role played by primary healthcare professionals in diagnosing and managing patients with deconditioning and frailty.4 Deconditioning can also mimic long COVID, particularly in older patients and people living with disability.5
This article provides recommendations for screening and diagnosis in general practice and highlights the role of multidisciplinary rehabilitation as an important adjunct to medical management for the rehabilitation of patients who experience deconditioning.
What is deconditioning?
Deconditioning is an umbrella term that describes the physical, physiological and functional changes following a period of inactivity and bed rest, usually in the setting of illness or injury.6 These changes can cause functional decline and contribute to, or exacerbate, disability (Table 1).6-10 The onset of physiological changes can occur after as little as two days of inactivity, with an association between its severity and the duration of inactivity. A longer duration of inactivity is also associated with a longer than expected recovery period. Deconditioning can be reversed by an increase in activity. Severity and recovery are associated with a patient’s premorbid levels of fitness (or frailty) and the degree of superimposed inactivity.6
The deleterious effects of hospitalisation and bed rest on older adults have been described in the literature since the 1980's, with 30 to 60% of affected patients requiring assistance with activities of daily living during admission. More than 50% of patients aged 75 years and older require assistance even after hospitalisation and only half of those patients are able to resume their usual functional activities three months after discharge.11-14 In 2021, deconditioning was the most common diagnosis in those requiring inpatient rehabilitation in Australia and it remains the most common cause of people requiring community rehabilitation services.15
What is frailty?
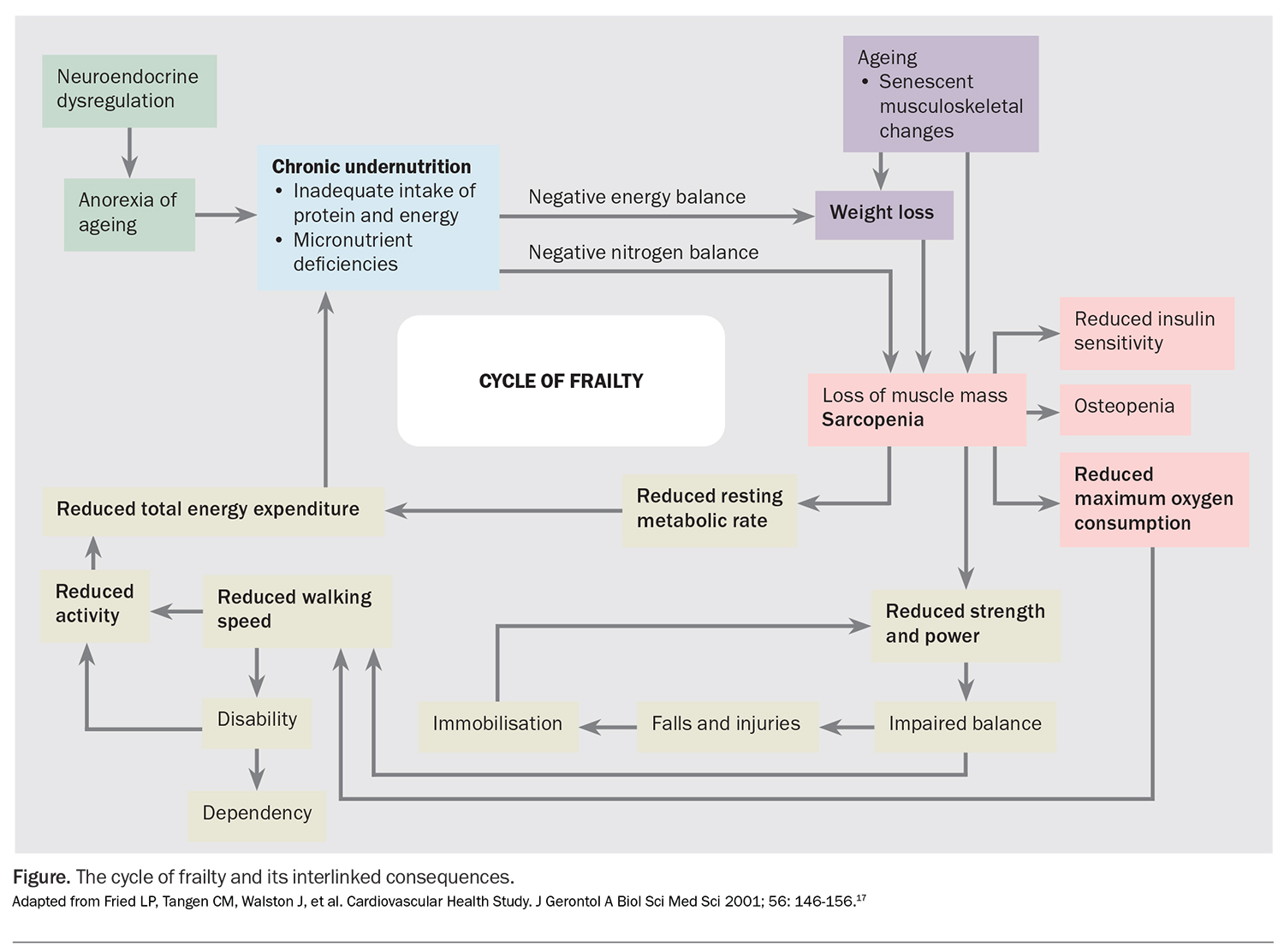
Frailty is a syndrome in which age-related physiological changes are accelerated or exacerbated in multiple body systems, resulting in a loss of functional homeostatic reserve and a reduced resilience to minor stress events. Individuals with frailty are predisposed to adverse health consequences, including sarcopenia, osteoporosis, anorexia, fatigue, and a higher falls risk.16 These interlinked consequences and the cycle of frailty is best summarised in the Figure.17
A frailty continuum exists with a significant difference in the level of dependency and disability in the robust (not at risk), prefrail (at risk, exhibiting some frailty characteristics) or frail older adult.17 Early detection of a patient in the prefrail state reduces risk of progression to frailty and associated dependency and disability.18 Due to a lower premorbid level of fitness, the prefrail or frail patient is also at increased risk of experiencing deconditioning.
It was estimated in 2018 that about 20% of Australians aged 65 years and older experienced frailty, with a further 48% qualifying as prefrail. Women were found to be twice as likely to experience frailty compared with men and incidence was found to increase with advancing age.19 In 2019, about 50% of patients who accessed reconditioning programs in Australia were classed as having mild to severe frailty.15 Australian statistics were higher than global reports, with global incidence of frailty and prefrailty being reported as 43.4%.20
Frailty was found to affect almost 40% of patients aged 80 years and older in intensive care units, with most requiring high level residential care (even after recovery from an acute illness). It was also associated with increased morbidity and mortality in this age group.21
Studies have shown that older Australians living in residential aged care facilities appear to have higher levels of frailty, with increased time in facilities and age being predictive factors for physical frailty; this suggests that the condition is not being adequately addressed.22
Assessment and diagnosis of frailty and deconditioning
Frailty
No single biomarker exists to predict or diagnose frailty, which remains a clinical diagnosis. Several frailty screening and diagnostic tools exist, which vary in length and format (questionnaire vs physical assessment). There is no consensus on a gold standard assessment tool.
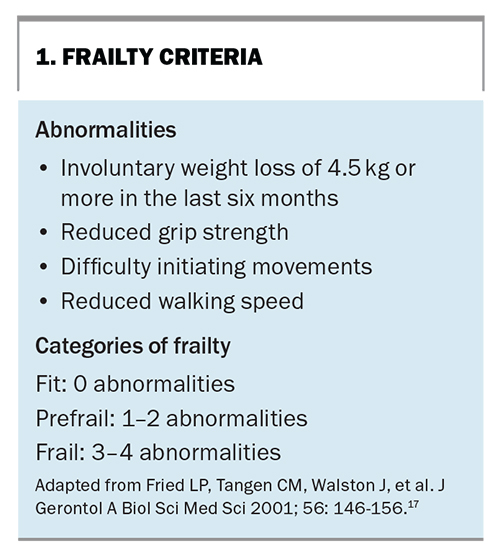
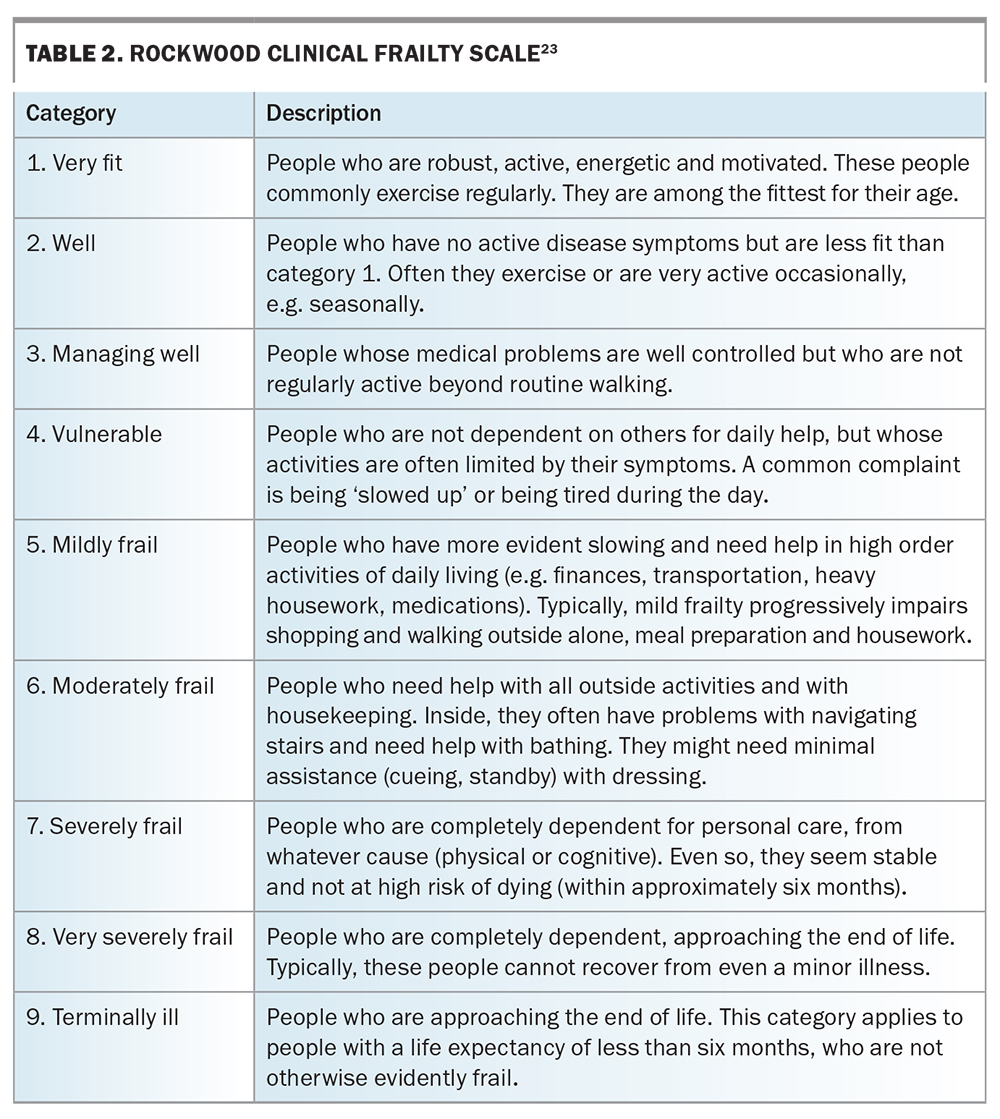
One of the earliest tools (developed in 2001) defines a frailty phenotype and provides diagnostic criteria, including weight loss, exhaustion, weakness, slowness and low physical activity (Box 1).17 The Clinical Frailty Index (developed in 2005 and further modified in 2012) is another well-established tool that provides additional grading of the severity of frailty (Table 2).23 A reported drawback of using these diagnostic tools for frailty screening in clinical practice is the time and labour required for their completion. The FRAIL Scale (developed in 2017) is a validated, shortened questionnaire, which may be a more practical alternative in the primary care setting (Box 2).24
Frailty assessment is performed by different clinical specialities for risk stratification and the identification of high-risk patients. Active screening of a person in the prefrail state identifies those in need of further multidisciplinary care, with the aim of reversing the early physical and physiological changes to reduce their risk of progression to established frailty. Once frailty is established, reversal of changes with treatment is slower and patients may not return to their premorbid baseline; however, a prevention of further decline is still achievable.
Deconditioning
A recent systematic review found that no single assessment tool exists that adequately includes all the components needed for a diagnosis of hospital-acquired deconditioning. However, the review outlined the relevant body systems and areas of physical examination to assess in patients who are suspected of having deconditioning.25 These findings have been adapted with recommendations to GPs on the screening and assessment of deconditioning (Table 3).25-28
Implications of walking speed
Walking relies on a combination of functioning musculoskeletal, sensory, neural, cognitive and nutritional factors. The speed of walking has been correlated with functional ability, physiological changes and balance confidence. Therefore, walking speed may be a key clinical indicator of deconditioning and frailty.29
Management
Deconditioning and frailty are both complex multisystem conditions. Clinical practice guidelines for frailty outline the strong and conditional recommendations for its management.30 Although the management of frailty is beyond the scope of this article, there are key concepts of managing frailty that also apply in the prevention of physical and physiological changes associated with deconditioning. These concepts include multidisciplinary care, exercise, nutrition and adequate supports.
Multidisciplinary care
Deconditioning and frailty are complex multisystem conditions that require patient-centred multidisciplinary care to address the systemic changes involved (Box 3). GPs are experts in co-ordinating and overseeing this style of healthcare, with the provision of screening, routine healthcare, chronic disease management plans, care plans and referrals.
Exercise
The reintroduction of activity and exercise is important. Exercise should be individually prescribed, graded and progressive to ensure it is safe and approachable. For example, lower limb strengthening and balance exercises combined with walking can improve physical performance and quality of life in older patients in hospital, compared with standard care.31
Although exercise involves a combination of flexibility, balance, resistance and aerobic training, some guidelines suggest resistance and balance training should precede aerobic training.29,32 An allied health professional (e.g. physiotherapist or exercise physiologist) can provide an individualised, graded exercise program that is monitored and adapted based on the patient’s condition and progress.
Nutrition
Evidence for the benefits of nutritional intervention is mixed due to various confounding factors, such as the duration and type of nutritional intervention and the pretreatment status of participants. Nutritional intervention should be combined with exercise and other multidisciplinary care.29 Higher levels of daily protein intake are required in the setting of muscle accretion (from 1.6 to 2.2 g/kg/day).33,34 The International Society of Sports Nutrition suggests even higher levels of daily protein intake (2.3 to 3.1 g/kg/day) are necessary for patients who are exercising during hypocaloric states and should be considered in patients with an underlying catabolic condition.34
Multiple nutritional interventions are available, most commonly as nutritional education and counselling, supplementation of micronutrients (e.g. vitamin D, multivitamin or omega-3 fatty acids), daily food fortification with protein supplements and specific nutritional supplemental formulas.35
Preliminary research has suggested that leucine (an amino acid found in unprocessed beef, oily and white fish and bread) may play an important role in preventing age-related physical decline, with studies suggesting higher dietary leucine intake is associated with a reduced risk of frailty and improvements in functional performance and lean muscle mass.36,37 A dietitian or nutritionist can offer tailored advice and regular monitoring to patients.
Adequate supports
Psychosocial factors should also be considered. Both deconditioning and frailty increase the likelihood of dependency and disability. Patients may struggle with activities of daily living (including self-care and domestic tasks) but not have access to formal (services) or informal (family and friends) supports. Patients may also experience secondary psychological stressors, such as associated mood changes, difficulty with adjustment following illness and loss of independence, as well as new sleep disturbances and insomnia. Cognitive changes can impact a patient’s function and their safety in the community should be assessed. If not addressed, these additional factors can affect treatment compliance and outcomes.
Conclusion
Deconditioning results from prolonged inactivity, immobility, or bed rest and is associated with acute or chronic illness and social isolation. It is a complex syndrome involving multiple systems, resulting in physical and physiological changes, functional decline and disability.
The impact of deconditioning following hospitalisation and bed rest is well established, but recent data from the COVID-19 pandemic have highlighted that the risk also extends to people in the community – particularly, older or vulnerable adults and those at risk of frailty. The GP has an important role in identifying and diagnosing deconditioning. Early identification and multidisciplinary management are associated with faster recovery in affected patients. MT
COMPETING INTERESTS: None.
References
1. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019; 394: 1365-1375.
2. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet 2019; 394: 1376-1386.
3. Dent E, Daly RM, Hoogendijk EO, Scott D. Exercise to prevent and manage frailty and fragility fractures. Curr Osteoporos Rep 2023; 21: 205-215.
4. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health 2020; 5: e256.
5. Ferreira EVM, Oliveira RKF. Mechanisms of exercise intolerance after COVID-19: new perspectives beyond physical deconditioning. J Bras Pneumol 2021; 47: e20210406.
6. Avoiding Deconditioning. Scotland: NHS Highland – Remote and Rural Healthcare Education Alliance (RRHEAL.); 2021. Available online at: https://learn.nes.nhs.scot/48905/rrheal/nhs-highland-virtual-lectures/avoiding-deconditioning (accessed October 2023).
7. Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007; 297: 1769–1774.
8. Parker S, Rollinson R, Gilad M, Holmes K, Sygall N, Faux S. The ravages of bed rest: rehabilitation after prolonged immobility. Medicine Today 2008; 9(8): 36-44.
9. Rudwill F, O’Gorman D, Lefai E, at al. Metabolic inflexibility is an early marker of bed-best-induced glucose intolerance even when fat mass is stable. J Clin Endocrinol Metab 2018; 103: 1910-1920.
10. Kara M, Kaymak B, Frontera W, et al. Diagnosing sarcopenia: functional perspectives and a new algorithm from the ISarcoPRM. J Rehabil Med 2021; 53: 1-14.
11. McVey L, Becker P, Saltz CC, Feussner JR, Cohen HJ. Effect of a geriatric consultation team on functional status of elderly hospitalized patients. A randomized, controlled clinical trial. Ann Intern Med 1989; 110: 79–84.
12. Warshaw GA, Moore JT, Friedman SW, et al. Functional disability in the hospitalized elderly. JAMA 1982; 248: 847–850.
13. Margitić S, Inouye S, Thomas JL, Cassel CK, Regenstreif DI, Kowal J. Hospital Outcomes Project for the Elderly (HOPE): rationale and design for a prospective pooled analysis. J Am Geriatr Soc 1993; 41: 258–267.
14. Fortinski RH, Covinsky KE, Palmer RM, Landefeld CS. Effects of functional status changes before and during hospitalization on nursing home admission of older adults. J Gerontol A Biol Sci Med Sci 1999; 54: 521-526.
15. The state of rehabilitation in Australia: the AROC annual report. Wollongong: Australasian Rehabilitation Outcomes Centre and The University of Wollongong; 2019. Available at: https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow272218.pdf (accessed October 2023).
16. Clegg A, Young J. The frailty syndrome. Clin Med (Lond) 2011; 11: 72-75.
17. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: 146-156.
18. Santamaría-Peláez M, González-Bernal J, González-Santos J, Soto-Cámara R. Differences between robust, frail, prefrail and dependent institutionalized older people. Aten Primaria 2021; 53: 101968.
19. Thompson MQ, Theou O, Karnon J, Adams RJ, Visvanathan R. Frailty prevalence in Australia: Findings from four pooled Australian cohort studies. Australas J Ageing 2018; 37: 155-158.
20. Ofori-Asenso R, Chin KL, Mazidi M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open 2019; 2: e198398.
21. Darvall J, Bellomo R, Paul E, et al. Frailty in very old critically ill patients in Australia and New Zealand: a population-based cohort study. Med J Aust 2019; 211: 318-323.
22. Milte, R, Petersen J, Boylan J, et al. Prevalence and determinants of physical frailty among people living in residential aged care facilities: a large-scale retrospective audit. BMC Geriatr 2022; 22: 424.
23. Moorehouse P, Rockwood K. Frailty and its quantitative clinical evaluation. J R Coll Physicians Edinb 2012; 42: 333-340.
24. Gleason LJ, Benton EA, Alvarez-Nebreda ML, Weaver MJ, Harris MB, Javedan H. FRAIL questionnaire screening tool and short-term outcomes in geriatric fracture patients. J Am Med Dir Assoc 2017; 18: 1082-1086.
25. Gordon S, Grimmer KA, Barras S. Assessment for incipient hospital-acquired deconditioning in acute hospital settings: a systematic literature review. J Rehabil Med 2019; 51: 397-404
26. Edelstein JE. Lower-limb orthoses for older adults In: Geriatric Physical Therapy. 3rd ed. St Louis: Mosby; 2012. pp. 412-425.
27. Protein: diet and nutrition health advice. Canberra: Dietitians Australia; 2022. Available online at: https://dietitiansaustralia.org.au/health-advice/protein/ (accessed October 2023).
28. Rogeri PS, Zanella R Jr, Martins GL, et al. Strategies to prevent sarcopenia in the aging process: role of protein intake and exercise. Nutrients 2021; 14: 52.
29. Walston J, Buta B, Xue QL. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med 2018; 34: 25-38.
30. Dent E, Lien C, Lim WS, et al. The Asia-Pacific clinical practice guidelines for the management of frailty. J Am Med Dir Assoc 2017; 18: 564-575.
31. McCullagh R, O’Connell E, O’Meara S, et al. Augmented exercise in hospital improves physical performance and reduces negative post hospitalization events: a randomized controlled trial. BMC Geriatr 2020; 20: 46.
32. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2013.
33. Stokes T, Hector AJ, Morton RW, McGlory C, Phillips SM. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 2018; 10: 180.
34. Jäger R, Kerksick CM, Campbell BI, et al. International Society of Sports Nutrition Position Stand: protein and exercise. J Int Soc Sports Nutr 2014; 14: 20.
35. Manal B, Suzana S, Singh DK, et al. Nutrition and frailty: a review of clinical intervention studies. J Frailty Aging 2015; 4: 100–106.
36. Vega-Cabello V, Caballero FF, Rodriguez-Artalejo F, Lopez-Garcia E, Struijk EA. Leucine intake and risk of impaired physical function and frailty in older adults. J Gerontol A Biol Sci Med Sci 2023; 78: 241-249.
37. Martínez-Arnau FM, Fonfría-Vivas R, at al. Effects of leucine administration in sarcopenia: a randomized and placebo-controlled clinical trial. Nutrients 2020; 12: 932.