Towards the optimal care of Parkinson’s disease – a guide for GPs

Parkinson’s disease is one of the archetypal and most common neurodegenerative disorders. However, our appreciation of its disease manifestations, its evolution over time and the need for a multidisciplinary approach to assessment and management has grown tremendously over recent decades. It is no longer ‘just a movement disorder’, but a multisystem condition that requires the attentive and agile collaboration of primary care and neurology.
- Parkinson’s disease is growing in prevalence and incidence and presents a major challenge for our healthcare system as the population ages.
- Parkinson’s disease is not just a movement disorder; it also affects multiple organ systems, including the gastrointestinal tract, mood, cognition and sleep.
- The best possible care of people living with Parkinson’s disease must involve multimodal assessment and treatments, and is best served by engaging a multidisciplinary team.
- The diagnosis of Parkinson’s disease is still predominantly clinical, and the use of imaging and biomarkers is largely still a research tool.
- The role of primary care in identifying and helping to manage the many possible manifestations of Parkinson’s disease, especially in later life, is vital to timely and patient-focused care.
First described by Dr James Parkinson in 1817, Parkinson’s disease now represents the second most common neurodegenerative disorder globally after Alzheimer’s disease. Its burden of death and disability has grown faster over the past 30 years than that of any other neurological disease.1-3
Parkinson’s disease is not a unitary disease. It has a variable age of onset and rate of progression and a broad array of clinical manifestations of patient responses to, and tolerance of, a growing list of treatment options, such that some experts have proposed (only somewhat facetiously) that there is no single archetypal Parkinson’s disease but rather six million individual variants in the world today.4
The optimal care of people with Parkinson’s disease involves the holistic and patient-centred care of an attentive GP, in alliance with a responsive neurologist and a team of allied health professionals, offers immense and sustained benefits to these patients. This article focuses on the GP’s role in the management of patients with Parkinson’s disease. Comprehensive discussion of advanced therapies, such as deep brain stimulation (DBS) or subcutaneous and gastrointestinal infusions, is outside the scope of the article.
Aetiology and pathogenesis
To date, a single, universal disease mechanism has remained elusive for Parkinson’s disease and likely does not exist, although ageing is the most common known risk factor for this disease. A complex interplay of genetic factors and environmental triggers is thought to result in a degenerative process that essentially leads to the gradual loss of dopaminergic neurons in the substantia nigra. The specific combination of predisposing and precipitating factors that is unique to each patient is likely responsible for much of the observed variability in the spectrum of symptoms and disease progression.
Genetics
So-called ‘monogenic’ Parkinson’s disease is relatively rare, with over 20 known genes contributing about 5 to 10% of disease incidence.5 Meanwhile, genome-wide association studies have identified more than 90 common genetic variations implicated in ‘polygenic’ Parkinson’s disease, and yet, these variations only explain 22% of its heritable component, meaning that a large proportion of hereditary factors remain undiscovered.6 It is possible that this genetic risk is responsible for a substantial fraction of what is currently labelled ‘sporadic’ or ‘idiopathic’ Parkinson’s disease.7
Environmental exposures
Epidemiological research has revealed several consistent exposure risks for Parkinson’s disease. Among the most well-established of these is exposure to pesticides (and perhaps as a proxy for this, a rural lifestyle in general), influenza infection and previous head trauma. Weaker associations are seen with type 2 diabetes, high consumption of dairy products, methamphetamine use and a history of certain cancers, especially melanoma. A range of other factors, including blood cholesterol levels, body mass index and hormonal or nutritional status, have demonstrated no reproducible association with Parkinson’s disease risk.8
Diagnosing Parkinson’s disease
The diagnosis of Parkinson’s disease remains, for most practical purposes, clinical. There is no imaging signature or blood biomarker that can substitute for the attentive observation of a suitably trained doctor. This was again reinforced in the most recent diagnostic criteria developed by the International Parkinson and Movement Disorder Society.9
The first step is to confirm the presence of the clinical syndrome known as ‘parkinsonism’; this requires the presence of bradykinesia (or slowed movement) plus either a resting tremor, rigidity or both. Bradykinesia can often be identified first, as the patient takes longer than usual to rise from their chair in the waiting room and make it into the doctor’s office. However, when subtle, it may be elicited more easily by asking the patient to perform rapid alternating movements of the fingers, wrists or lower limbs. With sustained repetition, a ‘decrement’ in amplitude and velocity of these movements becomes evident.
A parkinsonian resting tremor is defined as a 4 to 6 Hz tremor that emerges when a limb is completely relaxed, but may also ‘re-emerge’ during prolonged activity or when the patient is distracted. Occasionally, a postural tremor can be misidentified as a resting tremor if the arms are held under tension in the lap rather than completely relaxed.
Finally, rigidity (referring to increased muscle tone opposing the passive movement of a joint) can be demonstrated by gentle flexion and extension or circumduction movements at the wrist, among other simple manoeuvres, and typically worsens when the patient is asked to perform a simple movement with the other limb (so-called ‘coactivation’). Unlike spasticity, the ‘extrapyramidal’ increased muscle tone seen in Parkinson’s disease will not worsen with faster movement of a joint (i.e. is independent of velocity) and usually has an additional ratcheting ‘cogwheel’ characteristic.
Atypical parkinsonism
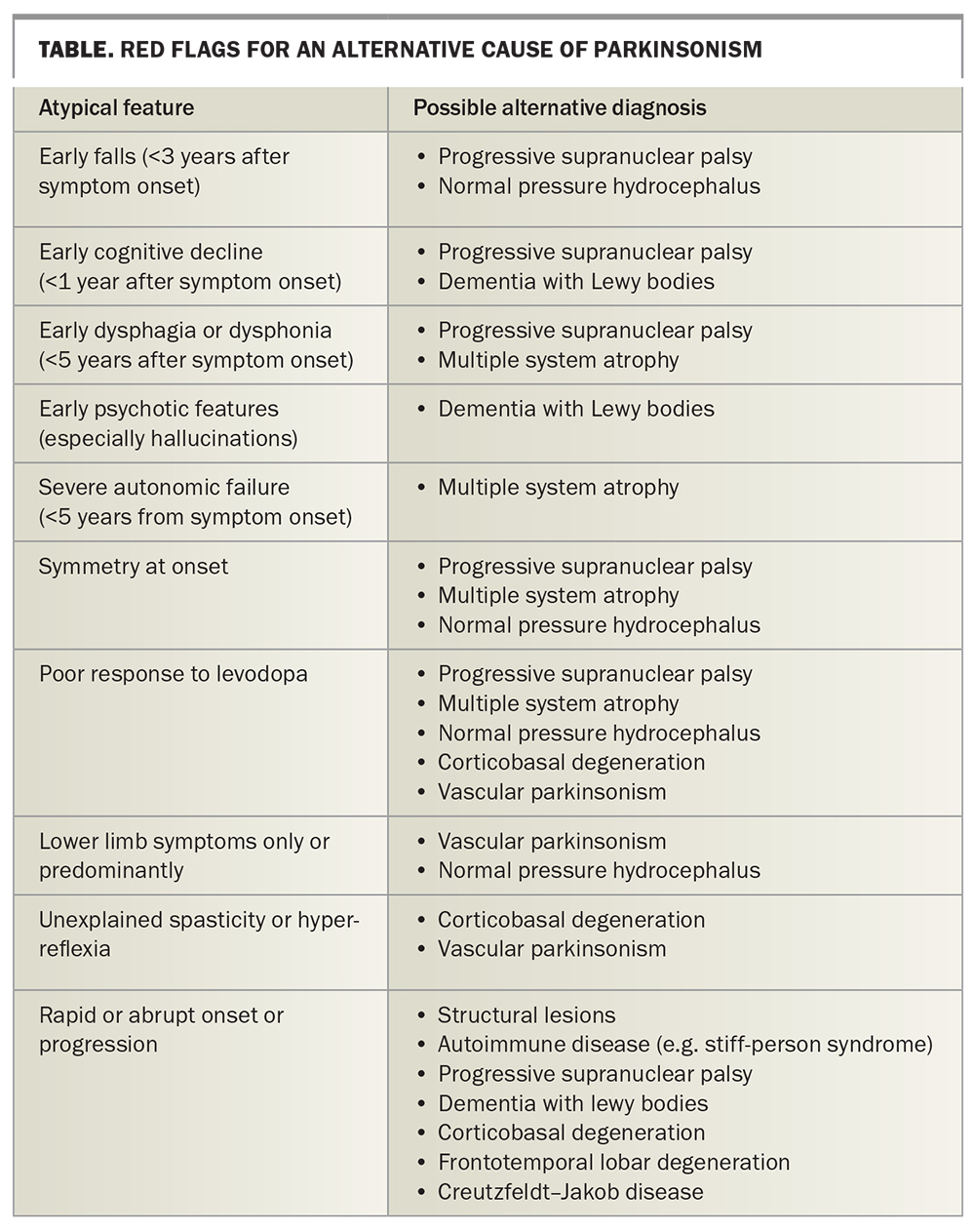
Having established the presence of the cardinal features of parkinsonism, the next step is to look for specific features (‘red flags’) that suggest atypical parkinsonism (Table). These include:
- rapid progression or, indeed, lack of progression of symptoms
- the presence of unexplained ‘pyramidal’ motor signs, such as spasticity or hyper-reflexia
- symptoms that are completely symmetric or limited to the lower limbs only
- the early onset of falls
- swallowing or speech dysfunction
- cognitive decline
- early features of severe autonomic dysfunction.
Any cerebral imaging abnormalities that support an alternative diagnosis, such as normal pressure hydrocephalus, severe microvascular ischaemia or focal cortical atrophy, should also be considered as red flags. A useful rule of thumb is to seek advice from a neurologist when parkinsonism is first suspected but also at any point subsequently if the patient’s symptoms do not evolve in an expected manner.
If no atypical features are found, the clinician should next enquire about supportive features of idiopathic Parkinson’s disease. The most salient supportive feature of idiopathic Parkinson’s disease remains a robust and persistent response to dopamine therapy. The emergence of dyskinesia (involuntary movements) with dopaminergic therapy is also considered supportive. The astute clinician may also look for features that would (in retrospect) diagnose the recently defined ‘prodromal Parkinson’s disease’.10 These features include a history of constipation, anxiety and depression, loss of olfaction and, most specifically, a history of rapid eye movement (REM) sleep behaviour disorder. It should be noted that patients with REM sleep behaviour disorder have an 80 to 90% lifetime risk of being diagnosed with Parkinson’s disease or a related ‘synucleinopathy’, such as multiple system atrophy, pure autonomic failure or Lewy body disease.
Neuroimaging
The primary role of neuroimaging in the patient with parkinsonism is to rule out alternative pathologies that might affect the substantia nigra or basal ganglia. Rarely, alternative structural lesions, such as normal pressure hydrocephalus, neoplasms, infections or vascular disease, can mimic Parkinson’s disease. It was, for example, a case of a tuberculoma in the substantia nigra that originally led to this structure being identified as the neuroanatomic substrate for the disease.11 It is reasonable to leave the ordering and interpretation of advanced imaging to the neurologist.
Natural history and clinical course
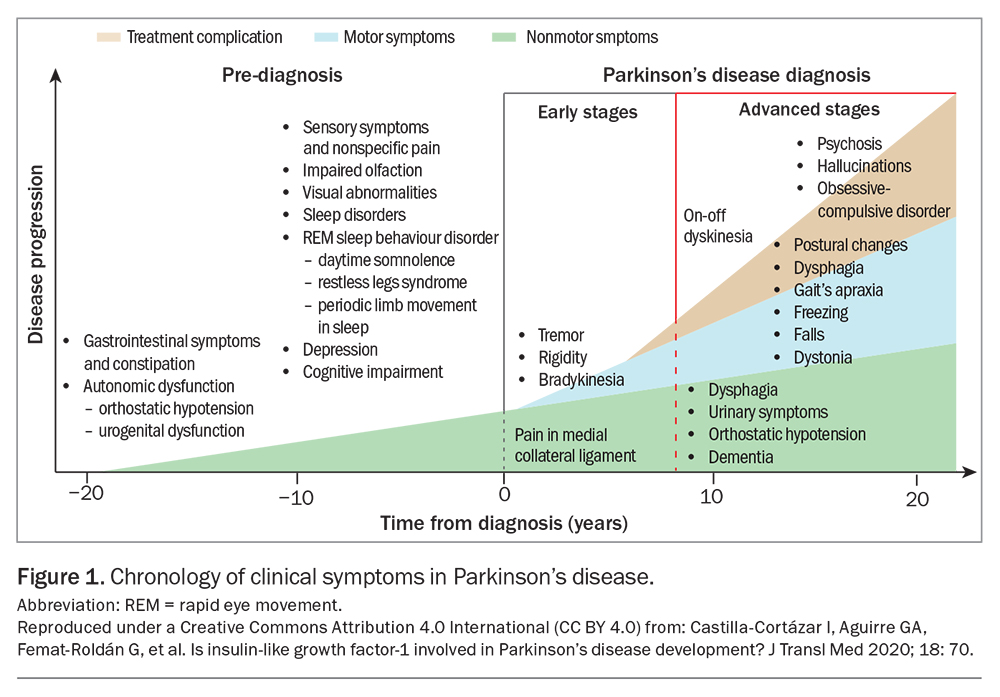
The progression of motor symptoms in Parkinson’s disease, including the emergence of treatment- and disease-related motor complications, generally follows a predictable course that relates to the progressive loss of dopaminergic reserve in the nigrostriatal pathways. In parallel, an underlying neurodegenerative process affects other neurological systems, leading to ‘nonmotor’ disease manifestations that often precede motor symptoms, tend to progress over time and be less responsive to dopaminergic therapy. These nonmotor features create a substantial burden of disease in patients with Parkinson’s disease and warrant deliberate attention.
Motor symptoms
In the early stages of disease, motor symptoms of bradykinesia, rigidity and tremor are often mild and troublesome, but not debilitating, and can be abolished with low doses of dopaminergic therapy. In many patients, this can be achieved with a single daily dose of a dopamine agonist, such as pramipexole, or a low total daily dose of levodopa. Often, the benefit of levodopa is prolonged, with dose effects lasting longer than would be expected based on simple pharmacokinetics (the so-called ‘long-dose response’). This period, when medical treatment can often return patients to an effectively asymptomatic state, can last for several years and has been termed the ‘honeymoon’ period.
Over time, patients will start to 'wear off' of their medication early. Wearing off refers to the re-emergence of Parkinson’s symptoms before the next dose, such that the levodopa dose frequency and total dose need to be increased. The long-dose response attenuates and motor symptoms fluctuate more closely with plasma drug levels. As the disease progresses and dopaminergic reserve declines further, the therapeutic window of levodopa shrinks; peak plasma levels cause involuntary movements (‘peak dose dyskinesia’) and trough levels lead to ‘off’ periods that occur earlier in the dose interval and last longer. This pattern is referred to as ‘motor fluctuations’ – the patient experiences too much or too little movement for an increasing proportion of their day. Patients may start falling more, which should remind the clinician to assess and optimise bone health. In patients who are physically and cognitively more robust, this is the time to start talking about advanced therapies, such as DBS or levodopa-containing intestinal gel infusion therapy. In patients who are more frail or have comorbidities, this is the time (if not earlier) to discuss residential care options and other aspects of advanced care planning.
Finally, as the disease progresses, the dose-response relationship becomes increasingly unpredictable, even chaotic. Some doses have no effect (so-called ‘dose failure’), and dyskinesias can emerge, both early and late in the dose interval (known as ‘diphasic dyskinesias’), making an estimation of under-versus overtreatment difficult. Meanwhile, higher and more frequent doses start to cause increasingly problematic nonmotor side effects, such as hallucinations and postural hypotension, and dose escalation is no longer possible. A larger fraction of the patient’s day is spent in the ‘off’ state, and quality of life and independence suffer tremendously. Neuropsychiatric issues, especially cognition, become increasingly prominent, and surrogate decision-makers are usually needed, hence the importance of establishing patient-centred priorities and care plans well in advance of this stage. At this point, optimising motor outcomes becomes far less important than the complex tasks of optimising bowel and bladder function, implementing falls prevention strategies and supporting mood and cognition. Needless to say, at this point the role of an attentive generalist (whether a GP or geriatrician) becomes increasingly indispensable.
Nonmotor symptoms
It has become increasingly clear over time that nonmotor features of Parkinson’s disease are pervasive, troublesome and in many ways dominate the clinical picture in both the earliest (‘prodromal’) and final stages of the disease (Figure 1). In the prodromal phase (i.e. the stage before the onset of cardinal motor symptoms), symptoms of hyposmia, constipation and REM sleep behaviour disorder are common and provide important retrospective clues to the diagnosis. There may be a constellation of autonomic features, such as postural hypotension or urinary and sexual dysfunction. Depression is common.
Initially, treatment of motor symptoms with dopamine agonists or levodopa can alleviate some of these nonmotor issues, especially dopamine-dependent features, such as mood and restless legs syndrome, but with time this treatment may worsen others, in particular orthostatic hypotension and cognition. Eventually, a balance needs to be struck between optimising increasingly precarious motor function and stemming the rising tide of treatment complications.
Fortunately, the many nonmotor features of Parkinson’s disease can be treated on their own merits in the usual fashion. As such, they provide ample scope for the attentive generalist to make a sizeable difference to their patient’s care.
Mood
Apathy, anxiety and low mood, are substantial burdens on the quality of life in patients living with Parkinson’s disease, with at least half of all patients experiencing each of these complaints (Box 1). The approach to managing mood disturbance in patients with Parkinson’s disease is largely similar to that for other patients, with the notable exception that, given the deficiency in dopamine, a dopamine-first approach can be effective. A patient who can tolerate a dopamine agonist, from the cognitive and psychiatric perspective (with special attention to dysregulated impulse control), may see their apathy and low mood resolve with a relatively low dose. For the patient already on levodopa, a diurnal pattern to the low mood may suggest the need for dose frequency changes.
For the patient who clearly ‘looks good but feels terrible’, a trial of antidepressant medication is reasonable, despite controversy over its relative effectiveness, and selective serotonin reuptake inhibitors remain the first-line treatment of choice.12 Sertraline, venlafaxine and paroxetine are the best studied selective serotonin reuptake inhibitors.
Cognition
Up to 80% of patients with Parkinson’s disease experience cognitive impairment and dementia during the course of their illness.13 The pattern of cognitive change is often characterised as ‘subcortical’, with patients demonstrating slowed thinking, impaired information processing and difficulty with memory retrieval.14 Compared with typical ‘cortical’ cognitive impairment (such as that seen in Alzheimer’s disease) in which deficits affect memory encoding, language and insight impairment occur early in the disease course. In the ageing patient, it is quite common for both pathologies to coexist and, therefore, no such distinction may be apparent.
Disease-modifying treatment for Parkinson’s disease-related cognitive change remains elusive, and symptomatic relief is hard to achieve through pharmacological means. Cholinesterase inhibitors, such as donepezil, rivastigmine and galantamine, have been shown to modestly improve cognitive function and global clinical status in patients with Parkinson’s disease and dementia but not in those with mild cognitive impairment.15 They have also been reported to worsen motor symptoms, particularly tremor, in more than 10% of patients. These agents are not currently PBS-approved for Parkinson’s disease-associated cognitive impairment or dementia.
Nonpharmacological therapies, therefore, have taken on a more central role in managing cognitive decline in people with Parkinson’s disease. Therapies, such as cognitive rehabilitation, and the use of tools, such as memory training and computerised cognitive training, may improve cognitive function, but are yet to yield consistent results in the limited studies to date.16 Physical therapies have shown the most robust and durable benefit on cognitive parameters.17
As with other neurodegenerative cognitive disorders, the most crucial intervention is often the timely provision of information, support and additional resources to the patient’s caregivers.
Gastrointestinal symptoms
Parkinson’s disease is a movement disorder in more ways than one. Most patients will experience clinically significant constipation, and impaired gastric emptying is also common. The significance of these features, which may not be considered by the patient to be out of the ordinary or particularly bothersome, is that they substantially affect the delivery and subsequent absorption of levodopa and other medications to the small bowel. The easiest and often most effective way to help a patient with an unexplained, subacute worsening of their Parkinson’s disease symptoms is to get their bowels moving and to leave their Parkinson’s disease medications alone (Box 223).
The treatment of constipation in Parkinson’s disease is with the usual measures, including lifestyle recommendations to increase dietary fibre and water intake and physical activity. Given the prevalence of constipation and its often subclinical nature, a common practice is to ask all patients to take an osmotic laxative (such as a macrogol-containing preparation) and a bulking agent (such as a fibre supplement) daily, whether they feel constipated or not, to prevent the unfortunate snowballing of a poorly perceived problem. Commonly prescribed bowel stimulant medications, such as sennosides, usually lead to disappointing results. Treatment is then titrated to effect, with the goal of one or two soft but formed bowel movements daily. This target ensures that any fluctuation in symptoms due to drug absorption issues is minimised and that doses of levodopa can then be adjusted with confidence.
Pain
Pain is a common and under-recognised nonmotor symptom of Parkinson’s disease, with at least half of all patients reporting moderate or severe pain at some point in their disease course.18 Parkinson’s disease-associated pain can manifest as typical musculoskeletal pain, neuropathic or radicular pain, dystonia-related cramps or an akathisia-like discomfort similar to restless legs syndrome that affects other body parts. Because of the age and comorbidities of the typical patient with Parkinson’s disease, such pain is often mistaken for arthritis, neuropathy or other more generic pain syndromes, such as sciatica.
Assessing pain in patients with Parkinson’s disease can be difficult, given the variabilities and competing diagnostic possibilities. In the first instance, any suggestion of a diurnal pattern or response to levodopa medication should be sought, since fluctuating pain can be an ‘off’ symptom in itself, both due to dystonia-related muscle tension and as a result of central pain sensitisation. Alternative causes should be sought and treated in parallel.
An optimal pain management strategy should be multimodal. Although significant benefits are seen with optimising dopaminergic therapy, using botulinum toxin injections for focal dystonia-associated pain and DBS, nonpharmacological approaches are also vital.19 Traditional physical and occupational therapy have proven benefits, but novel and recreational modalities, including regular walking, dance classes and Tai chi, have also been shown to help.20-22
Sleep
Many factors conspire to disrupt the sleep of a person with Parkinson’s disease. These may be disease-specific, such as restless legs syndrome, motor fluctuations (especially rigidity that impairs bed mobility) and Parkinson’s disease-related pain syndromes, or they may be more generic to the human condition and older people in particular, such as nocturia and generally less robust sleep-wake rhythms. Often, they are a combination of both, and the task is not to delineate their exact pathophysiology, but rather to ameliorate their effects by whatever means is most expedient, since inadequate sleep has substantial effects on quality of life, either directly, or by worsening other symptoms often seen in Parkinson’s disease, such as cognitive slowing and daytime somnolence.
The best approach to management is to identify patient-specific impediments to good sleep and treat them on their own terms. A dopamine agonist for restless leg syndrome, a slow-release levodopa tablet at bedtime for bed mobility issues and the optimisation of coexistent pain relief are reasonable first steps, in addition to the usual suite of sleep measures, such as increased physical activity during the day, avoiding stimulants and daytime naps and other poor sleep practices.
The role of the GP
Given the prevalence and diversity of nonmotor symptoms that emerge over the course of Parkinson’s disease, the value of good primary care is impossible to overstate. Vigilance for new symptoms and medication adverse effects, the timely engagement of appropriate allied health resources and frequent attention to the most salient therapeutic targets can help improve patient wellbeing, the clinical course of disease and quality of life.
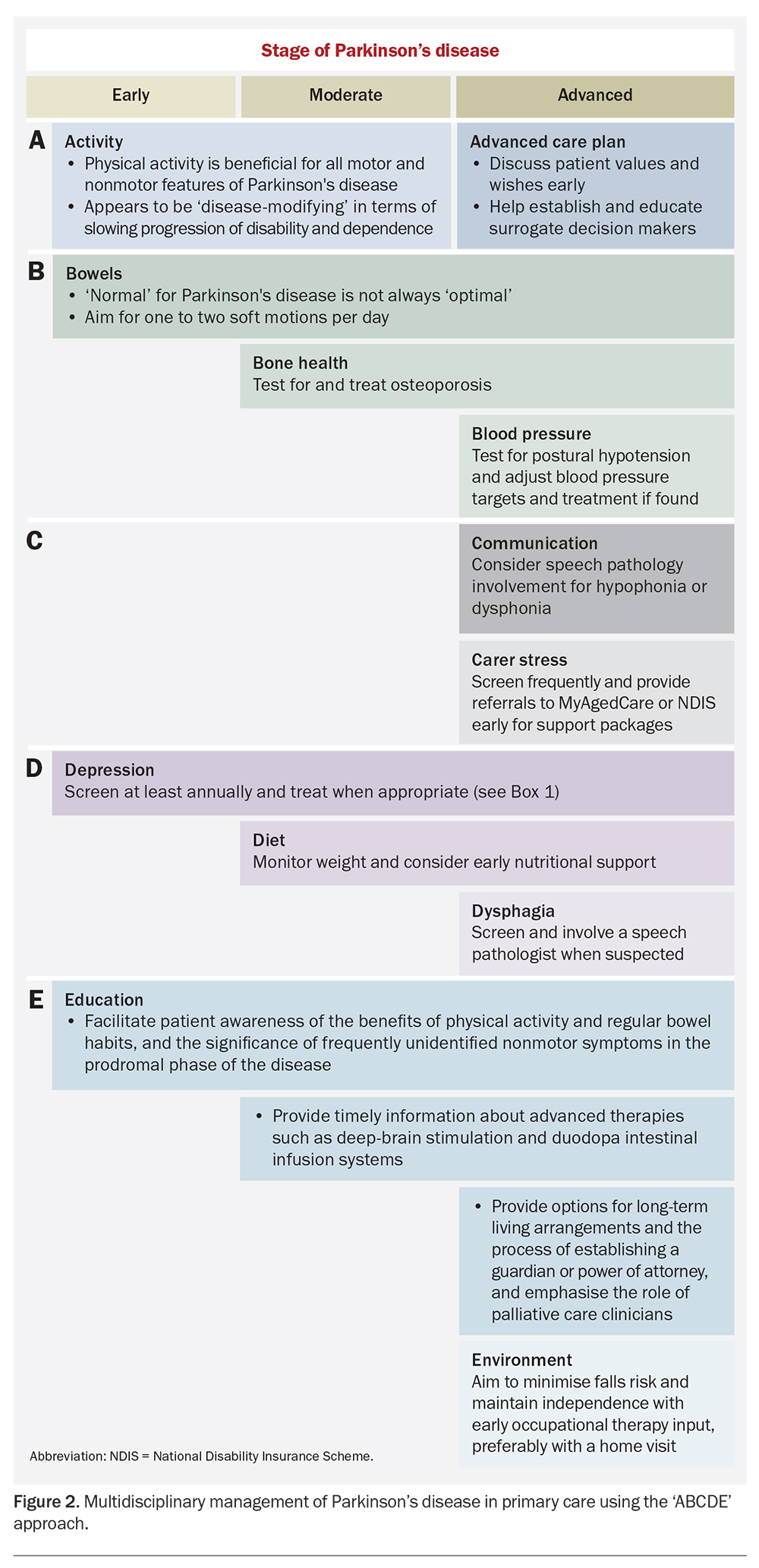
Given the variation in the evolution of Parkinson’s disease between patients, even at similar stages, a single approach to management for all patients is neither feasible nor helpful. Rather, a structured and systematic approach at each stage of a typical patient’s disease course is more useful. It may be helpful to use an ‘ABCDE’ approach, such as that outlined in Figure 2, to ensure that issues are raised and addressed in a consistent and systematic manner.
It is reasonable, in all but the most under-resourced healthcare settings, to leave the management of the motor aspects of Parkinson’s disease to the treating neurologist. Although many nonmotor aspects can and should also be addressed with the specialist, much of the day-to-day care provision can be done in primary care. In particular, implementing a comprehensive care plan, including referral to Parkinson’s disease-specific exercise programs (such as PD Warrior™or KOPD/Punching Parkos™; Box 3), Parkinson’s disease-oriented allied health practitioners and local Parkinson’s disease support groups, benefits immensely from the input of the consulting GP.
Conclusion
Parkinson’s disease is a multisystem condition that is unique to each patient and evolves substantially over a patient's lifetime. Clinical assessment remains indispensable, both to the initial diagnosis of Parkinson’s disease and to its ongoing care, especially with regard to the management of nonmotor symptoms, such as mood disorders, constipation, pain and sleep disturbance. A multidisciplinary approach that leverages the co-ordinated expertise and attention of primary care physicians, neurologists and allied health professionals is essential to optimising quality of life and independence for patients living with Parkinson’s disease. MT
COMPETING INTERESTS: Dr Rowe: None. Professor Sue is a non-executive Director of Living Cell Technologies (LCT); and a Director of Mitochondrial Therapeutics, the Australian Mitochondrial Medical Network and Cell Morphomics.
References
1. Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci 2002; 14: 223-236.
2. Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017; 124: 901-905.
3. GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017; 16: 877-897.
4. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet 2021; 397: 2284-2303.
5. Jia F, Fellner A, Kumar KR. Monogenic Parkinson’s Disease: genotype, phenotype, pathophysiology, and genetic testing. Genes (Basel). 2022; 13: 471.
6. Nalls MA, Blauwendraat C, Vallerga CL, et al; 23andMe Research Team; System Genomics of Parkinson’s Disease Consortium; International Parkinson’s Disease Genomics Consortium. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol 2019; 18: 1091-1102.
7. Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of Parkinson’s disease. Lancet Neurol 2020; 19: 170-178.
8. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 2016; 15: 1257-1272.
9. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015; 30: 1591-601.
10. Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord 2015; 30: 1600-1611.
11. Blocq P, Marinesco G. Sur un cas de tremblement parkinsonien hémiplégique symptomatique d’une tumeur du pédoncule cerebral. Comptes Rendus Séances Société Biol 1893; 5: 105-111.
12. Starkstein SE, Brockman S. Management of depression in Parkinson’s disease: a systematic review. Mov Disord Clin Pract 2017; 4: 470-477.
13. Aarsland D, Creese B, Politis M, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol 2017; 13: 217-231.
14. Mack J, Marsh L. Parkinson’s disease: cognitive impairment. Focus (Am Psychiatr Publ) 2017; 15: 42-54.
15. Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 2012(3): CD006504.
16. Orgeta V, McDonald KR, Poliakoff E, Hindle JV, Clare L, Leroi I. Cognitive training interventions for dementia and mild cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2020; 26; 2(2): CD011961.
17. Avenali M, Picascia M, Tassorelli C, Sinforiani E, Bernini S. Evaluation of the efficacy of physical therapy on cognitive decline at 6-month follow-up in Parkinson disease patients with mild cognitive impairment: a randomized controlled trial. Aging Clin Exp Res 2021; 33: 3275-3284.
18. Tai YC, Lin CH. An overview of pain in Parkinson’s disease. Clin Park Relat Disord 2019; 2: 1-8.
19. Ha AD, Jankovic J. Pain in Parkinson’s disease. Mov Disord 2012; 27: 485-491.
20. Sturkenboom IH, Graff MJ, Hendriks JC, et al; OTiP study group. Efficacy of occupational therapy for patients with Parkinson’s disease: a randomized controlled trial. Lancet Neurol 2014; 13: 557-566.
21. Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med 2009; 41: 475-481.
22. Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med 2012; 366: 511-519.
23. Lubomski M, Davis RL, Sue CM. Gastrointestinal dysfunction in Parkinson’s disease. J Neurol 2020; 267: 1377-1388.