Tremor: differentiating between causes

Tremors are the most common movement disorder encountered in clinical practice. They can be challenging to accurately assess and are frequently misdiagnosed. Although tremors can be benign, the consequences can have a negative impact on quality of life, which can be avoided with accurate diagnosis, prognostication and treatment.
- The International Parkinson and Movement Disorder Society recently reviewed their consensus criteria in 2018, advising for the classification of tremor according to axis 1 (clinical features) and axis 2 (aetiology).
- History-taking should encompass age of onset, affected body part, temporal evolution, past medical and family history, current medications, activating conditions and whether the tremor is associated with other neurological symptoms.
- Examination should include identification of whether the movement disorder is a tremor, determination of the location of the tremor, provocation of activating conditions with special tests (finger-nose testing, Archimedes spiral and handwriting) and recognition of any associated neurological signs, such as sensory or motor deficits elsewhere.
- The classification of tremors includes essential, drug-induced, parkinsonian, dystonic, functional, genetic, enhanced physiological, cerebellar, orthostatic and rubral aetiologies.
- Management should include pharmacological and nonpharmacological therapies, individualised to the patient, with consideration of specialist referral in the event of treatment resistance.
The International Parkinson and Movement Disorder Society reviewed their consensus criteria in 2018, revising previous guidelines established in 1998. Based on their recommendations, tremor is now defined as ‘an involuntary, rhythmic, oscillatory movement of a body part’ and is classified according to axes: clinical features (axis 1) and aetiology (axis 2).1 This article provides an approach to GPs for the clinical assessment of tremor in diagnosing, prescribing first-line treatment and informing neurological referrals, if appropriate.
Axis 1: clinical features
History
Age of onset
Tremor can begin in infancy (birth to 2 years); childhood (3 to 12 years); adolescence (13 to 20 years); early adulthood (21 to 45 years); middle adulthood (46 to 60 years); or late adulthood (>60 years). Age of onset can guide clinical reasoning.
Affected body part and temporal evolution
It is important to take note of the first affected body part, including symmetry, and whether further areas have been affected over time. Tremor can also be audible, with involvement of the voice, palate and tongue. The tremor may be focal (only one body region is affected), segmental (two or more contiguous body parts, e.g. head and arms), hemitremor (affecting one side of the body), or generalised (affecting the upper and lower body).
Past medical history
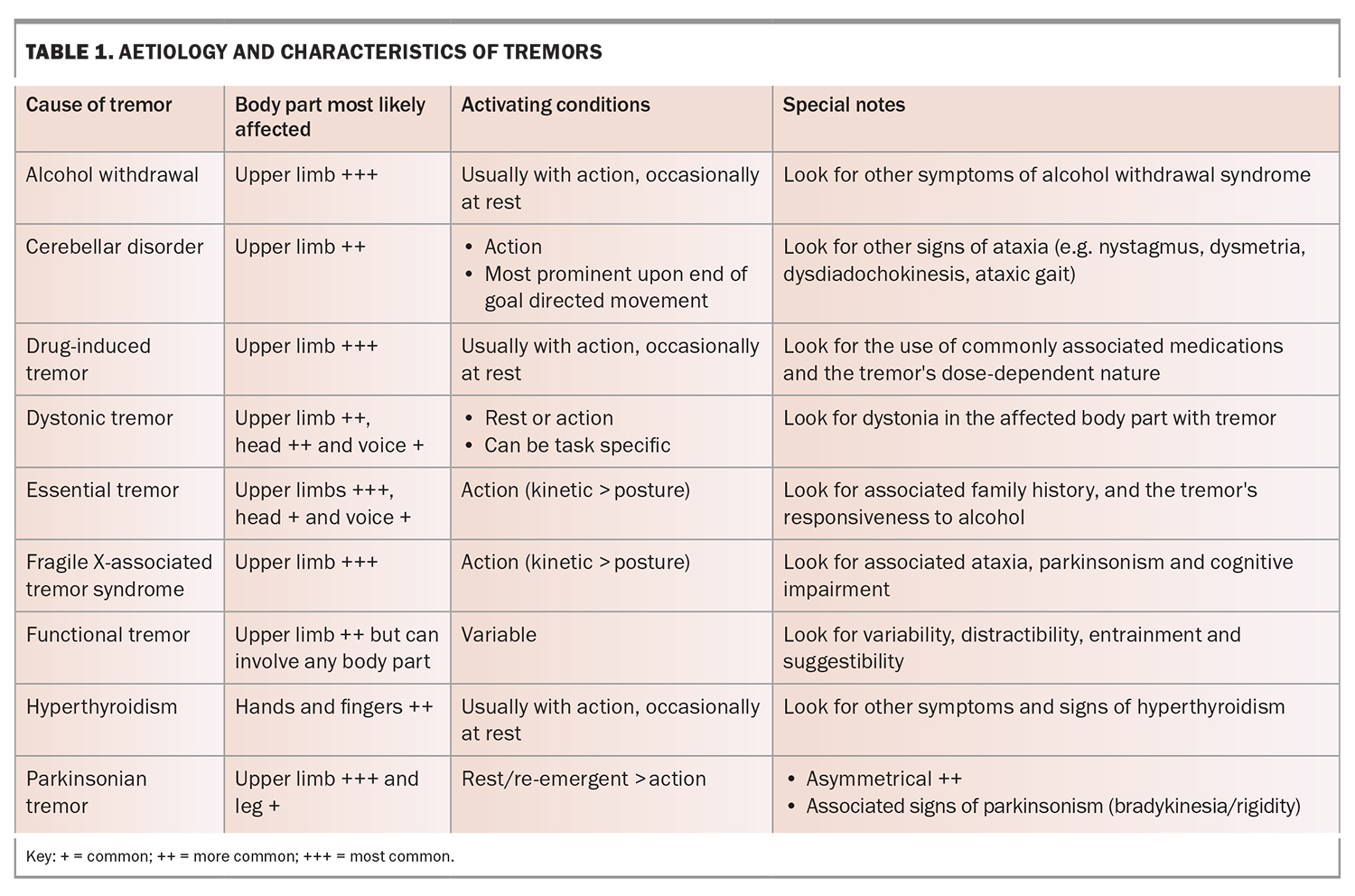
Tremor can be a symptom of many medical conditions or medications that may not relate
to neurological disorders (Table 1). Tremor can be a side effect of many psychiatric medications and is associated with substance use disorders, particularly withdrawal states (e.g. delirium tremens). Parkinson’s disease is commonly associated with anxiety, depression, psychosis and cognitive decline.
Family history
Take a brief family history of tremor and other neurological disorders (especially ataxia, Parkinson’s disease, dementia) with emphasis on first-degree relatives. Around two-thirds of patients with essential tremor have a first-degree relative with the same disorder.2 Moreover, 10 to 20% of patients with Parkinson’s disease have a first-degree relative with Parkinson’s disease.3
Medications and toxins
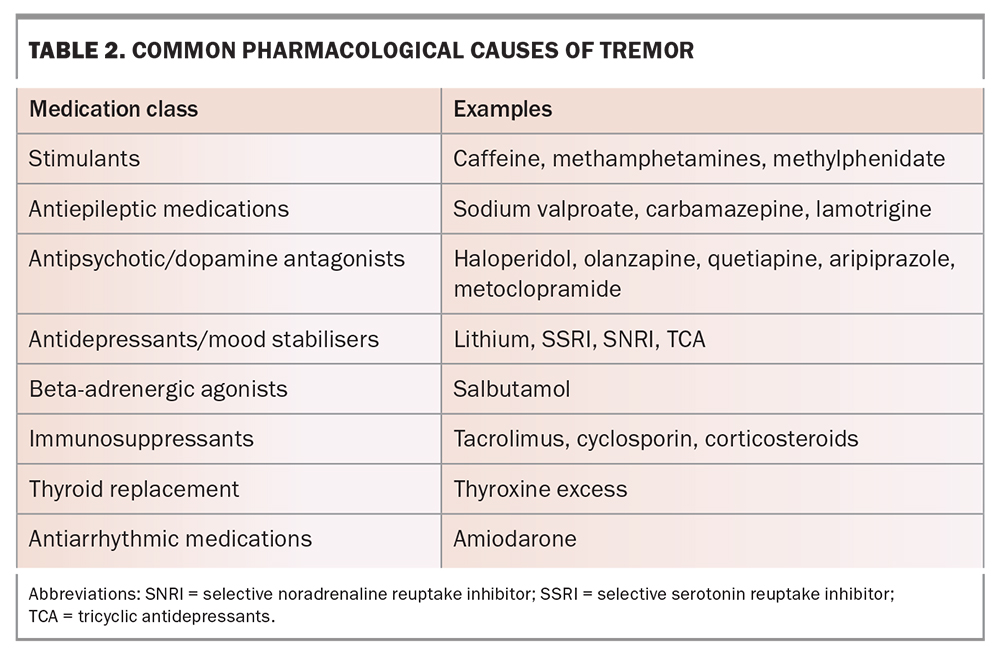
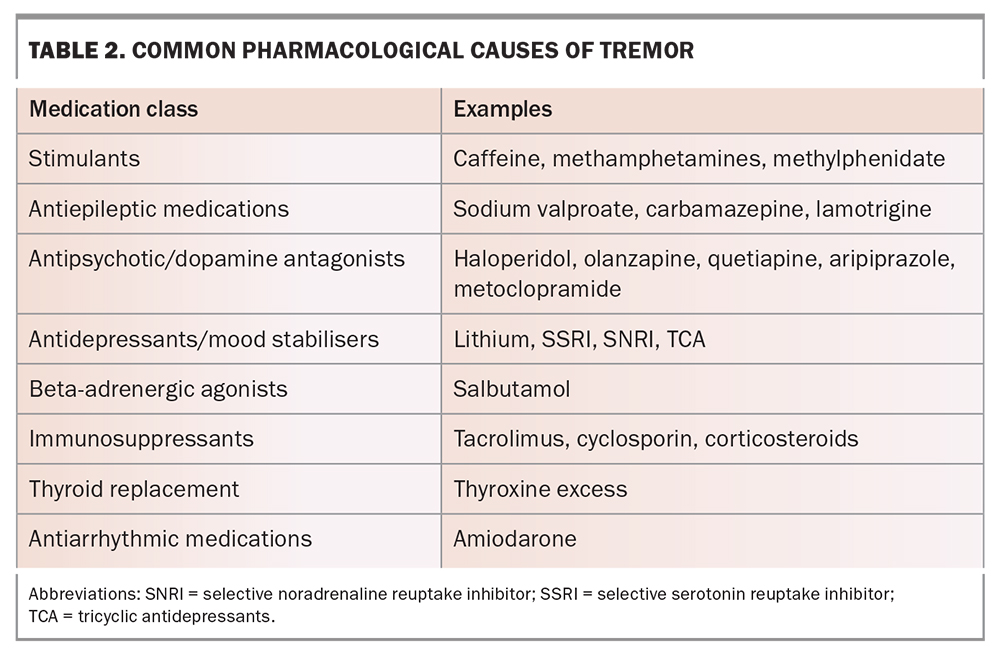
Multiple medications and toxins can cause tremor (Table 2). Temporal association of tremor onset or change in relation to medication commencement should be noted.
Activating conditions
Typically, tremor may be more noticeable at rest or with action. Ask the patient whether the tremor is apparent when at rest (e.g. when relaxed watching TV, sitting or lying down) or if it mainly affects action; for example, if the tremor is noted in the upper limbs when holding a cup of water. It is also worthwhile noting if the tremor only occurs during specific tasks, such as writing, playing a musical instrument, standing up or walking.
Other neurological symptoms
Determine whether the tremor is an isolated syndrome or involves other neurological symptoms. Patients should be asked about symptoms including weakness, stiffness in muscles, mobility problems (e.g. imbalance, falls, slowing down), sensory abnormalities, visual abnormalities, speech abnormalities and cognitive difficulties.
Examination
Is it a tremor?
First, confirm that the movement disorder is a tremor and not another involuntary movement (e.g. chorea, which is typically slower and less predictable than tremor [<4 Hz]). Tremor is involuntary, rhythmic and oscillatory in quality.
Where is the tremor?
Use the entire consultation period to characterise the anatomical distribution of the tremor and then examine if it affects other parts of the body, such as the face, palate, head, trunk or legs.
Are activating conditions involved?
Tremors at rest are best observed when the affected body part is fully relaxed. This can occur when the patient is sitting or lying down (depending on the body part). A parkinsonian tremor may be activated with a mental distraction task, such as asking the patient to perform serial sevens (counting backwards from 100 by sevens). Conversely, a functional tremor may be modified, diminished or lost with a mental distraction task.
An action tremor can be a postural or kinetic tremor. A postural tremor in the upper limbs is examined in two positions: holding the hands out in front, palms down and elbows extended with fingers slightly spread; and in the wing-beating position, with hands just below the chin, palms down, elbows flexed and at the same level of the shoulders.
A kinetic tremor is examined with finger-nose testing, having the patient draw spirals or assessing their handwriting. Any specific trigger for the tremor, such as playing a musical instrument, should also be assessed.
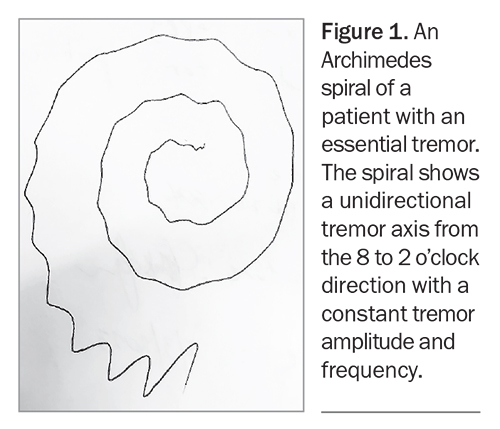
The Archimedes spiral test involves drawing a spiral on paper without resting the side of the hand on the paper, and then asking the patient try to emulate it; first with their dominant, then their nondominant hand (Figure 1). This test is often one of the most illuminating tasks because it helps to pinpoint more information about a patient’s specific tremor characteristics and is extremely useful to track changes over time and in response to therapy.
The writing and drawing of patients with Parkinson’s disease tends to be smaller and slower, with tightly bunched letters and curves (micrographia; Figure 2).4 In contrast, the script seen in patients with essential tremor when completing handwriting tasks tends to be larger, have a higher frequency and be more regular. The handwriting seen in patients with dystonic tremor has a lower frequency and is jerkier and more asymmetric.
Are there associated neurological or other symptoms?
Relevant associated neurological signs should be looked for on examination, including parkinsonism (bradykinesia, rigidity), cerebellar ataxia (nystagmus, dysarthria, dysmetria, dysdiadochokinesis, tandem gait) and dystonia (abnormal posturing of body parts potentially affected by tremor). Nonmotor symptoms that are typically associated with Parkinson’s disease often precede the diagnosis of Parkinson’s disease by many years (Box).
Wearables
The Parkinson KinetiGraph® is a biosensor worn on the wrist that is designed to continuously monitor and record movement activity in the patient’s home environment. It is used as part of an ancillary test, usually ordered by a specialist when the diagnosis is unclear or the response of the tremor to dopaminergic therapy is clinically uncertain. The biosensor is worn on the side of the body most severely affected by symptoms of Parkinson’s disease, including tremor and bradykinesia.5 Similarly, surface electromyography has sometimes been used as a diagnostic adjunct for tremor analysis but access is relatively limited.1
Axis 2: aetiology
Essential tremor
Essential tremor affects about 1% of the population and has a bimodal age of onset (early onset group younger than 24 years and older onset group above age 46 years).2 Essential tremor is usually bilateral, relatively symmetrical, and involves the upper limbs (kinetic more common than postural). It is typically not present at rest. Less commonly, it affects the head and voice; it rarely affects the legs. There may also be associated ‘soft’ neurological signs, such as mild memory impairment, impaired balance (tandem gait), subtle dystonic posturing or parkinsonism. It has an insidious onset and progression. There is frequently a family history of similar tremors, and about two-thirds of affected patients recognise that the tremor is improved temporarily by ingesting alcohol.2 Pharmacotherapy can be initiated by the primary care clinician if the patient experiences social embarrassment or disability. Referral to a neurologist is recommended if the patient responds poorly to treatment or has moderate to severe disability despite pharmacotherapy, or if there is uncertainty about the diagnosis.
Drug-induced tremor
Many medications and toxins can cause tremor (Table 2). A history of induction or exacerbation of tremor after the commencement of a medication should be sought. Furthermore, worsening of the tremor usually occurs in a dose-dependent manner and cessation of the medication should improve the tremor. The phenomenology of a drug-induced tremor can vary but typically involves a bilateral upper limb jerky action tremor. There will usually be associated neurological and nonneurological signs of drug toxicity.6,7
Parkinsonian tremor
Parkinsonian tremor tends to occur and be at its worst when at rest, is asymmetrical and unilateral at onset. It commonly involves the fingers and thumb (pill-rolling) and is progressive. It ceases or greatly subsides with action, but this may be followed by a
re-emergence of the tremor after several seconds of sustained posture (re-emergent tremor). It is important to look for associated features of parkinsonism (bradykinesia, rigidity, decreased arm swing, facial hypomimia, shuffling gait and postural instability).6,7 It can be difficult to differentiate parkinsonian tremor secondary to idiopathic Parkinson’s disease from antidopaminergic medication, so it is important to ask about the nonmotor features listed in the Box.8
Dystonic tremor
Dystonic tremor occurs in a body part affected by dystonia. The most common example is cervical dystonia with head tremor. The clues to diagnosing dystonic tremor is identifying the dystonia, which is characterised by abnormal (usually asymmetrical) posturing or positioning of the body part with associated asymmetric hypertrophy of the involved muscles. There may be an accompanying ‘geste antagoniste’ or sensory trick where a voluntary manoeuvre applied to the body part can temporarily improve the dystonia, such as touching the face or head in the case of cervical dystonia. The tremor is usually jerky in nature and there may be a null point (i.e. a certain position of the body part that reduces the tremor).9
Functional tremor
Functional tremors (and functional neurological disorders) are increasingly common in the post-COVID era.10 Clues in the patient's history include abrupt onset of the tremor, inconsistency of the symptoms and, potentially, psychological stressors prior to onset of the tremor. On examination, the tremor can be inconsistent and variable with respect to its anatomical distribution, frequency, direction and activation characteristics.6,7
Functional tremor includes any of the following features:
- distractibility, where the tremor improves with mental (e.g. serial sevens) or motor distraction (e.g. alternate finger tapping)
- entrainability, where the rhythm of the tremor can be changed to match a rhythmic movement in another limb
- suggestibility, where the tremor can improve or be exacerbated in response to a placebo stimuli.
Tremor secondary to genetic conditions
Tremor is a common neurological symptom associated with genetic conditions. Fragile
X-associated tremor ataxia syndrome (FXTAS) is significantly less common than parkinsonian tremors and essential tremors, with an estimated lifetime prevalence of 1 in 8000. FXTAS is an X-linked neurodegenerative disorder caused by a CGG repeat expansion (55 to 200) in the FMR1 gene. It presents with insidious onset of neurological symptoms after the age of 40 in men. The tremor seen is an action tremor in the upper limbs, with a similar appearance to an essential tremor. It is often associated with cerebellar ataxia, parkinsonism and cognitive impairment (executive dysfunction and memory impairment).11 Testing is covered by Medicare and it is important to identify because of its implications for family members.11,12
Enhanced physiological tremor
Enhanced physiological tremor has a frequency range of 8 to 12 Hz. It is bilateral, symmetrical and often manifests as a fine, rhythmic tremor in the hands. It can be exacerbated by stress or anxiety.6,7
Cerebellar tremor
Cerebellar tremor is a type of tremor that arises due to damage or dysfunction of the cerebellum. It is typically characterised as an intention tremor because it becomes more pronounced as the affected individual attempts to perform purposeful or goal-directed movements. The tremor is typically absent at rest or during complete relaxation. The frequency of cerebellar tremor is usually low, typically ranging between 2 to 5 Hz. The amplitude or intensity of cerebellar tremor can vary, ranging from mild to severe. Cerebellar tremor is often accompanied by other signs of cerebellar dysfunction, such as ataxia (unco-ordinated movements), dysmetria (impaired ability to judge distances) and dysarthria (difficulty in articulating speech).6,7
Cerebellar tremor can be caused by:
- cerebellar lesions, such as cerebellar stroke, multiple sclerosis, tumours, or brain trauma
- progressive degenerative disorders affecting the cerebellum, such as spinocerebellar ataxias or multiple system atrophy
- alcohol abuse, resulting in cerebellar degeneration
- genetic disorders, such as FXTAS or autosomal dominant spinocerebellar ataxias.
Orthostatic tremor
Orthostatic tremor is a rare neurological condition characterised by a high-frequency (13 to 18 Hz) tremor that occurs when standing or maintaining an upright posture. The tremor typically affects the legs but can also involve the trunk and sometimes the arms. It is distinct from essential tremor or Parkinson’s disease, although it can sometimes coexist with these conditions. It may respond to gabapentin or pregabalin and nonpharmacological approaches, including canes and walkers.6,7
Rubral tremor
Also known as a Holmes tremor, rubral tremor arises due to damage or dysfunction in the red nucleus of the midbrain, which plays a role in motor control. It typically occurs with rest, posture, action and intention, and has a low frequency of 2 to 4 Hz. It is commonly associated with other cerebellar features, such as dysmetria, dysdiadochokinesia and ataxia.6
Treatment of tremor
Removal of exacerbating factors
In the case of tremor that is caused or exacerbated by a secondary factor, this should be addressed first (e.g. removal of the offending medication). In the case of functional tremor, psychiatric input and psychological therapy may be useful.6
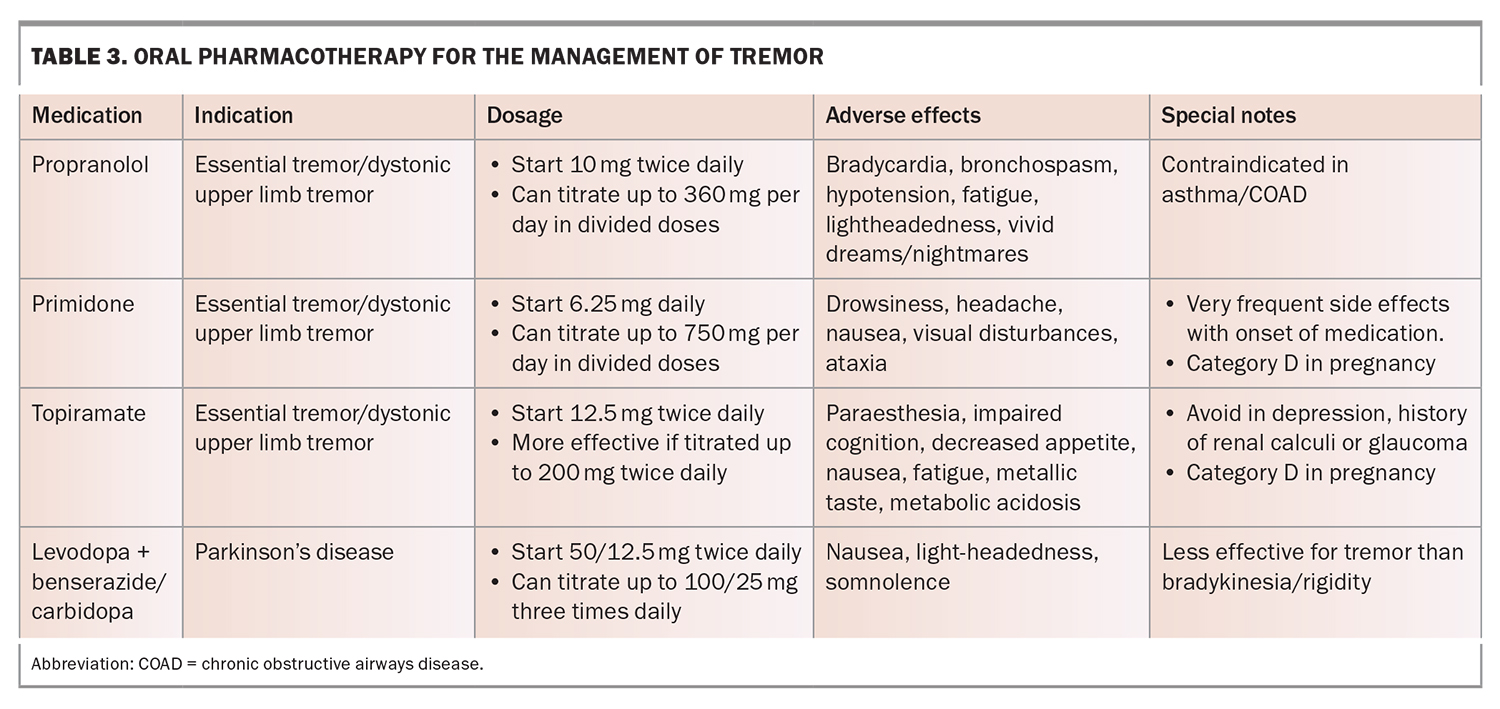
Oral pharmacotherapy
Several oral medications are available for the treatment of tremor, as outlined in Table 3. Oral treatments for essential tremor have not changed for decades and include propranolol, primidone and topiramate. The choice of agent is largely dictated by the adverse effect profile. Response can be variable but, generally, there is only a mild to moderate improvement (up to 50% at best).2,6 Propranolol is usually the best-tolerated option. Several T-type calcium channel blockers that look promising for the treatment of essential tremor are in development.13
The only other oral pharmacotherapy for tremor is dopaminergic therapy in the treatment of Parkinson’s disease; with its distinct pathophysiological process of dopaminergic neuron loss, dopaminergic therapy (most commonly in the form of levodopa) can lead to significant improvements in tremor. However, it must be noted that the improvement in tremor is usually less than for bradykinesia and rigidity at the same dose of dopaminergic therapy.
Chemodenervation
Botulinum toxin therapy is typically first-line therapy for focal dystonic tremor affecting the head, jaw or vocal cords and can also be considered for medically refractory essential tremor or dystonic upper limb tremor. This therapy is given usually at three-monthly intervals and must be given by experienced injectors, as dosage and choice of muscles must be individualised. Tremor improvement is seen after one week. The main potential adverse effect of toxin therapy is weakness of the injected muscles.
Functional neurosurgical treatments
Medically refractory tremor can respond well to deep brain stimulation (DBS). Tremor aetiology is not crucial in determining response to DBS; it can be effective for dystonic tremor as well as parkinsonian tremor, beyond the best response achievable with dopaminergic medication.14 DBS for tremor entails implantation of unilateral/bilateral electrodes into the contralateral thalamic ventral intermediate nucleus (ViM) or posterior subthalamic area (PSA) of the brain, which are then connected to an implantable pulse generator sitting in the chest wall, similar to a cardiac pacemaker. The mechanism of improvement in tremor with DBS is believed to relate to the stimulation causing disruption to the maladaptive function of cerebellothalamic pathways. Tremor improvement can be 50 to 90% (greater than with oral pharmacotherapy), with evidence of sustained benefit in the long term.14 Parkinsonian tremor can additionally respond to DBS of the subthalamic nucleus.
More recently, magnetic resonance imaging-guided high-intensity focused ultrasound (MRIgFUS) has emerged as a nonsurgical alterative to DBS.15 It involves image-guided thermocoagulation of the ViM nucleus using high intensity ultrasound. The efficacy of this treatment is similar to DBS, although there are no head-to-head trials. MRIgFUS has the advantage of being less invasive than DBS and has less perioperative risks but treatment related adverse effects may be more persistent, because of its lesional nature (whereas stimulation from DBS can be adjusted). MRIgFUS is better established for unilateral treatment of tremor.15
Conclusion
Tremor is the most common movement disorder encountered in general practice. It can have a significant impact on a patient’s quality of life. Diagnosis requires judicious history-taking and a careful examination of the characteristics of the tremor. Using this approach to make an accurate diagnosis allows for appropriate initial management and significantly reduces the risk of adverse outcomes. Uncertainty regarding the diagnosis or resistance to treatment should indicate referral to a neurologist. MT
COMPETING INTERESTS: Dr Andrew Evans has received honoraria for providing presentations to AbbVie, Abbott, STADA, Pfizer and Ipsen; has served on advisory boards for AbbVie, STADA and Ipsen; and has purchased stock with CSL and GKC Australia. Dr Yeung: None.
References
1. Bhatia KP, Bain P, Bajaj N, et al. Tremor Task Force of the International Parkinson and Movement Disorder Society. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018; 33: 75-87.
2. Shanker V. Essential tremor: diagnosis and management. BMJ 2019; 366:l4485.
3. Deng H, Wang P, Jankovic J. The genetics of Parkinson disease. Ageing Res Rev 2018; 42: 72-85. doi:10.1016/j.arr.2017.12.007
4. Shukla AW, Ounpraseuth S, Okun MS, Gray V, Schwankhaus J, Metzer WS. Micrographia and related deficits in Parkinsons disease: a cross-sectional study. BMJ Open 2012; 2: e000628.
5. Objective measurement in Parkinsons disease: a descriptive analysis of Parkinsons symptom scores from a large population of patients across the world using the Personal KinetiGraph®. Pahwa et al. Journal of Clinical Movement Disorders 2020; 7: 5 https://doi.org/10.1186/s40734-020-00087-6.
6. Puschmann A, Wszolek ZK. Diagnosis and treatment of common forms of tremor. Semin Neurol 2011; 31: 65-77.
7. Louis ED. Tremor. Continuum (Minneap Minn) 2019; 25: 959-975. https://doi.org/10.1212/CON.0000000000000748.
8. Evans AH, Chai CH. Evaluation of Nonmotor Symptoms in Diagnosis of Parkinsonism and Tremor. Parkinsons Dis 2016. https://doi.org/10.1155/2016/9182946.
9. Fasano A, Bove F, Lang AE. The treatment of dystonic tremor: a systematic review. J Neurol Neurosurg Psychiatry 2014; 85: 759-769.
10. Cartella S, Terranova C, Arena I, Quartarone A, Girlanda P. Impact of Covid-19 on essential tremor and dystonic tremor: Experience of an Italian centre. J Neurol Sci 2021; 429: 119437. https://doi.org/10.1016/j.jns.2021.119437.
11. Hagerman R, Hagerman P. Fragile X-associated tremor/ataxia syndrome: pathophysiology and management. Curr Opin Neurol 2021; 1; 34: 541-546. https://doi.org/10.1097/WCO.0000000000000954.
12. Is Fragile X Testing Covered by Medicare? Fragile X Association of Australia; 2023. Available online at: https://www.fragilex.org.au/fragile-x-disorders/fragile-x-syndrome/testing-diagnosis/testing-for-fragile-x/is-testing-covered-by-medicare/.
13. Matthews LG, Puryear CB, Correia SS, et al. T-type calcium channels as therapeutic targets in essential tremor and Parkinson’s disease. Ann Clin Transl Neurol 2023; 10(4): 462-483. https://doi.org/10.1002/acn3.51735.
14. Kremer NI, Pauwels RWJ, Pozzi NG, et al. Deep Brain Stimulation for Tremor: Update on Long-Term Outcomes, Target Considerations and Future Directions. J Clin Med 2021; 5; 10:3468. https://doi.org/10.3390/jcm10163468.
15. Agrawal M, Garg K, Samala R, Rajan R, Naik V, Singh M. Outcome and Complications of MR Guided Focused Ultrasound for Essential Tremor: A Systematic Review and Meta-Analysis. Front Neurol 2021; 7; 12:654711. https://doi.org/10.3389/fneur.2021.654711.